Abstract
Background
Proteinuria induced by lenvatinib is a class effect that occurs secondary to VEGFR suppression. Withholding of lenvatinib is required in cases with severe proteinuria. Urine protein–creatinine ratio (UPCR, g/gCre) has recently attracted attention as an alternative to 24-h urine collection for assessing proteinuria. The aim of this study was to examine the correlation between the results of proteinuria assessed by the dipstick test and UPCR, and to investigate the influence of proteinuria grading with UPCR on lenvatinib dose adjustment compared to that with only the dipstick test.
Method
Three hundred and ten urine samples from 63 patients with advanced thyroid cancer under treatment with lenvatinib, which were tested by both the dipstick test and UPCR were analyzed. Lenvatinib was withheld when there was evidence of CTCAE grade 3 proteinuria, and restarted when it resolved. The frequency of proteinuria, correlation between the results of the dipstick test and UPCR test, and the effect of dose withholding in cases with results of 3 + in the dipstick test were calculated.
Results
Proteinuria was seen in 56 (88.9%) patients. Of the 154 dipstick 3 + samples, only 56 (36.4%) were judged as more than 3.5 g/gCre by UPCR (grade 3 proteinuria), although none of the 1 + and only 3.7% of 2 + samples were judged as grade 3 proteinuria. We were able to prevent unnecessary lenvatinib interruption due to proteinuria in 63.6% of dipstick 3 + samples by assessment of UPCR.
Conclusions
Urinalysis by combination of the dipstick test and UPCR assessment might be a better strategy for preventing unnecessary interruption of lenvatinib.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the years, anti-vascular endothelial growth factor (VEGF)/VEGF receptor (VEGFR) agents have been developed as anticancer agents, since angiogenesis plays a role in tumor growth. Lenvatinib, which is a multikinase inhibitor of VEGFR 1–3, fibroblast growth factor receptors 1–4, platelet-derived growth factor-alpha, KIT and RET, is one such agent [1,2,3]. VEGFR-2, in particular, is the most important mediator of tumor angiogenesis, along with being an important mediator of nephrin, a protein essential for proper functioning of the renal filtration barrier [3,4,5]. Hence, VEGF-2 suppression induces not only strong tumor suppression, but also proteinuria [3]. Thus, with the rapid development of these agents, the management of proteinuria has gained more attention than before.
Lenvatinib is indicated as monotherapy in patients with locally recurrent, progressive, and radioiodine refractory differentiated thyroid carcinoma (RR-DTC) [6] and unresectable hepatocellular carcinoma (HCC) [7]. Further indications of lenvatinib are also expected in future, such as its combination with everolimus in patients with advanced renal cell carcinoma (RCC) [8]. Appropriate use of lenvatinib involves an adequate proteinuria management strategy to obtain maximum clinical benefit from the drug. Interruption and reduction of the lenvatinib dose are recommended when high-grade proteinuria occurs during treatment, making regular proteinuria monitoring in patients on lenvatinib therapy mandatory [4, 9].
However, protocols and strategies for monitoring and management of the proteinuria induced by lenvatinib remain unestablished. According to National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) v4.0 guidelines (available at: http//ctep.cancer.gov/reporting/ctc.html Accessed November 1, 2019), grade 3 proteinuria is defined as urinary protein levels greater than 3.5 g/gCre in a 24-h urine sample. This 24-h urine protein test relies on the patient collecting overnight urine samples, which is laborious, and might be influenced by patient compliance [9]. Urinary protein is occasionally evaluated only by use of a qualitative dipstick analysis, due to the convenience of this method in real practice. However, performing only qualitative dipstick urinalysis ignores the influence of urine concentration, which impacts test accuracy [10, 11]. Hence, evaluation of proteinuria using only qualitative dipstick urinalysis might be insufficient as an indicator of the need for dose adjustment of lenvatinib.
Urine protein–creatinine ratio (UPCR, g/gCre), which is a simple ratio of the level of protein (mg/dl) and creatinine (mg/dl) in a single-void urine sample, is known to correlate significantly with 24-h proteinuria [10, 12]. Hence, in our practice, we adopted UPCR instead of 24-h urine collection as a method of grading proteinuria. The aim of this study was to examine the correlation between the results of proteinuria assessed by the dipstick test and UPCR, and to investigate the influence of proteinuria grading on lenvatinib dose adjustment in patients with advanced thyroid cancer treated with lenvatinib.
Patients and methods
This study involved 63 patients with advanced thyroid cancer who received lenvatinib therapy at Ito Hospital, Tokyo, Japan from May 2015 to February 2018, and whose results of urinalysis were obtained. The patients’ characteristics and renal parameters are shown in Table 1. The estimated glomerular filtration rate (eGFR, mL/min/1.73 m2) of all patients was ≥ 30 mL/min/1.73 m2 at baseline. Data up to 27 August 2018 were assessed and retrospectively reviewed.
Adverse events (AEs) were assessed based on the CTCAE version 4.0. When treatment-related grade 3 or intolerable grade 2 AEs were seen, lenvatinib was interrupted until the events resolved to grade ≤ 2 or baseline, following which lenvatinib was restarted, but with sequential dose reductions, if necessary.
Urinalysis was performed at baseline and at every regular visit, at least every 2 weeks for the first 2 months and every month thereafter, if the patient’s condition was clinically stable. Each urine sample first underwent a qualitative test using a commercially available dipstick (MULTISTIX SG, CLINITEK Status + Analyzer; Siemens Healthcare Diagnostics Inc., Deerfield, IL, USA). Samples that tested positive for proteinuria, namely 1 + or more (+ 2, and + 3) (only “3 + ” before July 2017) on the dipstick test were sent for UPCR testing (Micro TP-AR 2 Pyrogallol Red method; FUJIFILM Wako Pure Chemical Corp., Osaka, Japan, Aqua-auto Kainos CRE-III plus; Kainos Laboratories, Inc., Tokyo, Japan, and JCA-BM9130; JEOL Ltd., Tokyo, Japan,) on the same day. Dose adjustment was decided based on the results of UPCR as follows: lenvatinib was interrupted when ≥ 3.5 g/gCre, i.e. grade 3 proteinuria, was seen, and was restarted when proteinuria improved to < 3.5 g/gCre, i.e. equal to or less than grade 2 (Fig. 1). Although CTCAE criteria define grade 3 proteinuria as higher than 3.5 g/24 h in a 24-h urine sample, we adopted only UPCR as the quantitative test in this study, based on the accepted theory that the results of UPCR correlate with the results of 24-h-timed urine collection [10, 12].
A total of 310 adequately collected urine samples from 63 patients that were tested by both the dipstick test and UPCR were retrospectively analyzed. Patient characteristics are as shown in Table 1. Seven patients with baseline proteinuria of “1 + ” by the dipstick test were included, none of whom were found to have any past renal disease. First, the frequency and timing of proteinuria in these patients were investigated. In patients with positive baseline proteinuria, they were considered to have developed treatment-related proteinuria only when it became more severe. Non-progressive proteinuria was not counted as proteinuria. Then, the correlation between the results of the qualitative dipstick test and quantitative UPCR test was examined using the Dunn test. In the case of a result of 3 + in the dipstick test, the number of samples that actually had grade 3 proteinuria was calculated to investigate the effect of withholding the drug in patients with a false positive test result. Overall survival (OS) of 39 differentiated thyroid carcinoma (DTC) patients stratified based on the presence or absence of grade 3 proteinuria were investigated using the Kaplan–Meier method by focusing on the relationship between proteinuria and survival benefit with our management strategy.
All study participants provided their informed consent for study participation, and the study design was approved by our institutional ethics review board.
Results
Incidence of proteinuria
The incidence and median time to first onset of any grade and grade 3 proteinuria in the 63 patients irrespective of thyroid cancer histology were 84.1% [53 patients, 2.7 (0.1–26.6) months] and 28.6% [18 patients, 5.9 (0.7–21.6) months], respectively. Table 2 shows the incidence and timing of proteinuria in patients stratified based on histological type of thyroid cancer, i.e. differentiated, anaplastic, and medullary thyroid carcinoma (DTC, ATC, and MTC).
Correlation between qualitative and quantitative results
Figure 2 shows the results of dipstick testing in relation to quantitative UPCR in the 310 urine samples. Of the 47 samples that tested 1 + by dipstick testing, 42 (89.4%) were judged as less than 1 g/gCre by UPCR (equal to or lower than grade 1 proteinuria) and none (0%) corresponded to grade 3 proteinuria (≥ 3.5 g/gCre). Of the 109 samples that tested 2 + by dipstick testing, 105 (96.3%) were judged as less than 3.5 g/gCre by UPCR (equal to or lower than grade 2 proteinuria), and four samples (3.7%) corresponded to grade 3 proteinuria, suggesting the need for withholding lenvatinib in these patients. Of the 154 samples with 3 + proteinuria by the dipstick test, only 56 (36.4%) were judged to have proteinuria of more than 3.5 g/gCre by UPCR (grade 3 proteinuria), and a whopping 98 (63.6%) samples showed lower than grade 3 proteinuria. Furthermore, a total of 13 samples from 9 patients that tested as grade 3 + on qualitative dipstick analysis revealed severe proteinuria of over 6 g/gCre (Fig. 2).
Correlation between qualitative dipstick and quantitative urine protein–creatinine ratio (UPCR) test values. The number (%) of samples with 1 + , 2 + , and 3 + proteinuria by the dipstick test that were found to have proteinuria of more than 3.5 g/gCre by UPCR (grade 3 proteinuria) was none (0%), 4 (3.7%), and 56 (36.4%), respectively. Dunn test: r = 0.5349, p < 0.0001
Relationship between proteinuria and survival outcomes
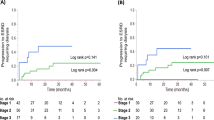
With our oncological treatment strategies, there were no significant differences in OS between the groups of patients with grade 3 versus grade ≤ 2 proteinuria among the 39 DTC patients (Fig. 3). Five (7.9%) of all the patients studied discontinued lenvatinib due to uncontrollable proteinuria, and none of the patients suffered from nephrotic syndrome, although a patient with timed decrease in eGFR died due to cancer. None of the patients required dialysis initiation.
Discussion
Lenvatinib, which is a strong suppressor of VEGFR-2, is known to frequently induce proteinuria [6, 7], especially in Japanese subjects [13]. Proteinuria is well known as a relatively commonly shared class effect among all therapeutics targeting the VEGF pathway [1, 2]. Early detection and close monitoring to prevent the worsening of proteinuria and development of renal-associated problems is required. The first step in monitoring for proteinuria is the qualitative dipstick test. If this indicates significant proteinuria, 24-h timed urine collection is unquestionably the gold standard for accurate evaluation of proteinuria [9]. Although this test is burdensome in terms of its inconvenience and the inaccuracy involved in repeated collection of 24-h urine samples, CTCAE criteria recommend this test for grading of proteinuria. In real practice, the qualitative dipstick test is the only test adopted in some cases. However, it is not well known that higher levels of urinary protein secretion are associated with a wide range of quantitative results [11]. This is a very important problem because the results of proteinuria grading are directly linked to lenvatinib dose adjustment.
UPCR is a quantitative test, in which the level of urinary protein (mg/dl) is divided by the urinary creatinine level (mg/dl) in a spot urine sample, that has been shown to have acceptable sensitivity and specificity compared with the standard 24-h urine protein test and is more convenient for clinical usage [14, 15]. It is based on the premise that in a given patient, the urinary creatinine excretion rate is fairly constant in the presence of a stable glomerular filtration rate and does not depend on urine concentration [12]. The utility of UPCR has already been demonstrated in the REFLECT trial of HCC patients [16], and it has been recommended by different international guidelines [9, 17]. Based on these recommendations, we investigated the feasibility of performing the CTCAE-recommended proteinuria grading using UPCR instead of a 24-h urine sample in thyroid cancer patients treated with lenvatinib (Fig. 1).
Our study revealed that only a third of samples that were 3 + for proteinuria using the urine dipstick test were CTCAE grade 3 by UPCR (Fig. 2). When our UPCR results were applied to our treatment strategy, overestimation of proteinuria was found in as many as 63.6% of samples, which could have led to unnecessary lenvatinib interruption in these patients. These findings show that grade 3 proteinuria should never be defined purely on the basis of a result of “3 + ” in the dipstick test, as this could lead to unnecessary cessation of lenvatinib therapy. Furthermore, it is interesting to note that some “3 + ” samples showed > 6 g/gCre proteinuria. Assessing UPCR would ensure that such severe proteinuria is not overlooked. Patients showing high levels of proteinuria need special long-term evaluation of the amount and duration of proteinuria, renal function, and physical findings (e.g., leg edema and hypoalbuminemia). In our study, UPCR values of samples that tested as 1 + and 2 + on qualitative analysis were also assessed. The results revealed that qualitative results of 1 + and 2 + correlate well with UPCR grade 1 and grade 2 proteinuria, respectively. On the other hand, a qualitative result of 3 + shows wide-ranging quantitative results. These results are compatible with those of past basic researches [10, 15] and is closely related with urinary concentration [11].
The dipstick test is a conventional urinalysis widely used to screen proteinuria, which is deemed to show positive results only when protein excretion exceeds 0.3 g/l. The numerical values of urinary protein corresponding to the results of dipstick assessment are roughly as follows: 1 + represents a value of 0.3 g/l, 2 + corresponds to 1 g/l, 3 + corresponds to 3 g/l, and 4 + with > 3 g/l [2]. This indicates that the range of quantitative results covered by the qualitative results becomes wider as the concentration increases. Therefore, we should recognize again that more severe proteinuria is associated with greater uncertainty of the results of qualitative tests.
Our management of proteinuria in thyroid cancer patients on lenvatinib therapy is based on CTCAE grading and involves withholding lenvatinib in patients with proteinuria, as recommended by the REFLECT guidelines, although we assess proteinuria using UPCR and not 24-h urine collection [7]. The results of the current study indicated that this strategy did not negatively affect the survival prognosis of thyroid cancer patients with grade 3 proteinuria (Fig. 3). Several reports that evaluated patients receiving treatments for different malignancies stated that grading of AEs is useful in the prediction of patient prognosis [18, 19]. However, these results cannot necessarily be applied to our subjects, because patients with thyroid cancer treated with lenvatinib generally have a prolonged treatment period. In our investigation, of the 15 DTC patients who showed grade 3 proteinuria, 8 patients experienced initial grade 3 more than 6 months after treatment initiation, six of whom experienced more than 1 year later. These results also supported by the fact that the rates of proteinuria significantly differ between patients with DTC and ATC, which would significantly affect treatment duration. We believe that the greater likelihood of development of proteinuria with long-term treatment is more relevant than the prognostic implications of the early development of proteinuria.
Furthermore, the impact of proteinuria on renal function with long-term lenvatinib exposure has not been revealed yet, with little evidence available on whether it induces renal failure [4, 20]. Since proteinuria identified in health check-ups predicts the risk of end-stage renal disease in the general population [21, 22], it suggests the need for further investigation of renal outcome in patients with proteinuria. A field called “onconephrology” is now developing to further study such issues [23, 24].
The balance between allowed harm and provided benefit from lenvatinib treatment is an important aspect of therapy. Needless to say, prolongation of OS with anti-cancer treatment is given the highest priority, along with consideration of renal prognosis that is commensurate with oncological prognosis in patients treated with lenvatinib, since there is no specific supportive care appropriate for reducing proteinuria. Sorafenib, another agent approved for DTC [25], rarely induces proteinuria and is proved to have no effect on renal function [26, 27]. We need to consider these points comprehensively and continue treatment while balancing the benefits and harms of treatment. To this end, we believe that it is better to monitor urine protein levels with UPCR than with only the dipstick test.
There are two limitations to this study. One is that the criteria of UPCR testing were not consistent throughout the observation period. The other is that we did not perform a comparison between the results of 24-h collection and UPCR. There is still controversy over the correlation between these two methods [28,29,30]. The timing of random urine collection, renal function, type of kidney disease, and handling of urine samples might all affect the accuracy of UPCR [14, 30, 31]. Urinary protein excretion rate is associated with changes in posture, physical activity, protein intake and hemodynamic factors [29], and urinary creatinine concentration is associated with muscle size [15]. The effects of these factors on cancer patients under treatment have not been investigated.
To the best of our knowledge, our study is the first to describe the utility of UPCR for the assessment of proteinuria in patients treated with lenvatinib in actual clinical practice. Our study revealed that two-thirds of urine samples that test as 3 + in dipstick tests show less than CTCAE grade 3 proteinuria with UPCR. Hence, combination of qualitative and quantitative tests is likely to be significantly more meaningful in real practice than just using the urinary dipstick test for facilitating proper dose adjustment and obtaining maximum efficacy of lenvatinib. In conclusion, urinalysis by combination of the dipstick test and UPCR might be a better strategy for avoiding unnecessary interruption of treatment in thyroid cancer patients treated with lenvatinib.
References
Stjepanovic N, Capdevila J (2014) Multikinase inhibitors in the treatment of thyroid cancer: specific role of lenvatinib. Biologics 8:129–139. https://doi.org/10.2147/BTT.S39381
Izzedine H, Massard C, Spano JP et al (2010) VEGF signalling inhibition-induced proteinuria: mechanisms, significance and management. Eur J Cancer 46(2):439–448. https://doi.org/10.1016/j.ejca.2009.11.001
Salgia R (2011) Prognostic significance of angiogenesis and angiogenic growth factors in nonsmall cell lung cancer. Cancer 117(17):3889–3899. https://doi.org/10.1002/cncr.25935
Cosmai L, Gallieni M, Liguigli W et al (2017) Renal toxicity of anticancer agents targeting vascular endothelial growth factor (VEGF) and its receptors (VEGFRs). J Nephrol 30(2):171–180. https://doi.org/10.1007/s40620-016-0311-8
Kitamoto Y, Tokunaga H, Miyamoto K et al (2002) VEGF is an essential molecule for glomerular structuring. Nephrol Dial Transplant 17(Suppl 9):25–27. https://doi.org/10.1093/ndt/17.suppl_9.25
Schlumberger M, Tahara M, Wirth LJ et al (2015) Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med 372(7):621–630. https://doi.org/10.1056/NEJMoa1406470
Kudo M, Finn RS, Qin S et al (2018) Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 391(10126):1163–1173. https://doi.org/10.1016/S0140-6736(18)30207-1
Motzer RJ, Hutson TE, Glen H et al (2015) Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: a randomised, phase 2, open-label, multicentre trial. Lancet Oncol 16(15):1473–1482. https://doi.org/10.1016/S1470-2045(15)00290-9
Capdevila J, Newbold K, Licitra L et al (2018) Optimisation of treatment with lenvatinib in radioactive iodine-refractory differentiated thyroid cancer. Cancer Treat Rev 69:164–176. https://doi.org/10.1016/j.ctrv.2018.06.019
Ralston SH, Caine N, Richards I et al (1988) Screening for proteinuria in a rheumatology clinic: comparison of dipstick testing, 24 hour urine quantitative protein, and protein/creatinine ratio in random urine samples. Ann Rheum Dis 47(9):759–763. https://doi.org/10.1136/ard.47.9.759
Yang CY, Chen FA, Chen CF et al (2015) Diagnostic accuracy of urine protein/creatinine ratio is influenced by urine concentration. PLoS ONE 10(9):e0137460. https://doi.org/10.1371/journal.pone.0137460
Ginsberg JM, Chang BS, Matarese RA et al (1983) Use of single voided urine samples to estimate quantitative proteinuria. N Engl J Med 309(25):1543–1546. https://doi.org/10.1056/NEJM198312223092503
Kiyota N, Schlumberger M, Muro K et al (2015) Subgroup analysis of Japanese patients in a phase 3 study of lenvatinib in radioiodine-refractory differentiated thyroid cancer. Cancer Sci 106(12):1714–1721. https://doi.org/10.1111/cas.12826
Ruggenenti P, Gaspari F, Perna A et al (1998) Cross sectional longitudinal study of spot morning urine protein:creatinine ratio, 24 hour urine protein excretion rate, glomerular filtration rate, and end stage renal failure in chronic renal disease in patients without diabetes. BMJ 316(7130):504–509. https://doi.org/10.1136/bmj.316.7130.504
Chen YT, Hsu HJ, Hsu CK et al (2019) Correlation between spot and 24h proteinuria: Derivation and validation of equation to estimate daily proteinuria. PLoS ONE 14(4):e0214614. https://doi.org/10.1371/journal.pone.0214614
Evans TRJ, Kudo M, Finn RS et al (2019) Urine protein:creatinine ratio vs 24-hour urine protein for proteinuria management: analysis from the phase 3 REFLECT study of lenvatinib vs sorafenib in hepatocellular carcinoma. Br J Cancer. https://doi.org/10.1038/s41416-019-0506-6
Levey AS, Eckardt KU, Tsukamoto Y et al (2005) Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 67(6):2089–2100. https://doi.org/10.1111/j.1523-1755.2005.00365.x
Cho JY, Paik YH, Lim HY et al (2013) Clinical parameters predictive of outcomes in sorafenib-treated patients with advanced hepatocellular carcinoma. Liver Int 33(6):950–957. https://doi.org/10.1111/liv.12168
Akutsu N, Sasaki S, Takagi H et al (2015) Development of hypertension within 2 weeks of initiation of sorafenib for advanced hepatocellular carcinoma is a predictor of efficacy. Int J Clin Oncol 20(1):105–110. https://doi.org/10.1007/s10147-014-0691-5
Cavalieri S, Cosmai L, Genderini A et al (2018) Lenvatinib-induced renal failure: two first-time case reports and review of literature. Expert Opin Drug Metab Toxicol 14(4):379–385. https://doi.org/10.1080/17425255.2018.1461839
Lea J, Greene T, Hebert L et al (2005) The relationship between magnitude of proteinuria reduction and risk of end-stage renal disease: results of the African American study of kidney disease and hypertension. Arch Intern Med 165(8):947–953. https://doi.org/10.1001/archinte.165.8.947
Usui T, Kanda E, Iseki C et al (2018) Observation period for changes in proteinuria and risk prediction of end-stage renal disease in general population. Nephrology (Carlton) 23(9):821–829. https://doi.org/10.1111/nep.13093
Capasso A, Benigni A, Capitanio U et al (2019) Summary of the international conference on onco-nephrology: an emerging field in medicine. Kidney Int 96(3):555–567. https://doi.org/10.1016/j.kint.2019.04.043
Cosmai L, Porta C, Perazella MA et al (2018) Opening an onconephrology clinic: recommendations and basic requirements. Nephrol Dial Transplant 33(9):1503–1510. https://doi.org/10.1093/ndt/gfy188
Brose MS, Nutting CM, Jarzab B, investigators D et al (2014) Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet 384(9940):319–328. https://doi.org/10.1016/S0140-6736(14)60421-9
Tatsugami K, Oya M, Kabu K et al (2018) Efficacy and safety of sorafenib for advanced renal cell carcinoma: real-world data of patients with renal impairment. Oncotarget 9(27):19406–19414. https://doi.org/10.18632/oncotarget.24779
Tatsugami K, Oya M, Kabu K et al (2018) Evaluation of efficacy and safety of sorafenib in kidney cancer patients aged 75 years and older: a propensity score-matched analysis. Br J Cancer 119(2):241–247. https://doi.org/10.1038/s41416-018-0129-3
Medina-Rosas J, Yap KS, Anderson M et al (2016) Utility of Urinary Protein-creatinine ratio and protein content in a 24-hour urine collection in systemic lupus erythematosus: a systematic review and meta-analysis. Arthritis Care Res (Hoboken) 68(9):1310–1319. https://doi.org/10.1002/acr.22828
Singh R, Bhalla K, Nanda S et al (2019) Correlation of spot urinary protein: creatinine ratio and quantitative proteinuria in pediatric patients with nephrotic syndrome. J Family Med Prim Care 8(7):2343–2346. https://doi.org/10.4103/jfmpc.jfmpc_403_19
Akin D, Ozmen S (2019) An unresolved issue: the relationship between spot urine protein-to-creatinine ratio and 24-hour proteinuria. J Int Med Res 47(3):1179–1184. https://doi.org/10.1177/0300060518819602
Kobayashi S, Amano H, Terawaki H et al (2019) Spot urine protein/creatinine ratio as a reliable estimate of 24-hour proteinuria in patients with immunoglobulin A nephropathy, but not membranous nephropathy. BMC Nephrol 20(1):306. https://doi.org/10.1186/s12882-019-1486-8
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors have any potential conflicts of interest associated with this research.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Masaki, C., Sugino, K., Kobayashi, S. et al. Urinalysis by combination of the dipstick test and urine protein–creatinine ratio (UPCR) assessment can prevent unnecessary lenvatinib interruption in patients with thyroid cancer. Int J Clin Oncol 25, 1278–1284 (2020). https://doi.org/10.1007/s10147-020-01678-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-020-01678-x