Abstract
Background
The expression of programmed death ligand 1 (PD-L1) is considered a predictive biomarker of anti-programmed death 1 (PD-1)/PD-L1 cancer therapies. However, changes in PD-L1 expression of tumor cells during clinical courses have not been fully evaluated. We evaluated changes in PD-L1 expression for non-small cell lung cancer (NSCLC) patients who received anticancer treatments during clinical courses.
Methods
In 76 NSCLC patients, PD-L1 expression was evaluated before and after anticancer treatment by immunohistochemical (IHC) analysis using an anti-PD-L1 antibody. We defined two cut-off points of PD-L1 expression (1 and 50%) and three corresponding IHC groups (A: 0%, B: 1–49%, and C: ≥50%). IHC group B and C were considered to be positive expression, and we defined the difference of IHC group between pre- and post-treatment as ‘major change’ in PD-L1 expression.
Results
Before anticancer treatment, PD-L1 expression was observed in 38/76 (50%) patients, and was significantly less common in patients harboring mutations in the epidermal growth factor receptor gene (EGFR) than in those without (P = 0.039). After anticancer treatment, PD-L1 expression was observed in 36/76 (47%) patients. Major increases in PD-L1 expression were seen in 11 (14%), and major decreases in 18 (24%) patients. Among 13 patients harboring EGFR mutations treated with EGFR tyrosine-kinase inhibitor (EGFR-TKI), five (38%) showed major increases.
Conclusion
Major changes of PD-L1 expression in tumor cells were observed in 38% of NSCLC patients who received anticancer treatments. And, treatments with EGFR-TKI may increase PD-L1 expression in NSCLC patients harboring EGFR mutations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer chemotherapy is currently undergoing some major changes. Although recent advances in targeted therapy against oncogenic epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase (ALK) have shown promising results [1,2,3,4,5,6,7,8,9,10], lung cancer remains the leading cause of cancer-related mortality worldwide [11]. Immune checkpoints are the new targets not only of non-small cell lung cancer (NSCLC) but also for many malignant tumors. Moreover, monoclonal antibodies targeting programmed death 1 (PD-1) or programmed death ligand 1 (PD-L1), such as nivolumab, pembrolizumab, atezolizumab, and durvalumab, have exerted promising antitumor effects in recent clinical trials [12,13,14,15,16].
The expression of PD-L1 is often detected in many malignant tumors, and the results of recent studies suggest that this can predict the efficacy of PD-1/PD-L1 antibody therapies. In a phase III, randomized trial of nivolumab versus docetaxel in advanced non-squamous cell NSCLC, nivolumab was shown to improve overall survival (OS) compared with docetaxel [12]. Furthermore, nivolumab was associated with greater efficacy than docetaxel across all end points in subgroups according to prespecified levels of PD-L1 expression in tumor cells. In a phase III, randomized trial of pembrolizumab versus chemotherapy in advanced NSCLC with PD-L1 expression at least 50% of tumor cells, pembrolizumab was shown to improve progression-free survival (PFS) and OS compared with chemotherapy [14]. Based on these results, PD-L1 expression has been suggested as a predictive biomarker of PD-1 /PD-L1 antibody therapy. However, although some reports have investigated relationships between PD-L1 expression and the survival of NSCLC patients [17,18,19,20,21,22,23], changes in the PD-L1 expression of tumor cells during clinical courses have not been fully evaluated. The present study, therefore, examined changes in PD-L1 expression for NSCLC patients undergoing anticancer treatments during clinical courses.
Patients and methods
Patients and samples
We retrospectively screened 130 consecutive patients from medical records who were histologically diagnosed with NSCLC, including those at post-treatment, at Shizuoka Cancer Center between November 2002 and December 2014. In this study, patients were included if they had sufficient formalin-fixed paraffin-embedded tumor tissue samples (≥ 10 slices) to evaluate PD-L1 expression. Pleural fluid cell blocks were admissible to be used as tissue samples. If a patient had ≥ 3 samples, we selected two samples, one had been taken at diagnosis, and the other was the latest after treatment.
Patients were eligible if they had received anticancer treatment, including surgery, cytotoxic chemotherapy, EGFR tyrosine-kinase inhibitor (EGFR-TKI), ALK inhibitor, and curative thoracic radiotherapy. EGFR mutation was examined by commercial clinical laboratories, Cycleave method and Scorpion Amplification Refractory Mutation System (Scorpion ARMS method) [24, 25]. We evaluated changes in PD-L1 expression according to therapeutic intervention. Patients who received immune checkpoint inhibitor or palliative radiotherapy only were excluded. OS was defined as the period from histological diagnosis of NSCLC until the date of death or last contact. All patients provided written informed consent, and this study was conducted in accordance with the provisions of the Declaration of Helsinki and was approved by the Institutional Review Board of Shizuoka Cancer Center.
Immunohistochemical analysis of PD-L1 expression
Formalin-fixed paraffin-embedded samples were sectioned at a thickness of 4 µm, mounted onto glass slides, then incubated with an anti-rabbit monoclonal antibody against PD-L1 (E1L3N, Cell Signaling Technology, Danvers, MA) for immunohistochemical (IHC) analysis. Briefly, each slide was deparaffinized, and rehydrated sections were treated with 0.3% hydrogen peroxide in methanol for 30 min to block endogenous peroxidase activity. For antigen retrieval, sections were autoclaved in Tris–EDTA buffer (pH 9.0) for 40 min at 95 °C. After rinsing, the sections were incubated overnight at 4 °C with a solution containing anti-PD-L1 antibody (1: 1000 dilution). Peroxidase-labeled rabbit polymer antibody (EnVision, K4002, Dako, Carpineria, CA, USA) was added for 30 min at room temperature, followed by incubation for 4 min at room temperature with 3, 3′ diaminobenzidine tetrahydrochloride (K3468; Dako) for the peroxidase reaction. Finally, nuclear counterstaining was performed with Mayer’s hematoxylin.
IHC analysis of PD-L1 expression was evaluated by two independent pathologists (R.W. and T.S.) who received no clinical information. For eligibility of the specimen, we evaluated macrophages in the specimen as inner control of PD-L1 immunostaining. Samples in which more than 1% of tumor cells had a stained cell membrane were considered to be positive for PD-L1 expression. Moreover, samples in which more than 50% of tumor cells were stained were considered to be strongly positive for PD-L1 expression, according to previous reports and clinical trials of pembrolizumab [14, 26]. We, therefore, defined three IHC groups based on the percentage of tumor cells stained for PD-L1 as follows: 0%, IHC group A (negative); 1–49%, IHC group B (positive); and 50–100%, IHC group C (positive/high). In the case of disagreement, the pathologists reviewed their findings to establish a consensus. We defined the difference of IHC group between pre- and post-treatment as ‘major change’ in PD-L1 expression. For example, a change from group A (negative) to B (positive) or from group C (positive/high) to B (positive), is defined as ‘major change’. In addition, the proportions of a major change in IHC group were calculated for each treatment.
Statistical analysis
Correlations between PD-L1 expression and patient characteristics were analyzed using Fisher’s exact test for categorical variables. We evaluated OS using the Kaplan–Meier method and compared OS between patients using the log-rank test. Cox proportional hazards models were used to adjust for potential confounding factors. We compared the distribution of PD-L1 expression between pre-treatment samples and post-treatment samples using Wilcoxon signed rank test. Differences in the proportions of major changes were considered significant at P < 0.05. All analyses were performed using JMP 10 for Windows statistical software (SAS Institute Japan Inc., Tokyo, Japan).
Results
Patient characteristics
Of the 130 patients screened from medical records, 76 were eligible for inclusion in the study. Thirty-five patients were excluded because they had an insufficient number of samples to evaluate. And, 19 patients were excluded because inner macrophages were integrally negative PD-L1 immunostaining. Clinical characteristics of eligible patients are shown in Table 1. The median age of patients at diagnosis was 67 years (range 39–89 years). Thirty (39%) of the patients were female, and 22 (29%) were never smokers. Sixty (79%) of the patients were diagnosed with adenocarcinoma, and 10 (13%) with squamous cell carcinoma. Stage distributions were: stage I or II in 43 patients (57%), and stage III or IV in 33 (43%). A total of 13 patients had tumors harboring deletions in EGFR exon 19, 7 carried the L858R mutation in EGFR exon 21.
Samples and treatments
The distribution of pre-treatment samples was as follows: 54 (71%) surgically resected specimens, 22 (29%) biopsy samples. Post-treatment samples included 25 (33%) surgically resected specimens, 38 (50%) biopsy samples, 8 (11%) pleural fluid cell blocks, and 5 (7%) autopsy samples. Biopsy samples were more frequently seen at post-treatment compared with pre-treatment.
Table 2 shows anticancer treatments performed before obtaining post-treatment samples. Forty-three patients received cytotoxic chemotherapy and 13 received EGFR-TKI treatment during the clinical course. Thirty patients received non-systemic anticancer treatments, including radiation therapy alone or surgery in the absence of postoperative adjuvant chemotherapy.
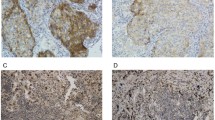
Immunohistochemical analysis of PD-L1 expression in pre-treatment tumor specimens
PD-L1 expression was observed in several tumor cells and most peripheral macrophages. As noted previously, samples in which inner macrophages were negative PD-L1 immunostaining were excluded. PD-L1 staining patterns in the membrane of tumor cells are shown in Fig. 1. Thirty-eight (50%) patients had tumor cells with positive PD-L1 staining (IHC group B, C) in pre-treatment samples. The relationships between PD-L1 expression in pre-treatment samples and patient demographics are shown in Table 1. PD-L1 expression was significantly more common in wild type than those with EGFR mutations (P = 0.039). There was no significant correlation between PD-L1 expression and smoking status or histology.
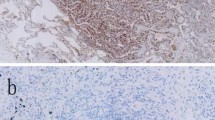
Major increase in PD-L1 expression after treatment. Negative PD-L1 immunohistochemical staining (IHC group A) was observed at diagnosis of a non-smoking 73-year-old male with adenocarcinoma (a), and positive PD-L1 immunohistochemical staining (IHC group C) was observed at postoperative recurrence (b)
Changes in PD-L1 expression
Thirty-six (36%) patients had tumors with positive PD-L1 staining (IHC group B, C) in post-treatment samples. The distribution of PD-L1 expression in tumor cells at post-treatment were similar to those at pre-treatment (P = 0.706). Defined major increases in PD-L1 expression were seen in 11 patients (14%) and major decreases in 18 (24%) (Fig. 2). Five (38%) of 13 NSCLC patients harboring EGFR mutations who were treated with EGFR-TKI showed major increases of PD-L1 expression, and one (8%) showed major decreases. Regarding EGFR-TKI therapy, median duration of EGFR-TKI therapy was 16.2 (95% CI; 8.5–31.6) months in five patients who had major increases of PD-L1 expression after EGFR-TKI therapy, and was 10.7 (95% CI; 3.0–13.4) months in 8 patients who had no major increases of PD-L1 expression after EGFR-TKI therapy. There was no significant difference in duration of EGFR-TKI therapy between two groups (P = 0.110). Eight (19%) of 43 patients treated with cytotoxic chemotherapy showed major increases in PD-L1 expression, and 8 (19%) showed major decreases. Only two (7%) of 30 patients who were not treated with cytotoxic chemotherapy or EGFR-TKI showed major increases in PD-L1 expression, and nine (30%) showed major decreases. In comparison to non-systemic treatment patients, patients who were treated by EGFR-TKI had significantly more ‘major increase’ in PD-L1 expression (P = 0.031).
Changes of the PD-L1 IHC group in NSCLC patients treated with anticancer treatment. Major increases in PD-L1 expression were seen in 11 (14%) patients, and major decreases were seen in 18 (24%). Among 13 patients harboring EGFR mutations treated with EGFR-TKI, five (38%) showed major increases, and one (8%) showed major decreases
Survival analysis
In patients with stage I or II cancers, the median follow-up time from diagnosis was 45.6 months. Patients with PD-L1-positive tumors (IHC group B, C) had a tendency for worse survival than those with PD-L1-negative expression (IHC group A) (median 75.6 months versus 103.1 months, P = 0.065; Fig. 3).
Overall survival of stage I or II NSCLC patients of PD-L1 IHC group A (n = 19) and IHC group B, C (n = 24). The survival of patients with positive PD-L1 expression (IHC group B, C) tended to be worse than those without PD-L1 expression (IHC group B, C) (median 75.6 months versus 103.1 months, log-rank test, P = 0.065)
Discussion
Recently, monoclonal antibodies targeting PD-1 or PD-L1 have been shown to have potential as a new therapeutic strategy for NSCLC. Several clinical trials have found that PD-L1 expression may be a predictive factor of therapeutic response; as such, it is important to determine changes in tumor cell PD-L1 expression during clinical courses because this may have an impact on treatment strategies, including patient selection and treatment sequences. In this study, we showed that major changes in PD-L1 expression of tumor cells were observed in 38% of NSCLC patients receiving anticancer treatments during the clinical course. In particular, 38% of NSCLC patients harboring EGFR mutations treated with EGFR-TKI showed major increases and 8% showed major decreases. And, patients treated with EGFR-TKI had significantly more ‘major increase’ in PD-L1 expression compared to patients with non-systemic treatment (Fig. 2, P = 0.031).
Previous reports have explored the changes occurring in PD-L1 expression during the clinical courses of NSCLC patients. Gainor et al. retrospectively compared PD-L1 expression status using pre- and post-EGFR-TKI treatment biopsy samples in NSCLC patients harboring EGFR mutations [27]. Using the same rabbit monoclonal anti-PD-L1 antibody (Clone E1L3N) as in the present study, nine (15%) of 62 patients showed PD-L1 expression in tumor cells before EGFR-TKI treatment, compared with 16 (25%) of 64 after EGFR-TKI treatment (P = 0.040). In individual paired specimens, increases in PD-L1 expression were seen in 10 (17%) of 58 NSCLC patients harboring EGFR mutations after EGFR-TKI treatment, and decreases were seen in three (5%) of 58. Han et al. also reported that seven (39%) of 18 gefitinib-resistant patients showed an increase in tumor cell PD-L1 expression, while 11 (61%) of 18 gefitinib-resistant patients had no change in PD-L1 expression [28]. These results are consistent with our own study, and support the hypothesis that EGFR-TKI therapy may increase PD-L1 expression in some NSCLC patients harboring EGFR mutations. However the mechanism of these changes has not been evaluated. In melanoma, Jiang et al. reported that activation of the MAPK pathway in BRAF-resistant melanoma cells promote PD-L1 expression. In NSCLC patients harboring non-T790M mutation acquired resistance to EGFR-TKI, PI3K/AKT pathway and MEK/ERK pathway are involved in resistance to EGFR-TKI [29]. And, some study using NSCLC cell lines harboring EGFR mutations suggested that PD-L1 expression was mediated by activated PI3K-AKT and MEK-ERK signaling pathway [28, 30, 31]. We could not evaluate changes in these proteins in signaling pathways due to lack of enough specimens, these changes may be related to increased PD-L1 expression in this study as reported previously.
Conversely, Akbay et al. reported that expression of PD-L1 was more common in NSCLC patients harboring EGFR mutations, and was reduced by EGFR-TKI treatment in NSCLC cell lines with activated EGFR mutations [32]. Indeed, several studies showed that PD-L1 expression was more common in patients with than without EGFR mutations [33,34,35]. These discrepancies may be explained by differences in patient characteristics, such as disease stage and the presence or absence of anticancer treatment other than EGFR-TKI, which could lead to differences in PD-L1 expression.
In NSCLC patients harboring EGFR mutations, the efficacy of PD-1 antibody therapy remains unclear. Nivolumab previously demonstrated superiority over docetaxel regarding OS in advanced non-squamous NSCLC [12]. However, in patients with EGFR mutations, OS and progression-free survival tended to be worse following nivolumab compared with docetaxel treatment. In a phase II/III trial of pembrolizumab versus docetaxel in advanced NSCLC patients with PD-L1 expression, pembrolizumab showed superior OS compared with docetaxel [36], but progression-free survival tended to be worse in pembrolizumab-treated patients harboring EGFR mutations. And, a meta-analysis of three studies showed that immune check inhibitor did not improve OS compared to docetaxel in NSCLC harboring EGFR mutations [37]. These results suggest that anti-PD-1/PD-L1 antibody treatment may have a limited effect against NSCLC patients harboring EGFR mutations. One of the reasons for these low effects of immune checkpoint inhibitors against NSCLC harboring EGFR mutations is considered to be lower tumor mutation burden (TMB) in tumor cells. TMB in lung and other cancers is considered predictive of benefit for immune checkpoint inhibitor. Among patients with NSCLC treated with pembrolizumab, higher nonsynonymous TMB has been associated with greater clinical benefit [38]. Interestingly, EGFR-mutated lung cancer was shown to have low TMB in another study using next-generation sequencing [39]. Moreover, low TMB is considered to be associated with low expression of PD-L1 [40]. PD-L1 expression levels may change after anticancer treatment, especially EGFR-TKI treatment, and so, further studies are needed to clarify the relationship between the efficacy of PD-1/PD-L1 antibody in EGFR-mutated patients and PD-L1 expression in tumors immediately before PD-1/PD-L1 antibody treatment.
Of the 76 NSCLC patients in the present study, PD-L1 expression was observed in 50% of samples at pre-treatment. According survival analysis, the OS of stage I or II patients with positive PD-L1 expression tended to be worse than those without PD-L1 expression. This is consistent with the previous findings of NSCLC patients [23, 33]. Conversely, some reports suggest that PD-L1 expression is associated with favorable OS [18, 19]. The exact reason for this discrepancy is still unknown.
We used an anti-rabbit monoclonal antibody against PD-L 1 (clone E1L3N) for IHC analysis, shown by other retrospective studies to have reliable staining properties [27, 41, 42], but this antibody clone was not used in clinical trials of nivolumab or pembrolizumab. Recently, some studies for evaluating clinical comparability in antibody against PD-L1 have been reported [43, 44]. These studies show that there are high concordances of PD-L1 staining between E1L3N and 22C3. Therefore, we think the results of this study can be compared with other studies using different antibody clone. In PD-L1 (22C3) antibody testing, the archival FFPE specimen within five years is recommended because antigen degraded with age. While there is no clear criterion in PD-L1 (E1L3N) antibody testing. Therefore, for eligibility of the specimen, we evaluated macrophages in the sample as inner control of PD-L1 immunostaining.
Our study had several limitations. First, the sample size was small and it involved patients attending a single institution. Second, patient characteristics are very heterogeneous. This study included patients with all stages and anticancer treatments were different for each patient. Development of anticancer treatment with immune checkpoint inhibitor is ongoing for not only advanced stage but also early stage NSCLC. Therefore, it may be meaningful to evaluate PD-L1 expression in tumor cells including early stage cancer. Third, because of spatial heterogeneity in PD-L1 expression within different regions of the same tumor tissue, our results may not show an accurate distribution of PD-L1 expression, especially in biopsy samples. Ilie et al. previously reported that there is a relatively poor association of PD-L1 expression between lung biopsy samples and corresponding resected tumors [45]. Conversely, Kitazono et al. reported that the PD-L1 status showed a good concordance between biopsy samples and surgically resected specimens [46]. They concluded that even small samples derived from transbronchial needle aspiration biopsies were adequate for the assessment of PD-L1 expression. Forth, we did not evaluate the status of tumor-infiltrating lymphocytes (TILs). It is still not conclusive whether TILs is a biomarker for PD-1/PD-L1 antibody therapy or not. Recent studies have reported that TILs may be an important predictive biomarker in clinical trial of PD-L1 antibody [15, 47]. In our study, we could not evaluate TILs accurately, because many biopsy specimens were not suitable to evaluate infiltrating lymphocytes for collapsed, and it was difficult to make a decision whether mixed lymphocytes are ‘infiltrate’ or not. In the future, it may be necessary to evaluate TILs along with PD-L1 expression in clinical setting.
In conclusion, we demonstrated that major changes in PD-L1 expression were observed in 38% of NSCLC patients undergoing anticancer treatments during the clinical course. PD-L1 expression in tumor cells was less common in NSCLC patients harboring EGFR mutations than those without previous anticancer treatment. Moreover, EGFR-TKI treatment appeared to increase PD-L1 expression in tumor cells harboring EGFR mutations. Further studies are warranted to explore why conflicting PD-L1 expression findings have been identified in NSCLC patients with EGFR mutations during clinical courses.
References
Mitsudomi T, Morita S, Yatabe Y et al (2010) Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 11(2):121–128
Maemondo M, Inoue A, Kobayashi K et al (2010) Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 362(25):2380–2388
Zhou C, Wu YL, Chen G et al (2011) Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 12(8):735–742
Rosell R, Carcereny E, Gervais R et al (2012) Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 13(3):239–246
Sequist LV, Yang JC, Yamamoto N et al (2013) Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 31(27):3327–3334
Wu YL, Zhou C, Hu CP et al (2014) Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol 15(2):213–222
Mok TS, Wu YL, Ahn MJ et al (2017) Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med 376(7):629–640
Solomon BJ, Mok T, Kim DW et al (2014) First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 371(23):2167–2177
Hida T, Nokihara H, Kondo M et al (2017) Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): an open-label, randomised phase 3 trial. Lancet 390(10089):29–39
Shaw AT, Kim AT, Crino L et al (2017) Ceritinib versus chemotherapy in patients with ALK-rearranged non-small-cell lung cancer previously given chemotherapy and crizotinib (ASCEND-5): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 18(7):874–886
Siegel RL, Miller KD, Jemal A (2015) Cancer statistics, 2015. CA Cancer J Clin 65(1):5–29
Borghaei H, Paz-Ares L, Horn L et al (2015) nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 373(17):1627–1639
Brahmer J, Reckamp KL, Baas P et al (2015) nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 373(2):123–135
Reck M, Rodriguez-Abreu D, Robinson AG et al (2016) pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 375(19):1823–1833
Rittmeyer A, Barlesi F, Waterkamp D et al (2017) Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 389(10066):255–265
Garassino MC, Cho BC, Kim JH et al (2018) Durvalumab as third-line or later treatment for advanced non-small-cell lung cancer (ATLANTIC): an open-label, single-arm, phase 2 study. Lancet Oncol 19(4):521–536
Chen YB, Mu CY, Huang JA (2012) Clinical significance of programmed death-1 ligand-1 expression in patients with non-small cell lung cancer: a 5-year-follow-up study. Tumori 98(6):751–755
Velcheti V, Schalper KA, Carvajal DE et al (2014) Programmed death ligand-1 expression in non-small cell lung cancer. Lab Invest 94(1):107–116
Yang CY, Lin MW, Chang YL et al (2014) Programmed cell death-ligand 1 expression in surgically resected stage I pulmonary adenocarcinoma and its correlation with driver mutations and clinical outcomes. Eur J Cancer 50(7):1361–1369
Cooper WA, Tran T, Vilain RE et al (2015) PD-L1 expression is a favorable prognostic factor in early stage non-small cell carcinoma. Lung Cancer 89(2):181–188
Pan ZK, Ye F, Wu X et al (2015) Clinicopathological and prognostic significance of programmed cell death ligand1 (PD-L1) expression in patients with non-small cell lung cancer: a meta-analysis. J Thorac Dis 7(3):462–470
Wang A, Wang HY, Liu Y et al (2015) The prognostic value of PD-L1 expression for non-small cell lung cancer patients: a meta-analysis. Eur J Surg Oncol 41(4):450–456
Zhou ZJ, Zhan P, Song Y (2015) PD-L1 over-expression and survival in patients with non-small cell lung cancer: a meta-analysis. Transl Lung Cancer Res 4(2):203–208
Yatabe Y, Hida T, Horio Y et al (2006) A rapid, sensitive assay to detect EGFR mutation in small biopsy specimens from lung cancer. J Mol Diagn 8(3):335–341
Kimura H, Kasahara K, Kawaishi M et al (2006) Detection of epidermal growth factor receptor mutations in serum as a predictor of the response to gefitinib in patients with non-small-cell lung cancer. Clin Cancer Res 12(13):3915–3921
Garon EB, Rizvi NA, Hui R et al (2015) Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 372(21):2018–2028
Gainor JF, Sequist LV, Shaw AT et al. (2015) Clinical correlation and frequency of programmed death ligand-1 (PD-L1) expression in EGFR-mutant and ALK-rearranged non-small cell lung cancer (NSCLC). ASCO Meeting Abstracts 33(15_suppl):8012
Han JJ, Kim DW, Koh J et al (2016) Change in PD-L1 expression after acquiring resistance to gefitinib in EGFR-mutant non-small-cell lung cancer. Clin Lung Cancer 17(4):263–270
Meng F, Wang F, Wang L et al (2016) MiR-30a-5p overexpression may overcome EGFR-inhibitor resistance through regulating PI3K/AKT signaling pathway in non-small cell lung cancer cell lines. Front Genet 7:197
Ota K, Azuma K, Kawahara A et al (2015) Induction of PD-L1 expression by the EML4-ALK oncoprotein and downstream signaling pathways in non-small cell lung cancer. Clin Cancer Res 21(17):4014–4021
Chen N, Fang W, Zhan J et al (2015) Upregulation of PD-L1 by EGFR activation mediates the immune escape in EGFR-driven NSCLC: implication for optional immune targeted therapy for NSCLC patients with EGFR mutation. J Thorac Oncol 10(6):910–923
Akbay EA, Koyama S, Carretero J et al (2013) Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov 3(12):1355–1363
Azuma K, Ota K, Kawahara A et al (2014) Association of PD-L1 overexpression with activating EGFR mutations in surgically resected nonsmall-cell lung cancer. Ann Oncol 25(10):1935–1940
D’Incecco A, Andreozzi M, Ludovini V et al (2015) PD-1 and PD-L1 expression in molecularly selected non-small-cell lung cancer patients. Br J Cancer 112(1):95–102
Tang Y, Fang W, Zhang Y et al (2015) The association between PD-L1 and EGFR status and the prognostic value of PD-L1 in advanced non-small cell lung cancer patients treated with EGFR-TKIs. Oncotarget 6(16):14209–14219
Herbst RS, Baas P, Kim DW et al (2015) Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 387(10027):1540–1550
Lee CK, Man J, Lord S et al (2017) Checkpoint inhibitors in metastatic EGFR-mutated non-small cell lung cancer-a meta-analysis. J Thorac Oncol 12(2):403–407
Rizvi NA, Hellmann MD, Snyder A et al (2015) Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 348(6230):124–128
Spigel DR, Schrock AB, Fabrizio D et al (2016) Total mutation burden (TMB) in lung cancer (LC) and relationship with response to PD-1/PD-L1 targeted therapies. J Clin Oncol 34(15_suppl):9017–9017
Madore J, Strbenac D, Vilain R et al (2016) PD-L1 negative status is associated with lower mutation burden, differential expression of immune-related genes, and worse survival in stage III melanoma. Clin Cancer Res 22(15):3915–3923
Katsuya Y, Fujita Y, Horinouchi H et al (2015) Immunohistochemical status of PD-L1 in thymoma and thymic carcinoma. Lung Cancer 88(2):154–159
Schultheis AM, Scheel AH, Ozretic L et al (2015) PD-L1 expression in small cell neuroendocrine carcinomas. Eur J Cancer 51(3):421–426
Gaule P, Smithy JW, Toki M et al (2016) A quantitative comparison of antibodies to programmed cell death 1 ligand 1. JAMA Oncol 3(2):256–259
Rimm DL, Han G, Taube JM et al (2017) A prospective, multi-institutional, pathologist-based assessment of 4 immunohistochemistry assays for PD-L1 expression in non-small cell lung cancer. JAMA Oncol 3(8):1051–1058
Ilie M, Long-Mira E, Bence C et al (2016) Comparative study of the PD-L1 status between surgically resected specimens and matched biopsies of NSCLC patients reveal major discordances: a potential issue for anti-PD-L1 therapeutic strategies. Ann Oncol 27(1):147–153
Kitazono S, Fujiwara Y, Tsuta K et al (2015) Reliability of small biopsy samples compared with resected specimens for the determination of programmed death-ligand 1 expression in non-small-cell lung cancer. Clin Lung Cancer 16(5):385–390
Fehrenbacher L, Spira A, Ballinger M et al (2016) Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 387(10030):1837–1846
Acknowledgements
We thank all the clinical pathology technicians who supported this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Shota Omori received personal fees from AstraZeneca, Boehriner Ingelheim, Chugai-Pharm, Taiho-Pharm, MSD and Ono-Pharma; Dr. Hirotsugu Kenmotsu received personal fees from AstraZeneca, Chugai-Pharma, Bristol-Myers, Boehriner Ingelheim, Eli Lilly, Kyowa Hakko Kirin, Taiho-Pharma, MSD and Ono-Pharma; Dr. Haruki Kobayashi received personal fees from Eli Lilly, and Taiho-Pharma; Dr. Kazuhisa Nakashima received personal fees from Eli Lilly, Taiho-Pharma, Mochida-Pharma and Ono-Pharma; Dr. Kazushige Wakuda received personal fees from Taiho-Pharma, Boehriner Ingelheim and Ono-Pharma; Dr. Akira Ono received personal fees from Chugai-Pharma, Takeda-Pharma and Taiho-Pharma; Dr. Tateaki Naito received personal fees from Ono-Pharma; Dr. Haruyasu Murakami received personal fees from AstraZeneca, Astellas, Bristol-Myers, Boehriner Ingelheim, Chugai-Pharma, Eli Lilly, Taiho-Pharma, MSD, Pfizer, Novartis and Ono-Pharma; Dr. Masahiro Endo received personal fees from Ono-Pharma; Dr. Toshiaki Takahashi received personal fees from AstraZeneca, Boehriner Ingelheim, Chugai-Pharma, Taiho-Pharma, MSD, Eli Lilly, Pfizer and Ono-Pharma, outside the submitted work. The other authors declare no conflict of interests.
About this article
Cite this article
Omori, S., Kenmotsu, H., Abe, M. et al. Changes in programmed death ligand 1 expression in non-small cell lung cancer patients who received anticancer treatments. Int J Clin Oncol 23, 1052–1059 (2018). https://doi.org/10.1007/s10147-018-1305-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-018-1305-4