Abstract
Objective
To examine the antitumor activity of zoledronic acid (ZA) combined with androgen deprivation therapy (ADT) for men with treatment-naive prostate cancer and bone metastasis.
Methods
We enrolled 227 men with treatment-naive prostate cancer and bone metastasis. Participants were randomly assigned (1:1 ratio) to receive combined androgen blockade alone (CAB group) or ZA with combined androgen blockade (CZ group). Time to treatment failure (TTTF), time to the first skeletal-related event (TTfSRE), and overall survival (OS) rates were estimated using the Kaplan–Meier method. Hazard ratios (HRs) were calculated using the Cox proportional hazards model. Median follow-up duration was 41.5 months.
Results
Median TTTFs were 12.4 and 9.7 months for the CZ and CAB groups, respectively (HR 0.75; 95 % CI 0.57–1.00; p = 0.051). For men with baseline prostate-specific antigen levels <200 ng/mL, median TTTFs were 23.7 and 9.8 months for the CZ and CAB groups, respectively (HR 0.58; 95 % CI 0.35–0.93; p = 0.023). Median TTfSREs were 64.7 and 45.9 months for the CZ and CAB groups, respectively (HR 0.58; 95 % CI 0.38–0.88; p = 0.009). OS was similar between the groups.
Conclusions
This study failed to demonstrate that combined use of ZA and ADT significantly prolonged TTTF in men with treatment-naive prostate cancer and bone metastasis. However, it generates a new hypothesis that the combined therapy could delay the development of castration resistance in a subgroup of patients with low baseline prostate-specific antigen values <200 ng/mL. The treatment also significantly prolonged TTfSRE but did not affect OS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Direct and indirect antitumor activities of zoledronic acid (ZA), such as induction of cancer cell apoptosis, activation of gamma-delta T cells, and inhibition of angiogenesis, have been demonstrated in vitro and in preclinical settings [1, 2]. ZA also has a clinically proven effect of preventing skeletal complications [3–5].
Moreover, there is clinical evidence of antitumor activity of ZA in the early stages of solid cancers. Three large phase 3 trials have suggested that adjuvant ZA could improve the disease-free survival of patients with early stage breast cancer and a post-menopausal or low-estrogen status [6–8]. Several studies have been conducted to test the antitumor effects of bisphosphonates in patients with non-metastatic [9] or castration-sensitive metastatic [10–12] prostate cancers.
Considering the expected antitumor effects of ZA in the early stages of solid cancer, we hypothesized that early treatment combining ZA and androgen deprivation therapy (ADT) could delay the development of castration resistance and improve survival in patients with metastatic castration-sensitive prostate cancer (CSPC). Accordingly, we conducted a phase III multicenter, randomized, controlled study to compare the antitumor efficacy and safety of two types of treatment—combined ZA and CAB therapy and CAB-only therapy—in patients with treatment-naive prostate cancer and bone metastasis.
Patients and methods
Study design and participants
The ZAPCA trial is a randomized, open-label, phase III trial of patients with treatment-naive prostate cancer and bone metastasis at 45 institutions in Japan. Eligible patients were men aged at least 20 years with histologically confirmed adenocarcinoma of the prostate, at least one bone metastasis detected by bone scan, Eastern Cooperative Oncology Group performance status ≤2, and a baseline prostate-specific antigen (PSA) concentration of ≥30 ng/mL. Patients with histories of prior local curative therapy, prior ADT for >2 weeks, chemotherapy, or bisphosphonate treatment were not eligible. Key exclusion criteria were other active malignancies, active dental disorders, infections, uncontrollable cardiovascular disorder or hypertension, and current steroid treatment.
The ZAPCA trial was conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice guidelines and was approved by the institutional review board at each participating institution listed in the Notes. All patients provided written informed consent. The ZAPCA trial is registered with ClinicalTrials.gov, number NCT00685646.
Procedures
Patients were randomly assigned (1:1 ratio) to receive CAB alone (CAB group) or intravenous ZA combined with CAB (CZ group). Computer-based randomization was conducted at the Translational Research Informatics Center (TRI; Kobe, Japan) with stratification according to the treatment institution, baseline PSA concentration (<200 or ≥200 ng/mL), baseline extent of disease (EOD) grade [13] (≤2 or ≥3), and biopsy Gleason score (≤7 or ≥8). We determined 200 ng/mL as a stratifying value of PSA in reference to median or mean baseline PSA values in the SWOG8894 trial [14] or a Japanese randomized study [15]. The system automatically evaluated the eligibility of each patient and randomly assigned participants to each group.
In both groups, patients received 80 mg of bicalutamide orally once a day and a subcutaneous injection of luteinizing hormone–releasing hormone (LH–RH) agonist (3.75 or 11.25 mg of leuprolide acetate, or 3.6 or 10.8 mg of goserelin acetate, every 4 or 12 weeks, respectively). Surgical castration was allowed if patients experienced any problems with the LH–RH agonist. In the CZ group, ZA treatment was initiated 4 weeks after the first LH–RH agonist injection. ZA was administered intravenously every 4 weeks for up to 2 years. Doses of ZA were 4, 3.5, and 3.0 mg for patients with creatinine clearances of >60, 50–60, and 30–49 mL/min, respectively. When patients experienced impairment of renal functions in spite of dose reduction, the protocol treatment was cancelled and ZA had to be stopped. Patients in the CZ group were instructed to undergo dental monitoring at each institution and to use calcium and vitamin D supplements. Interruption of ZA was allowed when patients needed any dental treatment during the study period.
Patients continued their assigned treatment until disease progression, which was defined as PSA or clinical progression, the appearance of adverse events, or withdrawal of informed consent by the patient. PSA progression was defined as three consecutive increases (≥0.1 ng/mL) in PSA from the lowest level, and was measured at 4-week intervals. The date of PSA progression was defined as the day of the first increase. Clinical progression was defined as an increase of at least 20 % in the sum of the longest diameters of the target lesions, the appearance of one or more new lesions, a clear progression of non-target lesions, or the appearance of two or more new bone metastases by bone scan. Clinical progression was also determined if the condition of a patient was worsening due to prostate cancer. The protocol did not mandate the type of secondary treatment after cessation or completion of the assigned treatment. Patients in the CAB group were not prohibited from receiving ZA after cessation or completion of the assigned treatment.
Serum PSA was measured at least every 12 weeks, and if PSA progression was suspected, at least every 4 weeks. Bone scans were obtained before patient registration and yearly thereafter for up to 3 years. We defined a skeletal-related event (SRE) as a pathologic fracture, spinal cord compression, palliative irradiation of the bone, bone surgery, or change in anticancer therapy due to bone pain. Collected data were sent to the TRI data center using web-based case report forms and managed by data managers at the TRI center.
Study endpoints
The primary endpoint was the time to treatment failure (TTTF), defined as the interval between the date of randomization and the earliest date of PSA progression, clinical progression, first SRE, death for any reason, or cessation of protocol treatment for any reason. Secondary endpoint was the time to the first SRE (TTfSRE), defined as the interval between the date of randomization and the earliest date of the first SRE or death for any reason, and OS, defined as the interval between the date of randomization and death for any reason. Adverse events (AEs) were graded using the Common Terminology Criteria for Adverse Events, version 3.
Sample size
The sample size was calculated assuming that a control group response rate would be 30 % after 4 years [16], and the effect size to be observed was the response rate of 50 % [i.e., hazard ratio (HR) of 0.58]. The significant level and power were set to 0.05 and 80 %, respectively, and the registration and follow-up periods were both 3 years. The calculation indicated that 89 patients per group were required [17], and we decided to enroll 100 patients for each group to allow for possible drop-outs.
Statistical analyses
The Kaplan–Meier method was used to estimate TTTF, TTfSRE, and OS. A two-sided P value of <0.05 was considered statistically significant. HRs were estimated using the Cox proportional hazards model. All statistical analyses were performed using SAS version 9.3 software (SAS Institute Inc., Cary, NC, USA).
Results
Between May 2008 and December 2010, 227 patients were randomly assigned to one of two treatment groups. Eight patients did not obtain evaluable efficacy data and were excluded from the full analysis set (FAS)—three were found to be ineligible, two in the CZ group did not receive ZA, and three in the CZ group did not receive any treatment since the beginning of the study. Therefore, 110 patients in the CAB group and 109 in the CZ group were included in the FAS (Fig. 1). All 224 patients who received at least one dose of LH–RH agonist were included in the Safety Assessment Set (SAS).
The baseline characteristics of 219 patients in the FAS were well balanced between the treatment groups (Table 1). More than 80 % of patients in both groups had Gleason scores of 8–10. Nearly two-thirds of the patients had PSA values of >200 ng/mL. Median PSA values were 375.2 and 328.0 ng/mL in the CAB and CZ groups, respectively. No statistically significant differences were observed between the groups for any of the variables.
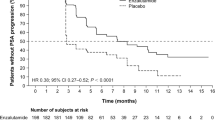
The median follow-up duration was 41.5 months. There were 192 TTTF events—139 PSA progressions (75 in the CAB group vs 64 in the CZ group), 9 clinical progressions (4 in the CAB group vs 5 in the CZ group), 7 SREs (4 in the CAB group vs 3 in the CZ group), 3 deaths in the CZ group, and 34 cessations of protocol treatment (15 in the CAB group vs 19 in the CZ group). The median TTTF was 9.7 months [95 % confidence interval (CI) 8.9–12.6] for the CAB group and 12.4 months (95 % CI 10.6–16.6) for the CZ group (HR 0.75; 95 % CI 0.57–1.00; log rank p = 0.051; Fig. 2a). In a subgroup analysis based on pre-specified stratification factors, the difference in TTTF between the CAB group (9.8 months; 95 % CI 6.6–17.0) and the CZ group (23.7 months; 95 % CI 15.3–31.3) was significant only in patients with baseline PSA values <200 ng/mL (HR 0.58; 95 % CI 0.35–0.93; log rank p = 0.023; Fig. 2b). However, subgroup analyses for PSA ≥200 ng/mL, EOD grade (≤2 or ≥3), and Gleason score (≤7 or ≥8) did not show improvements after CZ treatment similar to those observed for TTTF (Fig. S1).
There were 92 TTfSRE events—41 SREs (29 in the CAB group vs 12 in the CZ group) and 51 deaths without SREs (26 in the CAB group vs 25 in the CZ group). CZ treatment significantly prolonged TTfSRE by 18.8 months in comparison with CAB treatment (HR 0.58; 95 % CI 0.38–0.88; log rank p = 0.009; Fig. 3a). The median TTfSRE values were 45.9 and 64.7 months for the CAB (95 % CI 34.1–55.0) and CZ groups (95 % CI 48.5 to not yet estimable), respectively. There were 75 deaths until the last follow-up, including 53 deaths from prostate cancer (29 in the CAB group vs 24 in the CZ group). The median OS was 60.2 months for the CAB group (95 % CI 20.5 to not yet estimable) and not yet estimable for the CZ group (Fig. 3b). The difference in OS between the groups was not statistically significant (HR 0.78; 95 % CI 0.49–1.23; log rank p = 0.28). In subgroup analyses based on pre-specified stratification factors, CZ treatment significantly prolonged TTfSRE in patients with baseline EOD grade ≤2 (HR 0.48; 95 % CI 0.27–0.83; log rank p = 0.0076; Fig. 4a) and in patients with Gleason scores ≥8 (HR 0.52; 95 % CI 0.33–0.82; log rank p = 0.004; Fig. 4b). However, it did not prolong OS in any subgroup of patients (Fig. S2 and Fig. S3).
AEs of grade ≥3 are summarized in Table 2. The frequencies of these AEs were 25.9 and 33.0 % for the CAB and CZ groups (p = 0.30), respectively. Two patients in the CZ group (1.8 %) experienced osteonecrosis of the jaw (ONJ).
Discussion
The ZAPCA trial compared the results of early combination treatment with ZA and ADT with that of ADT treatment alone in men with treatment-naive prostate cancer and bone metastasis. We failed to show that the combined treatment improved TTTF over ADT alone, although the difference almost reached a statistical significance (p = 0.051). The effect was particularly clear in a subgroup of men with baseline PSA levels <200 ng/mL. Moreover, treatment with ZA and ADT combined significantly delayed TTfSRE compared with treatment with ADT alone. OS and the rates of AEs of grade ≥3 were similar in the two groups.
To date, there have been only three phase III randomized controlled trials testing the antitumor effect of early bisphosphonate treatment combined with ADT in men with bone-metastatic CSPC [10–12]. Medical Research Council PR05, a phase III placebo-controlled, randomized trial, was conducted to determine whether sodium clodronate improves bone progression-free survival in men with bone metastasis from prostate cancer [10, 18]. A long-term analysis with a median follow-up period of 11.5 years has demonstrated a statistically significant association between oral clodronate use and improved OS [10]. CALGB 90202 was a phase III placebo-controlled, randomized trial that evaluated the efficacy of earlier treatment with ZA in men with metastatic CSPC [11]. The primary endpoint was TTfSRE. Earlier treatment with ZA did not yield a significant improvement in TTfSRE. OS was also similar in the two treatment groups (with and without previous ZA treatment). Recently, another large-scale trial, STAMPEDE, was reported [12]. The trial failed to demonstrate the superiority of standard treatment combined with ZA over standard treatment alone in improving OS in patients with hormone-naive prostate cancer.
A meta-analysis including these three trials showed improvement of survival of men with M1 disease by addition of bisphosphonates (clodronate plus ZA) but not by ZA itself [19]. Overall, the patient population in the ZAPCA trial was similar to that in the PR05 trial, and more homogenous than those in the CALGB90202 and STAMPEDE trials. The populations in the ZAPCA and PR05 trials were uniformly patients with newly diagnosed prostate cancer with bone metastases, whereas the CALGB90202 and STAMPEDE trials allowed recurrent cases following prior local radical treatment. As local treatment might affect the survival of patients with metastatic diseases [20], the results of this meta-analysis should be cautiously interpreted. Considering that intravenous ZA is more effective in the suppression of bone resorption than oral clodronate [1, 21], the positive results reported in the PR05 trial strongly support the results of the ZAPCA trial, which used an intravenous ZA treatment that was more potent than oral clodronate. Therefore, further confirmatory studies are warranted to elucidate the effect of adding bisphosphonates to standard hormonal therapy.
The results of the ZAPCA trial described here suggest that early use of ZA combined with ADT delays the development of castration resistance in men with treatment-naive prostate cancer and bone metastasis. A subgroup analysis demonstrated that combined treatment with ZA and ADT significantly prolonged TTTF in men with baseline PSA levels <200 ng/mL. Serum PSA levels correlate with the EOD [22]. Indeed, in this study, PSA positively correlated with EOD grade (Table S1). These findings suggested that patients with a low PSA and low-volume bone metastasis might benefit more from early ZA use than other patient types. These results should be carefully interpreted because this subgroup analysis was based on data from a small subset of only 79 patients and therefore needs to be confirmed by other independent studies. However, there is some clinical evidence of the antitumor activities of bone-modifying agents in the early stages of solid cancers. Exploratory subgroup analyses in the PR05 trial demonstrated that the time from diagnosis to randomization is significantly associated with a positive response to clodronate therapy. This result indicates the importance of starting bisphosphonate treatment at the same time as ADT therapy [18]. A recent phase III trial demonstrated that denosumab delays progression to bone metastasis in men with non-metastatic CRPC [23]. Three large phase III trials suggested that adjuvant ZA improves the disease-free survival of patients with early stage breast cancer and a post-menopausal or low-estrogen status [6–8]. The ZAPCA trial also demonstrated that early ZA use combined with ADT significantly delayed the occurrence of SRE in men with treatment-naive prostate cancer and bone metastasis. This is not surprising, considering that ZA has been proven to prevent SREs when administered to patients with castration-resistant prostate cancer [4, 5]. However, the CALGB90202 trial could not demonstrate such a beneficial effect on preventing SREs by early ZA use [11]. Our subgroup analyses found that CZ treatment significantly prolonged TTfSRE in patients with baseline EOD grade ≤2 and in patients with Gleason scores ≥8. In the ZAPCA trial, 82 % of patients had prostate cancer with Gleason scores ≥8, compared with 58 % in the CALGB90202 trial. In addition, EOD grade was not specified in the CALGB90202 trial. We could speculate that the difference in results regarding TTfSRE might be to some extent affected by differences in the baseline characteristics of the patients in these two studies. Nevertheless, one of the goals in the treatment of men with treatment-naive prostate cancer and bone metastasis is to delay their disease progression without SREs affecting their quality of life. Thus, the findings of this trial are still of clinical significance, even though it did not show improvement in OS after ZA therapy.
AEs resulting from an early start and, consequently, longer term ZA use, are a potential concern. However, in the ZAPCA trial, the frequencies of AEs with grade ≥3 were similar in the two groups. No renal insufficiency occurred during the study period. Only 2 patients (1.8 %) experienced ONJ associated with ZA use; this rate is comparable to previously reported values [24]. However, patients should be carefully monitored even after the end of the study to detect late-onset AEs.
The ZAPCA trial had several limitations. First, this study was not placebo-controlled, which might have affected the results. Second, the sample size was relatively small. Third, this study enrolled only Japanese patients. It is unclear whether the results obtained here could be generalized to populations with different genetic or geographic backgrounds. However, the major strengths of the ZAPCA trial were its randomized controlled design and relatively homogeneous participant background, which minimized the potential biases. We believe that these strengths render the present findings clinically significant despite the limitations.
In conclusions, the ZAPCA trial failed to demonstrate that combined use of ZA and androgen deprivation therapy significantly prolonged TTTF in men with treatment-naive prostate cancer and bone metastasis. However, it generates a new hypothesis that the combined therapy could delay the development of castration resistance in a subgroup of patients with a low PSA values < 200 ng/mL. The combined treatment also significantly prolonged TTfSRE but did not affect OS.
References
Green JR (2004) Bisphosphonates: preclinical review. Oncologist 9(Suppl 4):3–13
Gnant M (2012) Zoledronic acid in the treatment of early-stage breast cancer: is there a final verdict? Curr Oncol Rep 14:35–43
Michaelson MD, Kaufman DS, Lee H et al. (2007) Randomized controlled trial of annual zoledronic acid to prevent gonadotropin-releasing hormone agonist-induced bone loss in men with prostate cancer. J Clin Oncol 25:1038–1042
Saad F, Gleason DM, Murray R et al. (2002) A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst 94:1458–1468
Saad F, Gleason DM, Murray R et al. (2004) Long-term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone-refractory prostate cancer. J Natl Cancer Inst 96:879–882
Gnant M, Mlineritsch B, Schippinger W et al. (2009) Endocrine therapy plus zoledronic acid in premenopausal breast cancer. N Engl J Med 360:679–691
Coleman R, de Boer R, Eidtmann H et al. (2013) Zoledronic acid (zoledronate) for postmenopausal women with early breast cancer receiving adjuvant letrozole (ZO-FAST study): final 60-month results. Ann Oncol 24:398–405
Coleman R, Cameron D, Dodwell D et al. (2014) Adjuvant zoledronic acid in patients with early breast cancer: final efficacy analysis of the AZURE (BIG 01/04) randomised open-label phase 3 trial. Lancet Oncol 15:997–1006
Wirth M, Tammela T, Cicalese V et al. (2015) Prevention of bone metastases in patients with high-risk nonmetastatic prostate cancer treated with zoledronic acid: efficacy and safety results of the Zometa European Study (ZEUS). Eur Urol 67:482–491
Dearnaley DP, Mason MD, Parmar MK et al. (2009) Adjuvant therapy with oral sodium clodronate in locally advanced and metastatic prostate cancer: long-term overall survival results from the MRC PR04 and PR05 randomised controlled trials. Lancet Oncol 10:872–876
Smith MR, Halabi S, Ryan CJ et al. (2014) Randomized controlled trial of early zoledronic acid in men with castration-sensitive prostate cancer and bone metastases: results of CALGB 90202 (alliance). J Clin Oncol 32:1143–1150
James ND, Sydes MR, Clarke NW et al. (2016) Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet 387:1163–1177
Soloway MS, Hardeman SW, Hickey D et al. (1988) Stratification of patients with metastatic prostate cancer based on extent of disease on initial bone scan. Cancer 61:195–202
Eisenberger MA, Blumenstein BA, Crawford ED et al. (1998) Bilateral orchiectomy with or without flutamide for metastatic prostate cancer. N Engl J Med 339:1036–1042
Kotake T, Usami M, Akaza H et al. (1999) Goserelin acetate with or without antiandrogen or estrogen in the treatment of patients with advanced prostate cancer: a multicenter, randomized, controlled trial in Japan. Jpn J Clin Oncol 29:562–570
Usami M, Akaza H, Arai Y et al. (2007) Bicalutamide 80 mg combined with a luteinizing hormone-releasing hormone agonist (LHRH-A) versus LHRH-A monotherapy in advanced prostate cancer: findings from a phase III randomized, double-blind, multicenter trial in Japanese patients. Prostate Cancer Prostatic Dis 10:194–201
Schoenfeld DA (1983) Sample-size formula for the proportional-hazards regression model. Biometrics 39:499–503
Dearnaley DP, Sydes MR, Mason MD et al. (2003) A double-blind, placebo-controlled, randomized trial of oral sodium clodronate for metastatic prostate cancer (MRC PR05 Trial). J Natl Cancer Inst 95:1300–1311
Vale CL, Burdett S, Rydzewska LH et al. (2016) Addition of docetaxel or bisphosphonates to standard of care in men with localised or metastatic, hormone-sensitive prostate cancer: a systematic review and meta-analyses of aggregate data. Lancet Oncol 17:243–256
Thompson IM, Tangen C, Basler J et al. (2002) Impact of previous local treatment for prostate cancer on subsequent metastatic disease. J Urol 168:1008–1012
Morgan GJ, Davies FE, Gregory WM et al. (2010) First-line treatment with zoledronic acid as compared with clodronic acid in multiple myeloma (MRC Myeloma IX): a randomised controlled trial. Lancet 376:1989–1999
Noguchi M, Kikuchi H, Ishibashi M et al. (2003) Percentage of the positive area of bone metastasis is an independent predictor of disease death in advanced prostate cancer. Br J Cancer 88:195–201
Smith MR, Saad F, Coleman R et al. (2012) Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: results of a phase 3, randomised, placebo-controlled trial. Lancet 379:39–46
Vahtsevanos K, Kyrgidis A, Verrou E et al. (2009) Longitudinal cohort study of risk factors in cancer patients of bisphosphonate-related osteonecrosis of the jaw. J Clin Oncol 27:5356–5362
Acknowledgments
The ZAPCA trial was supported by Grant for Urologic Research No. 200040700148 from Kyoto University Hospital. We thank all members of the ZAPCA Study Group for their cooperation. We also thank Dr. Masanori Fukushima in TRI for his valuable advice and critical reading of the manuscript and Mr. Koichi Yamashiro in TRI for his administrative and clerical support of this study. The affiliated members are Hakodate Goryokaku Hospital, Sunagawa City Medical Center, Sapporo Medical University, Akita University, Osaki Citizen Hospital, Tohoku University, Kesen-numa City Hospital, Yamagata Prefectural Central Hospital, Mito Medical Center, Jikei University School of Medicine, Jikei University Katsushika Medical Center, Kitasato University, Tokai University, Shimada Municipal Hospital, Shizuoka General Hospital, Shizuoka Hospital, Otsu Red Cross Hospital, Otsu Municipal Hospital, Shiga Medical Center for Adults, Shiga University of Medical Science, Kyoto University, Kyoto-Katsura Hospital, Kyoto City Hospital, Nara Medical University, Yamato Koriyama Hospital, Tenri Hospital, Osaka Red Cross Hospital, Kansai Electric Power Hospital, Osaka Kaisei Hospital, Kitano Hospital, Nishikobe Medical Center, Toyo-oka Hospital, Kurashiki Central Hospital, Kure Medical Center, Hiroshima University, JA Hiroshima General Hospital, Chugoku Rosai Hospital, Kagawa University, Oita University, Tsurumi Hospital, Nakamura Hospital, Yamaga Hospital, Ureshino Medical Center, Nagasaki University, and University of Miyazaki.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
Tomomi Kamba accepted an honorarium from Astellas Pharma. Toshiyuki Kamoto accepted research funding and honoraria from Astellas Pharma. Fuminori Sato accepted research funding from Janssen Pharmaceutical and Astellas Pharma. Naoya Masumori accepted honoraria from Novartis Pharma and Daiichi Sankyo, and research funding from Daiichi Sankyo. Shin Egawa accepted research funding from Astellas Pharma and Takeda Pharmaceutical. Hideki Sakai accepted research funding from Astellas Pharma and Takeda Pharmaceutical, and honoraria from Astellas Pharma and AstraZeneca. Osamu Ogawa accepted an honorarium from Astellas Pharma.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10147_2016_1037_MOESM1_ESM.tiff
Fig. S1. Kaplan−Meier plots for time to treatment failure in patients with baseline PSA ≥200 ng/mL (A), EOD ≤2 (B), EOD ≥3 (C), GS ≤7 (D) and GS ≥8 (E) (TIFF 5170 kb)
10147_2016_1037_MOESM2_ESM.tiff
Fig. S2. Kaplan−Meier plots for time to first SRE in patients with baseline PSA <200 ng/mL (A), PSA ≥200 ng/mL (B), EOD ≥3 (C) and GS ≤7 (D) (TIFF 4136 kb)
10147_2016_1037_MOESM3_ESM.tiff
Fig. S3. Kaplan–Meier plots for OS in patients with baseline PSA <200 ng/mL (A), PSA ≥200 ng/mL (B), EOD ≤2 (C), EOD ≥3 (D), GS ≤7 (E) and GS ≥8 (F) (TIFF 5596 kb)
About this article
Cite this article
Kamba, T., Kamoto, T., Maruo, S. et al. A phase III multicenter, randomized, controlled study of combined androgen blockade with versus without zoledronic acid in prostate cancer patients with metastatic bone disease: results of the ZAPCA trial . Int J Clin Oncol 22, 166–173 (2017). https://doi.org/10.1007/s10147-016-1037-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-016-1037-2