Abstract
Background
Improvements in operative technique and perioperative management have resulted in increasing numbers of elderly patients undergoing gastrectomy for gastric cancer (GC). We evaluated the accuracy of Estimation of Physiologic Ability and Surgical Stress (E-PASS) and modified (m)E-PASS scores in predicting postoperative complications in elderly patients with GC.
Methods
We retrospectively analyzed short-term outcomes in 413 patients who underwent gastrectomy for GC between 2005 and 2014. They were divided into two groups: Group N comprised 341 non-elderly patients <80 years of age and Group E comprised 72 elderly patients ≥80 years of age. We calculated the E-PASS and mE-PASS scores and evaluated the correlation between the comprehensive risk score (CRS) and occurrence of postoperative complications.
Results
Morbidity rates were 25.5 % in Group N and 31.9 % in Group E. In Group N, the CRS values of both the E-PASS (P < 0.0001) and mE-PASS (P < 0.0001) scores were significantly higher in patients with complications than in those without complications. In Group E, although the E-PASS CRS was significantly higher in patients with complications than in patients without complications (P = 0.01), the mE-PASS CRS fixed (CRSf) score was not significantly correlated with the occurrence of postoperative complications (P = 0.08).
Conclusion
Both E-PASS and mE-PASS can be used to predict the occurrence of postoperative complications in GC patients undergoing gastrectomy. However, the E-PASS CRS is more accurate for elderly patients because variations in intraoperative parameters such as operation time, blood loss, and extent of skin incision have a strong influence on the occurrence of postoperative complications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer (GC) is a major health problem and constitutes the second leading cause of cancer-related death [1]. The incidence of GC in Japan has been increasing recently, especially among the elderly [2]. The overall incidence of GC may be rising because peak incidence occurs in the seventh decade of life [3] and the life expectancy of the general population is increasing. Treatment for GC requires gastric resection [4] and, because of improved perioperative management, resection of GC has become the treatment of choice in elderly patients [5, 6].
Comorbidities are more common in elderly patients than in young ones and may result in increased postoperative morbidity and mortality. For elderly patients in particular, the benefits of surgical treatment must be balanced against postoperative morbidity and mortality [5]. However, age is not an inflexible criterion for deciding whether surgical treatment is indicated in elderly patients. Patient characteristics including biological age, physical presentation, and performance status should also be taken into consideration when evaluating the benefit–risk balance of surgery [7]. Several scoring systems can be used to assess the risk of mortality or of developing complications following special types of surgical procedures [8–10].
We previously constructed a prediction scoring system for postoperative morbidity and mortality in elective gastrointestinal surgery, which we designated “Estimation of Physiologic Ability and Surgical Stress” (E-PASS) [9]. Several cohort studies in GC and in other cancers have demonstrated reproducible outcomes using E-PASS scores to predict adverse postoperative events [9, 11–14]. However, E-PASS is limited by not being able to predict the occurrence of postoperative morbidity prior to surgery because the assessment includes some intraoperative factors. Therefore, we recently developed a modification of this system (mE-PASS) to estimate postoperative mortality rates before surgery using a reduced number of variables [15, 16] and reported on its accuracy for patients of all ages who underwent gastrectomy for GC [16].
However, the prognostic value of both E-PASS and mE-PASS for elderly patients who undergo gastrectomy for GC remains unclear. To accurately describe the physical condition of these patients, a practical scoring system that includes assessment of their preoperative condition and their response to invasive surgical procedures is essential. Therefore, we retrospectively analyzed the accuracy of both E-PASS and mE-PASS in patients who underwent gastrectomy for GC to identify which of these two scoring systems is better suited for use in elderly patients.
Methods
Patients and patient selection
A total of 413 patients who underwent gastrectomy and lymph node dissection at Kumamoto University Hospital between April 2005 and August 2014 were enrolled in this study. All patients had a pathologically confirmed diagnosis of gastric adenocarcinoma. The patients were divided into two groups by age: a non-elderly group (Group N, <80 years of age) of 341 patients and an elderly group (Group E, ≥80 years of age) of 72 patients. All patients gave written informed consent and the local Ethics Committee of Kumamoto University approved the study.
Methods and scoring systems
Disease stage was classified according to the Japanese Classification of Gastric Carcinoma (3rd English edition) [17], which is compatible with the Union for International Cancer Control (7th edition). Surgical procedures, including the extent of gastrectomy and lymph node dissection, were based on the Japanese Gastric Cancer Treatment Guidelines 2010 (version 3) [18].
E-PASS and mE-PASS physiological and operative variables were collected retrospectively. The preoperative risk score (PRS), surgical stress score (SSS), and comprehensive risk score (CRS) were calculated using Haga’s equations for E-PASS, as shown below [9].
where X1 is patient age; X2 is the presence (1) or absence (0) of severe heart disease; X3 is the presence (1) or absence (0) of severe pulmonary disease; X4 is the presence (1) or absence (0) of diabetes mellitus; X5 is the performance status index (0–4); and X6 is the American Society of Anesthesiologists physiological status classification (1–5).
where X1 is blood loss/body weight (g/kg); X2 is operation time (h); and X3 is extent of skin incision (0, minor incision for laparoscopic or thoracoscopic surgery; 1, laparotomy or thoracotomy alone; 2, both laparotomy and thoracotomy).
To determine the mE-PASS score, PRS was calculated using the same equation used for E-PASS. We used a fixed SSS (SSSf) value of 0.328 for total gastrectomy or 0.212 for partial gastrectomy in mE-PASS, which was the median SSS of the original E-PASS determination. The CRS fixed (CRSf) score was calculated as follows [15]:
Evaluation of outcomes
We calculated the E-PASS and mE-PASS scores and evaluated the correlation between the CRS and the occurrence of postoperative complications in the two groups. Postoperative complications are evaluated according to the Clavien–Dindo classification [19, 20]. Adverse events of grade 2 to 4 were expediently judged as postoperative complications. Grade 1 adverse events were excluded from the analysis because they require no medical treatment.
Continuous variables were expressed as mean ± standard deviation, and differences were assessed for significance using the Student t-test or the Mann–Whitney test. Categorical data were evaluated using chi-squared or Fisher exact tests, as appropriate. Univariate and multivariate logistic regression analyses were performed to identify predictors of postoperative complications. Receiver operating characteristic (ROC) curve analysis was performed to identify a cutoff value of each group for the E-PASS and mE-PASS score. Differences were considered to be significant at P < 0.05. All tests were performed using JMP software (SAS Institute Inc., Cary, NC, USA).
Results
Clinicopathological characteristics of patients
Clinicopathological characteristics of patients are summarized in Table 1. Sex ratio, body mass index, and presence of diabetes mellitus in the two groups were not significantly different, but the performance status index, American Society of Anesthesiologists physical status classification, and carcinoembryonic antigen level were significantly worse in Group E (P = 0.002, P = 0.03, and P = 0.006, respectively) than Group N. In pathological characteristics, only the depth of invasion was significantly different between the two groups (P = 0.03).
Surgical parameters, E-PASS score, and postoperative outcomes of patients
Surgical parameters, E-PASS score, and postoperative outcomes of patients are summarized in Table 2. There were no significant differences between the surgical parameters in each group, including type of gastrectomy, type of lymph node dissection, type of incision, and operation duration. PRS, CRS, and CRSf were significantly higher in Group E than Group N (P < 0.0001), but SSS was similar in the two groups (P = 0.08). There were no significant differences in postoperative outcomes in each group, including postoperative complications, length of stay, reoperation, and hospital death. The postoperative complications that occurred in each group following gastrectomy are summarized in Table 3. The morbidity rates in the 413 surgically resected patients were 25.5 % in Group N (n = 87) and 31.9 % in Group E (n = 23). The most frequent morbidities were pancreatic fistula in Group N (7.3 %) and anastomotic leakage in Group E (11.1 %). The only significant difference in postsurgical morbidity between the two groups (P = 0.003) was the rate of occurrence of respiratory complications.
Correlation between CRS and the occurrence of postoperative complications
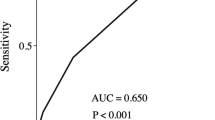
The presence or absence of complications in the combined groups, Group N, and Group E are plotted against the CRS and CRSf scores in Figs. 1, 2 and 3. In both the combined population and in Group N, the E-PASS CRS and mE-PASS CRSf values were significantly higher in patients with complications than in those without complications (all P < 0.0001, Figs. 1, 2). However, in Group E, although the E-PASS CRS was significantly higher in patients who experienced postoperative complications compared with those who did not (P = 0.01), the mE-PASS CRSf was not correlated with the occurrence of complications (P = 0.08, Fig. 3).
Univariate and multivariate analysis of the occurrence of complications
Univariate and multivariate analyses of the occurrence of complications in elderly patients are shown in Table 4. The performance of CRS and CRSf were assessed by ROC curve analysis. The ROC curve had an area under the curve (AUC) of 0.666 (P < 0.0001) in CRS and 0.653 (P < 0.0001) in CRSf. The univariate analysis revealed that blood loss (>240 ml), tumor size (>50 mm), and CRS (>0.23) were significantly associated with the occurrence of complications. Multivariate analysis including these variables revealed that only CRS (>0.23) was an independent predictive factor. In contrast, in non-elderly patients, multivariate analysis revealed that blood loss (>240 ml) and CRSf (>0.42) were independent predictive factors (Table 5). These analyses show that in elderly patients with GC, E-PASS was an independent predictive factor of the occurrence of complications, whereas in non-elderly patients mE-PASS was an independent predictive factor.
Discussion
In this study, we found that E-PASS was better than mE-PASS for estimating the occurrence of postoperative complications in elderly patients who underwent gastrectomy for GC. In a previous study, we reported that a CRS ≥0.5 was a good indicator of poor prognosis in GC patients over 80 years of age who underwent gastrectomy [21]. Ariake et al. also found that E-PASS-based CRS was a good predictor of comorbidity-related mortality in elderly patients who underwent gastrectomy for GC [7]. However, no studies have compared E-PASS with mE-PASS for estimating the likelihood of postoperative complications. This study revealed that the E-PASS CRS, but not the mE-PASS CRSf, was significantly correlated with the occurrence of postoperative complications in elderly gastrectomy patients. Multivariate analysis showed that in elderly patients a CRS >0.23 was an independent predictor of postoperative complications.
Polanczyk et al. reported that advanced age was an independent predictor of morbidity, mortality, and prolonged hospital stay in patients undergoing noncardiac surgery [22]. Therefore, it is especially important to assess physiological risk and surgical stress in elderly patients. Several scoring systems have been used to estimate the risk of postoperative complications and mortality, such as the Physiological and Operative Severity score [8]. However, the Physiological and Operative Severity index requires measuring as many as 19 perioperative physiological parameters, including some parameters that are not routinely measured before surgery. In contrast, the E-PASS scoring system includes only 10 parameters, which are routinely evaluated before surgery. E-PASS is simple and easily calculated [9]. The E-PASS scoring system has been shown to be useful for predicting postoperative risk not only in gastroenterological surgery [9, 11–14], but also in other fields such as thoracic [23, 24] and vascular surgery [25, 26].
Because the E-PASS scoring system includes intraoperative factors such as blood loss, operation time, and extent of skin incision, an accurate CRS can be obtained just after the completion of surgery. In fact, the surgeons have to calculate the CRS using estimated blood loss and operation time based on their past surgical experience. However, the mE-PASS scoring system can resolve this problem. Moreover, we found mE-PASS to be accurate in patients of all ages who underwent gastrectomy for GC [16]. The mE-PASS scoring system is particularly useful for two reasons. First, it can predict the occurrence of postoperative complications before surgery. In this study, the SSSf used in mE-PASS calculation was determined using the median SSS of 41 procedures in patients with E-PASS scores [15]. Using mE-PASS, surgeons can preoperatively discuss the risk of surgery and the selection of procedures with their patients. Second, mE-PASS reduces the number of variables from ten to seven, making it simpler than E-PASS [15, 16].
However, we should use these scoring systems with caution, especially in elderly patients, because intraoperative factors influence outcomes more strongly in elderly than in non-elderly patients. Various postoperative complications have been reported to occur when surgical stress exceeds the patient’s physiological reserve, making it impossible to maintain homeostasis [9]. Elderly patients may suffer from comorbid diseases that decrease their physiological reserves and should be considered before choosing surgical intervention. In addition, elderly patients frequently develop severe cardiac or pulmonary complications, even in the absence of preoperative comorbidities, because it is difficult for them to maintain homeostasis when they undergo invasive procedures [27]. Our study suggests that E-PASS is better than mE-PASS for predicting the occurrence of postoperative complications in elderly GC patients who undergo gastrectomy. Furthermore, using intraoperative factors obtained in individual patients, such as operation time, blood loss, and extent of skin incision, is more important in elderly than in non-elderly GC patients who undergo gastrectomy. In cases involving elderly patients, we suggest that a fixed estimate of intraoperative factors should not be used, and that it is necessary to evaluate their surgical stress individually and in detail. We suggest carefully evaluating each patient’s condition when deciding on the appropriate therapeutic procedure, particularly for the elderly.
The present study has limitations associated with its retrospective design and conduct at a single center, which might introduce several biases. Hence, a prospective multicenter study would be desirable to validate our present findings.
We conclude that E-PASS was a more accurate predictor than mE-PASS for estimating the occurrence of postoperative complications in elderly patients undergoing gastrectomy for GC. This is the first comparison of these two scoring systems. Although multiple scoring systems, including E-PASS and mE-PASS, are accurate for predicting the occurrence of postoperative complications, each must be used with knowledge of their advantages and disadvantages. Appropriate application of these systems enables accurate assessment of surgical risk and assists in choosing the proper treatment for each patient.
References
Jemal A, Bray F, Center MM et al (2011) Global cancer statistics. CA Cancer J Clin 61:69–90
Saito H, Osaki T, Murakami D et al (2006) Effect of age on prognosis in patients with gastric cancer. ANZ J Surg 76:458–461
Saif MW, Makrilia N, Zalonis A et al (2010) Gastric cancer in the elderly: an overview. Eur J Surg Oncol 36:709–717
Hundahl SA, Phillips JL, Menck HR (2000) The National Cancer Data Base Report on poor survival of US gastric carcinoma patients treated with gastrectomy: fifth edition American Joint Committee on Cancer staging, proximal disease, and the “different disease” hypothesis. Cancer 88:921–932
Orsenigo E, Tomajer V, Palo SD et al (2007) Impact of age on postoperative outcomes in 1118 gastric cancer patients undergoing surgical treatment. Gastric Cancer 10:39–44
Saidi RF, Bell JL, Dudrick PS (2004) Surgical resection for gastric cancer in elderly patients: is there a difference in outcome? J Surg Res 118:15–20
Ariake K, Ueno T, Takahashi M et al (2014) E-PASS comprehensive risk score is a good predictor of postsurgical mortality from comorbid disease in elderly gastric cancer patients. J Surg Oncol 109:586–592
Copeland GP, Jones D, Walters M (1991) POSSUM: a scoring system for surgical audit. Br J Surg 78:355–360
Haga Y, Ikei S, Ogawa M (1999) Estimation of Physiologic Ability and Surgical Stress (E-PASS) as a new prediction scoring system for postoperative morbidity and mortality following elective gastrointestinal surgery. Surg Today 29:219–225
Knaus WA, Draper EA, Wagner DP et al (1985) APACHE II: a severity of disease classification system. Crit Care Med 13:818–829
Banz VM, Studer P, Inderbitzin D et al (2009) Validation of the estimation of physiologic ability and surgical stress (E-PASS) score in liver surgery. World J Surg 33:1259–1265
Hashimoto D, Takamori H, Sakamoto Y et al (2010) Is an estimation of physiologic ability and surgical stress able to predict operative morbidity after pancreaticoduodenectomy? J Hepatobiliary Pancreat Sci 17:132–138
Hashimoto D, Takamori H, Sakamoto Y et al (2010) Can the physiologic ability and surgical stress (E-PASS) scoring system predict operative morbidity after distal pancreatectomy? Surg Today 40:632–637
Yoshida N, Watanabe M, Baba Y et al (2013) Estimation of physiologic ability and surgical stress (E-PASS) can assess short-term outcome after esophagectomy for esophageal cancer. Esophagus 10:86–94
Haga Y, Ikejiri K, Wada Y et al (2011) A multicenter prospective study of surgical audit systems. Ann Surg 253:194–201
Haga Y, Wada Y, Takeuchi H et al (2012) Evaluation of modified estimation of physiologic ability and surgical stress in gastric carcinoma surgery. Gastric Cancer 15:7–14
Japanese Gastric Cancer Association (2011) Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 14:101–112
Japanese Gastric Cancer Association (2011) Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer 14:113–123
Clavien PA, Barkun J, de Oliveira ML et al (2009) The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 250:187–196
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213
Haga Y, Yagi Y, Ogawa M (1999) Less-invasive surgery for gastric cancer prolongs survival in patients over 80 years of age. Surg Today 29:842–848
Polanczyk CA, Marcantonio E, Goldman L et al (2001) Impact of age on perioperative complications and length of stay in patients undergoing noncardiac surgery. Ann Intern Med 134:637–643
Yamashita S, Haga Y, Nemoto E et al (2006) Comparison of surgical outcome using the prediction scoring system of E-PASS for thoracic surgery. Jpn J Thorac Cardiovasc Surg 54:391–395
Yamashita S, Haga Y, Nemoto E et al (2004) E-PASS (The Estimation of Physiologic Ability and Surgical Stress) scoring system helps the prediction of postoperative morbidity and mortality in thoracic surgery. Eur Surg Res 36:249–255
Tang T, Walsh SR, Fanshawe TR et al (2007) Estimation of physiologic ability and surgical stress (E-PASS) as a predictor of immediate outcome after elective abdominal aortic aneurysm surgery. Am J Surg 194:176–182
Tang TY, Walsh SR, Fanshawe TR et al (2007) Comparison of risk-scoring methods in predicting the immediate outcome after elective open abdominal aortic aneurysm surgery. Eur J Vasc Endovasc Surg 34:505–513
Hayashi T, Yoshikawa T, Aoyama T et al (2012) Severity of complications after gastrectomy in elderly patients with gastric cancer. World J Surg 36:2139–2145
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical standards
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. Informed consent or substitute for it was obtained from all patients for being included in the study.
Conflict of interest
We declare that there is no grant support and any other conflict of interest.
About this article
Cite this article
Kitano, Y., Iwatsuki, M., Kurashige, J. et al. Estimation of Physiologic Ability and Surgical Stress (E-PASS) versus modified E-PASS for prediction of postoperative complications in elderly patients who undergo gastrectomy for gastric cancer. Int J Clin Oncol 22, 80–87 (2017). https://doi.org/10.1007/s10147-016-1028-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-016-1028-3