Abstract
Background
Little information has been published on the use of tyrosine kinase inhibitors for treatment of patients undergoing hemodialysis (HD). We investigated the efficacy and safety of sorafenib for metastatic renal cell carcinoma (mRCC) patients undergoing HD.
Methods
Twenty patients undergoing HD were treated with sorafenib as first-line therapy for mRCC at our hospital between April 2008 and August 2014. Patient medical records were retrospectively reviewed to evaluate the response to sorafenib and treatment-related toxicity.
Results
Fifteen and 5 patients were classified in the intermediate and poor risk groups, respectively, of the Memorial Sloan–Kettering Cancer Center risk model. Eighteen patients had 3 or more metastatic lesions, and 7 patients had metastases in 2 or more organs. Of 16 patients who had previously undergone nephrectomy, 8 were pathologically diagnosed with non-clear-cell carcinoma. The median duration of sorafenib therapy was 4.7 months. Sorafenib was discontinued owing to progressing disease for 15 patients and because of serious adverse events (AE) (≥grade 3) for 4 patients, i.e. subarachnoid hemorrhage, cerebral hemorrhage, sepsis, and syncope for 1 patient each. Median time to progression was 6.3 months, and median overall survival was 14.2 months.
Conclusions
In this study, many patients had unfavorable clinical features, for example poor risk classification and metastases in multiple organs. Although sorafenib treatment of HD patients seems feasible, careful monitoring is needed because of the tendency for a high incidence of serious AE, even when a reduced dose is administered.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Patients with end-stage renal disease (ESRD) are at substantially greater risk of developing renal cell carcinoma (RCC), the prevalence of which is reported to be 3–5 % for dialysis and/or renal transplant patients—100-fold higher than for the general population [1–4]. However, the biological behavior of RCC related to ESRD (ESRD-RCC) is usually reported to be less aggressive than that of RCC for other patients [3, 5], although a longer duration of dialysis might increase the possibility of developing more aggressive RCC with an unfavorable prognosis [6]. In fact, the natural history, prognosis, and optimum treatment of advanced ESRD-RCC remain unclear, with limited data available from only a few studies, each of which included only a small number of dialysis patients [7].
Sorafenib is an orally administered multi-targeted tyrosine kinase inhibitor (TKI) of Raf, vascular endothelial growth factor receptors 2 and 3, and platelet-derived growth factor receptor. In phase 3 of the treatment approaches in renal cancer global evaluation trial (TARGET) [8], sorafenib prolonged progression-free survival (PFS) compared with placebo in advanced clear-cell RCC patients with favorable-risk or intermediate-risk status, according to the Memorial Sloan-Kettering Cancer Center (MSKCC) prognostic score [9], and for whom 1 round of previous systemic therapy had failed. Most of these patients had previously received immunotherapy as systemic therapy, and over 99 % had clear-cell histology. As with most other clinical trials, patients with ESRD were excluded. Previous studies have shown there is no correlation between the degree of renal functional impairment and the steady-state area under the curve for sorafenib [10–12]. Case reports also suggest that the standard dose of sorafenib could be successfully used to treat metastatic RCC (mRCC) in dialysis patients without serious adverse events (AE) [13–15]. However, even retrospective analysis of the efficacy and safety of TKI for patients with ESRD requiring hemodialysis (HD) have been limited [16]. We continued to use sorafenib for treatment of HD patients with mRCC after evaluating its pharmacokinetics for 6 dialysis patients [17]. The purpose of this study was to re-evaluate the efficacy and safety of sorafenib for dialysis patients with mRCC by including more patients. We also assessed the prognostic significance of pretreatment serum C-reactive protein (CRP) level and duration of HD for overall survival (OS) of these patients.
Patients and methods
After institutional review board approval, the medical record archives between 2008 and 2014 at Tokyo Women’s Medical University Hospital were retrospectively reviewed. During this period, 22 patients on HD were treated for mRCC and screened for inclusion in the study. Of these, 20 patients were eligible for analysis; all had received sorafenib for mRCC as first-line therapy and pretreatment serum CRP levels were known.
In this series, sorafenib was initially administered at 200 mg per day; this was then increased to a maintenance dose of 400–600 mg daily, depending on AE. Treatment with sorafenib was continued until disease progression or observation of intolerable AE.
Before treatment, all patients underwent baseline evaluation, including pretreatment serum CRP level, tumor imaging, and chest computed tomography (CT), and they were followed-up for at least once a month during treatment. We defined pretreatment serum CRP levels >5 mg/dl as elevated CRP [18]. Objective clinical responses were assessed on the basis of the response evaluation criteria in solid tumors (RECIST) version 1.0 guidelines by use of CT every 2–3 months. AE were graded according to the National Cancer Institute’s Common Terminology Criteria for AE, version 4.0. Histological tumor types were categorized in accordance with the WHO 2004 classification.
All patients had a usable, surgically created, arteriovenous fistula, and they underwent standard HD 3 times a week for 3–4 h at their regular HD clinics.
Statistical analysis
Patients were divided into 2 groups on the basis of the duration of HD (≤120 vs >120 months). Variables for different groups were compared by use of the χ 2 test or the Mann–Whitney U test, as appropriate. OS curves were estimated by use of the Kaplan–Meier method and compared by use of the log-rank test. Associations between clinicopathological variables, including pretreatment serum CRP level, duration of HD, and treatment outcomes, were assessed in multivariate models by use of Cox proportional hazard regression models. A difference was considered significant when P < 0.05. Significance was calculated by use of JMP 11.0.0 software (SAS institute, Cary, NC, USA).
Results
Patient characteristics
Baseline demographics and clinical characteristics on the basis of the duration of HD are listed in Table 1. All patients were men, and their median age was 62.5 years. The median duration of HD was 283 months, and 13 patients (65 %) had been undergoing HD for over 120 months. Fifteen patients (75 %) were classified as being in the intermediate risk group, the other 5 patients (25 %) in the poor risk group, according to the MSKCC risk model. Eighteen patients (90 %) had 3 or more metastatic lesions, and 9 patients (45 %) had metastases in 2 or more organs. Sixteen patients (80 %) had previously undergone nephrectomy, with a median time from nephrectomy to metastasis of 8.5 months (range 0–131 months); 8 (40 %) of these patients were pathologically diagnosed with non-clear-cell carcinoma, 7 with papillary type 2 RCC and 1 with papillary type 1 RCC.
The mean duration of sorafenib therapy was 4.7 months. Five patients (25 %) received 600 mg daily, 10 patients (50 %) 400 mg daily, and 5 patients (25 %) 200 mg daily as a maintenance dose. Long-term dialysis patients (duration of HD > 120 months) were significantly more likely to have non-clear-cell carcinoma (P < 0.0001) and a significantly shorter duration of sorafenib therapy (P = 0.0063) compared with non-long-term dialysis patients (duration of HD ≤ 120 months).
Treatment outcomes
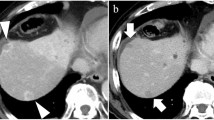
Of the 20 patients with measurable lesions, 1 (5 %) achieved a complete response, 2 (10 %) achieved a partial response, and 11 (55 %) had stable disease (Fig. 1) with an overall response of 15 % and disease control of 70 %. Three patients (15 %) had progressive disease (PD); the other 3 patients (15 %) could not be evaluated because their treatment was discontinued too early for assessment.
At the time of this analysis, only 1 patient (5 %) was still receiving treatment with sorafenib. Sorafenib was discontinued owing to PD for 15 patients (75 %) and serious AE (≥grade 3) for 4 patients (20 %). During the median follow-up period of 13 months, 15 patients (75 %) died. The median time to progression was 6.3 months, and median OS was 14.2 months (Fig. 2).
Survival outcomes by patient subgroup
Univariate analysis of different subgroups of the patients showed that the pretreatment CRP level (≤5 vs >5 mg/dl; P < 0.0001) and duration of HD (≤120 vs >120 months; P = 0.0087) were associated with OS whereas MSKCC risk category (intermediate vs poor) and histological type (clear cell carcinoma vs non-clear-cell carcinoma) were only marginally associated with OS (P = 0.078 and P = 0.063, respectively). Median OS was 2.8 months for patients with an elevated CRP level before treatment whereas median OS was 15.0 months for those without an elevated CRP level before treatment (P < 0.0001) (Fig. 3). Median OS was not reached for non-long-term dialysis patients compared with the median OS of 12.2 months for those undergoing long-term dialysis (P = 0.0087). Multivariate analysis identified pretreatment CRP level (hazard ratio (HR) = 90.7; P = 0.0007) and duration of HD (HR = 15.5; P = 0.029) as independent predictors of OS (Table 2).
Kaplan–Meier estimate of overall survival (OS) according to a MSKCC risk (P = 0.078), b histological type (P = 0.063), c a pretreatment C-reactive protein (CRP) level (P < 0.0001), and d duration of hemodialysis (P = 0.0087). CI confidence interval, NR not reached, CRCC clear cell renal cell carcinoma, NCRCC non-clear-cell renal cell carcinoma, HD hemodialysis
Sorafenib safety profile
The most common treatment-related AE observed during treatment are listed in Table 3. Commonly reported non-hematological AE of any grade were gastrointestinal toxicity, cardiac toxicity, skin toxicity, and fatigue. Hematological toxicity were also commonly reported, including grade 3 anemia (25 %). As described above, 4 patients (20 %) discontinued sorafenib therapy because of serious AE (≥grade 3), with 1 case each of subarachnoid hemorrhage, cerebral hemorrhage, sepsis, and syncope. All fatal AE (≥grade 4) were observed for long-term dialysis patients only.
Discussion
Patients with severe renal failure, including those undergoing HD, are usually excluded from clinical trials of anti-cancer agents. These trials are usually performed on highly selected patient groups by use of strict eligibility criteria, as in phase 3 of the TARGET [8] that led to the approval of sorafenib for advanced RCC. It is well documented that outcome is significantly poorer for patients who are ineligible for clinical trials than for those eligible to receive targeted therapy for mRCC [19]. Even retrospective analysis of the efficacy and safety of molecular-targeted agents has, however, been limited for dialysis patients [16]. Therefore, there is little evidence to support the use of targeted therapy for mRCC in patients with ESRD requiring HD.
Approval of sorafenib for Japanese patients with advanced RCC was followed by approval of sunitinib in 2008. In contrast with sunitinib, sorafenib did not extend PFS compared with interferon alpha as first-line therapy for mRCC. However, the incidence of AE was lower for patients treated with sorafenib than for those who received sunitinib [20, 21]. In pivotal phase 2 studies of Japanese patients with advanced RCC, AE were also less frequently observed after sorafenib treatment than after sunitinib treatment [22, 23]. Sorafenib is also reported to be well-tolerated, even by elderly patients [24] and by patients undergoing long-term treatment with sorafenib [25]. We have previously studied its pharmacokinetics in 6 dialysis patients [17]; on the basis of these findings, for patients undergoing HD we continued to use sorafenib instead of sunitinib, which is the recommended standard first-line therapy for good or intermediate risk clear-cell RCC patients, or temsirolimus, which is recommended as standard first-line therapy for poor-risk clear-cell RCC or non-clear-cell RCC patients, who are generally regarded as being susceptible to the side effects of targeted agents. To the best of our knowledge, this is the largest study of dialysis patients with mRCC treated with sorafenib, and the first to assess the prognostic effect of a pretreatment CRP levels and the duration of HD for these patients.
In this study we found that overall response was 15 % and disease control was 70 %. These results are comparable with those of previous pivotal studies on sorafenib [8, 26], which reported objective responses of 2–4 % and SD for 78–80 % of patients. Two recent phase 3 randomized controlled trials failed to show any survival benefit of axitinib or tivozanib compared with sorafenib as first-line therapy for mRCC patients [27, 28]. In these studies, median PFS for patients who received sorafenib were 6.5 and 9.1 months, respectively. Moreover, the patients in this study had a median OS of 14.2 months, which was longer than that for patients treated in our hospital before 2008, for whom median OS was 12.8 months. Our results appear promising and clinically meaningful, because we included a high proportion of patients on long-term dialysis, a condition associated with a high prevalence of severe comorbidity [29–31] and aggressive RCC [6]. In this study, non-long-term dialysis patients had significantly better OS and were administered sorafenib for significantly longer than long-term dialysis patients. Furthermore, fatal AE (≥grade 4) were observed for long-term dialysis patients only. Multivariate analysis showed that long-term dialysis was an independent risk factor for poor OS for dialysis patients receiving sorafenib for mRCC, although the sample size might be too small to evaluate it appropriately by multivariate analysis. On the basis of these findings we suggest that long-term dialysis patients should be started on sorafenib at a lower dose, which should then be gradually increased, with careful monitoring.
We also demonstrated that pretreatment CRP level is a significant prognostic factor for OS of dialysis patients with mRCC treated with sorafenib. Saito et al. reported that serum CRP and its kinetics have prognostic value for non-dialysis patients with mRCC treated with cytokine-based therapy [18], and Yasuda et al. reported its prognostic usefulness for patients with mRCC treated with TKI [32]. For dialysis patients we discovered preoperative serum CRP level and postoperative CRP normalization were independent predictors of survival after nephrectomy [33]. Experimental studies have shown that at least some renal tumors produce interleukin-6, which promotes the growth of RCC; therefore, the presence of a systemic inflammatory response could promote tumor aggressiveness [34, 35]. More recently, studies have shown that modification of the tumor microenvironment caused by these pro-inflammatory mediators is important in increasing inflammation-associated angiogenesis, invasion, metastasis, and immune suppression [36–38]. Taken together, the serum CRP level could reflect tumor burden or the aggressiveness of ESRD-RCC and sporadic RCC; it is, therefore, a potential biomarker for dialysis patients undergoing sorafenib treatment for mRCC. In this study, patients with elevated CRP levels before treatment had very poor OS, which might mean that the efficacy of sorafenib is very limited for such patients. CRP is a non-specific inflammatory marker, and for patients with ESRD, a complex condition with numerous metabolic changes, several inflammatory processes lead to CRP elevation [39]. Many factors, including tumor aggressiveness, atherosclerosis, and malnutrition, could contribute to the highly unfavorable outcome for patients with elevated CRP. Therefore, further studies are needed to evaluate other treatment strategies and best supportive care for these patients.
It is difficult to make definitive conclusions, owing to the retrospective nature of our single-center study, the small sample size, and the short follow-up period, all of which increase the risk of bias. However, our results indicate that treatment of dialysis patients with sorafenib might be feasible, but careful monitoring is needed because of the tendency for a high incidence of serious AE, especially for patients undergoing long-term HD, even when a reduced dose is administered.
References
Ishikawa I, Saito Y, Shikura N et al (1990) Ten-year prospective study on the development of renal cell carcinoma in dialysis patients. Am J Kidney Dis 16:452–458
Terasawa Y, Suzuki Y, Morita M et al (1994) Ultrasonic diagnosis of renal cell carcinoma in hemodialysis patients. J Urol 152:846–851
Denton MD, Magee CC, Ovuworie C et al (2002) Prevalence of renal cell carcinoma in patients with ESRD pre-transplantation: a pathologic analysis. Kidney Int 61:2201–2209
Schwarz A, Vatandaslar S, Merkel S et al (2007) Renal cell carcinoma in transplant recipients with acquired cystic kidney disease. Clin J Am Soc Nephrol 2:750–756
Neuzillet Y, Tillou X, Mathieu R et al (2011) Renal cell carcinoma (RCC) in patients with end-stage renal disease exhibits many favourable clinical, pathologic, and outcome features compared with RCC in the general population. Eur Urol 60:366–373
Nouh MA, Kuroda N, Yamashita M et al (2010) Renal cell carcinoma in patients with end-stage renal disease: relationship between histological type and duration of dialysis. BJU Int 105:620–627
Syrios J, Kechagias G, Tsavaris N (2013) Treatment of patients with metastatic renal cell carcinoma undergoing hemodialysis: case report of two patients and short literature review. BMC Nephrol 14:84
Escudier B, Eisen T, Stadler WM et al (2007) Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med 356:125–134
Motzer RJ, Bacik J, Murphy BA et al (2002) Interferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J Clin Oncol 20:289–296
Strumberg D, Richly H, Hilger RA et al (2005) Phase I clinical and pharmacokinetic study of the Novel Raf kinase and vascular endothelial growth factor receptor inhibitor BAY 43-9006 in patients with advanced refractory solid tumors. J Clin Oncol 23:965–972
Clark JW, Eder JP, Ryan D et al (2005) Safety and pharmacokinetics of the dual action Raf kinase and vascular endothelial growth factor receptor inhibitor, BAY 43-9006, in patients with advanced, refractory solid tumors. Clin Cancer Res 11:5472–5480
Moore M, Hirte HW, Siu L et al (2005) Phase I study to determine the safety and pharmacokinetics of the novel Raf kinase and VEGFR inhibitor BAY 43-9006, administered for 28 days on/7 days off in patients with advanced, refractory solid tumors. Ann Oncol 16:1688–1694
Ruppin S, Protzel C, Klebingat KJ et al (2009) Successful sorafenib treatment for metastatic renal cell carcinoma in a case with chronic renal failure. Eur Urol 55:986–988 (quiz 988)
Shinsako K, Mizuno T, Terada T et al (2010) Tolerable sorafenib therapy for a renal cell carcinoma patient with hemodialysis: a case study. Int J Clin Oncol 15:512–514
Rey PM, Villavicencio H (2008) Sorafenib: tolerance in patients on chronic hemodialysis: a single-center experience. Oncology 74:245–246
Masini C, Sabbatini R, Porta C et al (2012) Use of tyrosine kinase inhibitors in patients with metastatic kidney cancer receiving haemodialysis: a retrospective Italian survey. BJU Int 110:692–698
Kennoki T, Kondo T, Kimata N et al (2011) Clinical results and pharmacokinetics of sorafenib in chronic hemodialysis patients with metastatic renal cell carcinoma in a single center. Jpn J Clin Oncol 41:647–655
Saito K, Tatokoro M, Fujii Y et al (2009) Impact of C-reactive protein kinetics on survival of patients with metastatic renal cell carcinoma. Eur Urol 55:1145–1153
Heng DY, Choueiri TK, Rini BI et al (2014) Outcomes of patients with metastatic renal cell carcinoma that do not meet eligibility criteria for clinical trials. Ann Oncol 25:149–154
Motzer RJ, Hutson TE, Tomczak P et al (2009) Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol 27:3584–3590
Escudier B, Szczylik C, Hutson TE et al (2009) Randomized phase II trial of first-line treatment with sorafenib versus interferon Alfa-2a in patients with metastatic renal cell carcinoma. J Clin Oncol 27:1280–1289
Akaza H, Tsukamoto T, Murai M et al (2007) Phase II study to investigate the efficacy, safety, and pharmacokinetics of sorafenib in Japanese patients with advanced renal cell carcinoma. Jpn J Clin Oncol 37:755–762
Uemura H, Shinohara N, Yuasa T et al (2010) A phase II study of sunitinib in Japanese patients with metastatic renal cell carcinoma: insights into the treatment, efficacy and safety. Jpn J Clin Oncol 40:194–202
Procopio G, Bellmunt J, Dutcher J et al (2013) Sorafenib tolerability in elderly patients with advanced renal cell carcinoma: results from a large pooled analysis. Br J Cancer 108:311–318
Hutson TE, Bellmunt J, Porta C et al (2010) Long-term safety of sorafenib in advanced renal cell carcinoma: follow-up of patients from phase III TARGET. Eur J Cancer 46:2432–2440
Stadler WM, Figlin RA, McDermott DF et al (2010) Safety and efficacy results of the advanced renal cell carcinoma sorafenib expanded access program in North America. Cancer 116:1272–1280
Hutson TE, Lesovoy V, Al-Shukri S et al (2013) Axitinib vs. sorafenib as first-line therapy in patients with metastatic renal-cell carcinoma: a randomised open-label phase 3 trial. Lancet Oncol 14:1287–1294
Motzer RJ, Nosov D, Eisen T et al (2013) Tivozanib versus sorafenib as initial targeted therapy for patients with metastatic renal cell carcinoma: results from a phase III trial. J Clin Oncol 31:3791–3799
Harnett JD, Foley RN, Kent GM et al (1995) Congestive heart failure in dialysis patients: prevalence, incidence, prognosis and risk factors. Kidney Int 47:884–890
Agarwal R (2005) Hypertension and survival in chronic hemodialysis patients–past lessons and future opportunities. Kidney Int 67:1–13
Longenecker JC, Coresh J, Powe NR et al (2002) Traditional cardiovascular disease risk factors in dialysis patients compared with the general population: the CHOICE Study. J Am Soc Nephrol 13:1918–1927
Yasuda Y, Saito K, Yuasa T et al (2013) Prognostic impact of pretreatment C-reactive protein for patients with metastatic renal cell carcinoma treated with tyrosine kinase inhibitors. Int J Clin Oncol 18(5):884–889
Omae K, Kondo T, Tanabe K (2014) High preoperative C-reactive protein values predict poor survival in patients on chronic hemodialysis undergoing nephrectomy for renal cancer. Urol Oncol 33(2):67.e9–67.e13
Miki S, Iwano M, Miki Y et al (1989) Interleukin-6 (IL-6) functions as an in vitro autocrine growth factor in renal cell carcinomas. FEBS Lett 250:607–610
Koo AS, Armstrong C, Bochner B et al (1992) Interleukin-6 and renal cell cancer: production, regulation, and growth effects. Cancer Immunol Immunother 35:97–105
Trédan O, Galmarini CM, Patel K et al (2007) Drug resistance and the solid tumor microenvironment. J Natl Cancer Inst 99:1441–1454
Mancino A, Lawrence T (2010) Nuclear factor-kappaB and tumor-associated macrophages. Clin Cancer Res 16:784–789
Hanahan D, Coussens LM (2012) Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 21:309–322
Filiopoulos V, Vlassopoulos D (2009) Inflammatory syndrome in chronic kidney disease: pathogenesis and influence on outcomes. Inflamm Allergy Drug Targets 8:369–382
Acknowledgments
The authors thank Noriko Hata for secretarial assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
About this article
Cite this article
Omae, K., Kondo, T., Kennoki, T. et al. Efficacy and safety of sorafenib for treatment of Japanese metastatic renal cell carcinoma patients undergoing hemodialysis. Int J Clin Oncol 21, 126–132 (2016). https://doi.org/10.1007/s10147-015-0871-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-015-0871-y