Abstract
Background
This survey sought to determine Japanese gynecologists’ attitudes concerning administering hormone replacement therapy (HRT) for patients after surgery for endometrial cancer (EC).
Methods
Eight hundred and eighty-eight members of the Japanese Gynecologic Oncology Group (JGOG) were asked to respond to an anonymous questionnaire on the JGOG website. The survey asked whether or not HRT was to be administered when surgery was performed (including a hysterectomy and bilateral oophorectomy) to treat EC before or after menopause. If HRT was not to be administered, respondents were asked the reason why. Respondents were presented with the same hypothetical patients that were featured in a previous survey in Germany, and differences in the mindsets of Japanese and German physicians were compared.
Results
Responses from 363 individuals (response rate 40.9 %) were analyzed. Seventy-eight percent of physicians considered HRT for patients undergoing surgery before menopause. The most prevalent reason of refusal to prescribe HRT was the risk of EC recurrence. Forty-eight percent of physicians considered HRT for patients undergoing surgery after menopause. The most prevalent reasons of refusal of HRT were its limited benefit and the availability of alternative therapies. Sixty-five percent of Japanese physicians responded that they would administer HRT to patients with low risk of recurrence vs. 46 % of physicians in Germany (P < 0.0002). Forty-nine percent of Japanese physicians approved of prescribing HRT for patients with high risk of recurrence vs. 25 % of physicians in Germany (P < 0.0001).
Conclusion
Many Japanese gynecologists have a favorable attitude toward prescribing HRT after treatment of EC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Endometrial cancer (EC) is the most prevalent gynecologic cancer in the US and Europe. Worldwide, 142,000 women develop that cancer each year and 42,000 women die from it [1]. In Japan, 3,722 women developed EC in 2003, but over an 8-year period, that number doubled to 7,273 in 2011 [2]. In Japan, more women currently develop EC than develop uterine cervical cancer, and EC is the most prevalent malignancy among gynecologic cancers, much as it is in the US and Europe. This cancer often develops after menopause, but it develops in 25 % of patients before menopause and in 2.5–14.4 % of younger patients under the age of 40 [2, 3].
A hysterectomy and bilateral salpingo-oophorectomy is a standard procedure for surgical treatment of EC. Thus, after surgery, a number of patients suffer symptoms of ovarian insufficiency due to surgical menopause. According to Japanese statistics from 2011, 74.4 % of ECs are clinical stage I–II [2]. These cancers are considered to have a relatively good prognosis, so quality of life (QOL) after the conclusion of treatment must be considered. However, a number of gynecologists may hesitate to administer hormone replacement therapy (HRT) in light of its effects on a patient’s underlying condition.
Estrogen is essential to caring for women’s health. The fact that HRT in particular is an appropriate and effective way to care for postmenopausal women is beyond doubt. HRT is effective at treating obvious conditions such as menopause and degenerative disorders such as dyslipidemia and osteoporosis. Over the past few years, HRT has also been reported to be effective in treating a host of other conditions, such as metabolic disorders (e.g., gout and metabolic syndrome), and counteracting aging [4]. In contrast, a long-standing problem with estrogen is the risk of cancer developing in tissues that depend on estrogen. Estrogen stimulation alone is known to be a risk factor for EC. Clearly, some gynecologists may be concerned about the risk of cancer recurring as a result of HRT being administered after treatment.
Recently, a series of clinical trials reported that HRT should be considered even after treatment of EC, since HRT does not increase recurrence [5–10]. The Gynecologic Oncology Group (GOG) conducted a randomized, double-blind, noninferiority trial involving 1,236 patients who underwent surgery for clinical stage I–II EC, and the trial indicated that estrogen did not increase the risk of recurrence [10]. Given this finding, Hancke et al. [11] surveyed gynecologists in Germany with regard to whether or not they administered HRT to patients with climacteric symptoms after surgery for EC. According to that survey, 45.6 % of physicians responded that estrogen replacement therapy (ERT) would be contraindicated for patients with a low risk of recurrence of EC and 75.4 % responded that ERT would be contraindicated for patients with a high risk of recurrence. Respondents indicated that they would tend to hesitate to prescribe estrogen for patients after surgery for EC.
In 2000, the Committee of Gynecologic Practice of the American Congress of Obstetricians and Gynecologists issued a vague statement that use of estrogen for women with a previous history of EC should be determined on an individual basis in light of its benefits and risks [12]. In 2011, French guidelines for treatment of EC went a step further [13]. For the first time, guidelines clearly specified that HRT is not contraindicated and that it can treat postoperative symptoms of ovarian insufficiency in women under the age of 50. Those guidelines also specified that HRT should be administered to healthy women age 50 and over in accordance with its indications and contraindications. In Japan, guidelines for treatment of EC were published in 2013 [14]. Those guidelines also approve of HRT after treatment of EC. In actuality, however, whether or not Japanese gynecologists favor administering HRT to symptomatic patients after treatment of EC is unclear. This study sought to determine Japanese gynecologists’ current attitudes with regard to prescribing estrogen for patients after surgery for EC. In addition, subjects were presented with the same hypothetical patients that were featured in a previous survey in Germany, and differences in the mindsets of Japanese physicians and German physicians were compared.
Methods
Eight hundred and eighty-eight members of the Japanese Gynecologic Oncology Group (JGOG) were notified that a survey on administering HRT to patients after treatment of EC was being conducted from September to October 2013 after the approval by the Committee of the JGOG. Respondents were asked to complete an anonymous questionnaire on the JGOG website and were asked not to refer to guidelines or texts in order to ascertain the realities of care and individual mindsets.
Questions asked about respondent characteristics and aspects of care (sex, age, specialty, type of facility where the respondent worked, whether or not the respondent followed up on patients after surgery for EC). When surgery was performed (including a hysterectomy and bilateral oophorectomy) to treat EC before menopause, respondents were asked
1. Was HRT considered?
2. If HRT was to be administered, then what were their criteria?
3. If HRT was to be administered, then how soon after surgery?
4. Up to what point would they administer HRT?
5. What type of HRT was prescribed and what route of administration?
6. If HRT was not to be administered, what was the reason?
In addition, the same six questions were asked regarding when surgery was performed (including a hysterectomy and bilateral oophorectomy) to treat EC after menopause.
A hypothetical patient with a low risk of EC and a hypothetical patient with a high risk of EC were featured in a survey by Hancke et al. [11] in which 165 physicians participated. In our study, the same hypothetical patients were presented to compare the mindsets of Japanese physicians and German physicians with regard to HRT. Respondents were asked: (1) if they would administer HRT to these patients, and (2) which therapy they would actually choose. The low-risk patient was a 41-year-old woman who had undergone surgery 2 years prior. The patient had grade 2 endometrioid adenocarcinoma that was stage IB according to the old International Federation of Gynecology and Obstetrics (FIGO) classification system. The patient experienced painful intercourse, decreased libido, and moderate hot flashes. The high-risk patient was a 38-year-old woman who had undergone surgery 6 months prior. The patient had grade 3 endometrioid adenocarcinoma that was stage IIIA (ascitic fluid cytology is positive) according to the old FIGO classification system, so the patient underwent postoperative adjuvant therapy. The patient had persistent severe hot flashes and a family history of osteoporosis.
Data obtained from the web questionnaires were entered into a JGOG database. Collected responses were analyzed using the Statistical Package for the Social Sciences (SPSS) Statistics 22. Differences in frequency were evaluated using binomial distribution test or χ2 test. A p value of <0.05 was considered to be statistically significant.
Results
Respondent characteristics and aspects of care
Responses from 363 individuals were analyzed (response rate 40.9 %). Eighty-seven percent of the respondents were men. Seventy percent of respondents were in their 40 or 50 s. No respondent was younger than 29 or older than 70. In terms of their specialty, 73 % were gynecologic oncologists, 4 % specialized in perinatal medicine and women’s health, 2 % were reproductive endocrinologists, and 17 % were general gynecologists. In terms of their working conditions, 50 % of worked in a university hospital/teaching hospital, 36 % in a general hospital, and 11 % in a cancer center. Ninety-six percent of respondents followed up after surgery for EC.
When surgery was performed (including hysterectomy and bilateral oophorectomy) to treat endometrial cancer before menopause
Combining the responses that HRT would be actively administered and would be administered if the patient was symptomatic, 78 % responded that they would consider prescribing HRT (Fig. 1a, P < 0.001). The most prevalent criteria for administering HRT were having or lacking a previous history of breast cancer, age, and severity of symptoms. Seventy-five percent of respondents would administer HRT as soon as the patient was symptomatic or after the conclusion of postoperative adjuvant therapy. These two responses indicate that respondents wished to administer HRT relatively early on. Eighty-three percent of respondents would administer HRT up to age 50 or within 5 years of surgery. Seventy-nine percent of physicians would choose an HRT regimen of estrogen alone. The route of its administration would be via a transdermal patch for 58 % of respondents or orally for 32 %; 90 % of respondents chose one of those two routes. In contrast, about 20 % of physicians responded that they would not administer HRT (Fig. 1a, P < 0.001), with the most prevalent reason being the risk of recurrence.
Whether or not hormone replacement therapy (HRT) was considered. a When surgery was performed before menopause, 78 % of physicians responded that they would consider prescribing HRT; 19 % responded that they would not. The difference was statistically significant (‡P < 0.001). b When surgery was performed after menopause, 48 % responded that they would consider prescribing HRT; 51 % would not. The difference was not statistically significant
When surgery was performed (including a hysterectomy and bilateral oophorectomy) to treat endometrial cancer after menopause
Forty-eight percent of physicians responded that they would consider prescribing HRT for postmenopausal patients with EC (Fig. 1b); 51 % responded that they would not (Fig. 1b). The most prevalent criteria for administering HRT were severity of symptoms and having or lacking a previous history of breast cancer. Seventy-eight percent of respondents would administer HRT as soon as the patient was symptomatic or after the conclusion of postoperative adjuvant therapy. These responses followed the same trend as for premenopausal patients. Fifty-eight percent of respondents would administer HRT within 5 years of surgery. Eighty-one percent would choose an HRT regimen of estrogen alone. The route of administration would be via a transdermal patch for 61 % of respondents or orally for 32; 90 % of respondents chose one of those two routes. Trends in responses were similar to those observed for premenopausal patients. The most prevalent reason for not administering HRT (given by 56 % of respondents) was that HRT offered no advantages, followed by the risk of recurrence and the fact that alternative treatments were adequate.
Comparison of mindsets of Japanese and German physicians with regard to HRT
Low-risk patient
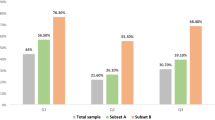
In Germany, 46 % of physicians agreed with the administration of HRT, but in Japan, 65 % of physicians responded that they would administer HRT (Fig. 2a, P < 0.0002). When Japanese respondents were asked which therapy they would actually choose for the patient, many preferred estrogen, whereas 23 % of those cited a traditional Japanese medicine in place of estrogen; 17 % would recommend a lubricating gel given a complaint of painful intercourse (Fig. 2b). Few physicians would prescribe isoflavone, a phytoestrogen (Fig. 2b). Very few Japanese physicians would seek a second opinion from a specialist regarding the suitability of HRT (Fig. 2b) and would determine the suitability of prescribing HRT themselves. In Germany, 63 % of physicians responded that they would prescribe topical estrogen given a complaint of painful intercourse due to vaginal atrophy [11], while in Japan, fewer than 14 % of physicians considered topical estrogen to be their therapy of choice (Fig. 2b). Many Japanese physicians would choose to use the oral or percutaneous route of administration. In Germany, frequent alternatives to estrogen were selective serotonin reuptake inhibitors (SSRIs) and isoflavone [11]. In Germany, 36 % of physicians would seek a second opinion from a specialist regarding the suitability of HRT [11].
Comparison of mindsets between Japanese and German physicians with regard to hormone replacement therapy (HRT) for patients with a low risk of endometrial cancer. a Respondents were asked if they would administer HRT to these patients: 48 % of German physicians agreed with the administration, whereas 65 % of Japanese physicians agreed; there was a significant difference in agreement (P < 0.0002). *German data were constructed on the basis of Hancke et al. [11]. b Responses of Japanese physicians regarding which therapy they would choose in the low-risk case
High-risk patient
In Germany, only 25 % of physicians agreed with the administration of HRT, while in Japan, 49 % of physicians responded that they would administer HRT (Fig. 3a, P < 0.0001). A look at the priority given to therapies indicated that most physicians (over half) would choose a traditional Japanese medicine (Fig. 3b), which would be the first choice for a high-risk patient, and would prescribe estrogen depending on how effective traditional medicine was. Few physicians would prescribe isoflavone (Fig. 3b), as was true for the low-risk patient, and few would choose to seek a second opinion (Fig. 3b). These findings suggested that respondents wished to determine the therapy themselves. In Germany, 74 % of physicians responded that they would prescribe isoflavone or SSRIs instead of estrogen [11]. In Germany, about half of the physicians wanted a second opinion [11].
Comparison of mindsets between Japanese and German physicians with regard to hormone replacement therapy (HRT) for patients with a high risk of endometrial cancer. a Respondents were asked if they would administer HRT to these patients: 25 % of German physicians agreed with the administration of HRT, whereas 49 % of Japanese physicians responded that they would administer HRT. There was a significant difference in agreement of administration of HRT between Japanese (49 %) and German (25 %) physicians (P < 0.0001). *German data were constructed on basis of Hancke et al. [11]. b Responses of Japanese physicians regarding which therapy they would actually choose in the high-risk case
Discussion
Based on this survey, Japanese gynecologists generally accept prescribing estrogen after surgery for EC. Respondents were members of the JGOG, so 70 % or more of the sample specialized in gynecologic oncology, though the sample also included physicians who routinely followed the course of patients after surgery for EC. The survey accurately reflected the attitudes of Japanese physicians with regard to HRT after cancer treatment.
Six studies primarily on clinical stage I–II EC examined postoperative HRT and the risk of that cancer recurring [5–10]. No studies reported that administration of HRT increased the rate of recurrence. In two studies, patients who received HRT had a significantly lower recurrence rate [5, 8]. The only prospective trial on HRT after surgery for clinical stage I–II EC was the GOG 137 randomized, double-blind, noninferiority trial [10]. That trial studied 618 patients who received estrogen therapy (ET) and 618 who received a placebo. Patients who received ET had a relative risk of recurrence of 1.27 [95 % confidence interval (CI) 0.916–1.77] compared with patients given the placebo; significant differences in the relative risk (RR) of recurrence were not noted. The GOG 137 trial planned to administer ET to a total sample of 2,108 patients for 3 years and then follow-up 5 years later, but the trial was halted when an interim report on the Women’s Health Initiative (WHI) study announced in 2002 that their study was being halted because estrogen and progestin therapy increased the risk of breast cancer [15]. The GOG 137 trial was halted at a median follow-up of 35.7 months but concluded that although this incomplete study cannot conclusively refute or support the safety of exogenous estrogen with regard to risk of endometrial recurrence, it is noteworthy that the absolute recurrence rate (2.1 %) and incidence of new malignancy were low [10]. Results of several retrospective studies have similarly indicated that HRT poses no risk whatsoever when administered after surgery for clinical stage I–II EC.
Previous clinical trials have several issues. One is that they lacked consistency with regard to doses, dosing, duration of administration, and whether or not progestin was also administered. Another is that there was no uniform period from the end of surgery to the start of HRT. In addition, numerous studies did not involve randomized controlled trials, so patients who wish to start HRT may be those who will benefit less from treatment. In other words, these women may be relatively healthier, reflecting the “healthy woman bias.” In the GOG 137 trial, 40 % of patients were menopausal women; 1 % was Asian, and 87 % had stage IA disease at this time, so there is little evidence of the effects of HRT in patients undergoing surgical menopause, patients with stage IB, those with more advanced disease, Asians, and patients who received postoperative chemotherapy. Thus, the suitability of HRT for Japanese survivors of EC who have undergone surgery and the risk of that cancer recurring must be determined. However, there is a concern that the physicians who administer HRT may hesitate to administer that therapy after treatment of EC. An in vitro experiment found that estrogen activated the estrogen receptors of EC cells and promoted cancer cell growth [16, 17]. If microscopic residual foci are present in the body, then administering estrogen may stimulate remaining cancer cells and lead to recurrence. However, our survey indicated that only 19 % of physicians were hesitant to prescribe HRT after surgery for EC, reflecting a fact that is evident from the results of previous clinical trials. About 60 % out of physicians who responded negatively to prescribing HRT said it was because of concerns about cancer recurring.
An interesting finding from the current survey is that German and Japanese gynecologists diverged in terms of their approval for prescribing HRT to patients with a low risk of EC and those with a high risk of EC. A German study surveyed 133 general gynecologists, 16 gynecologic oncologists, and 16 gynecologic reproductive endocrinologists in 2006 [11]. The study came soon after the recommendations from the WHI and it immediately followed publication of a paper on the GOG 137 trial, indicating that HRT did not increase the rate of recurrence of EC. The view that administering HRT after surgery for EC was contraindicated or hesitation to administer HRT was presumably firmly entrenched among German gynecologists. Cognizant of that fact, Hancke et al. [11] discussed how the therapy of choice at the time was an SSRI or phytoestrogen since evidence indicated that those substances were effective at alleviating climacteric symptoms such as hot flashes. Nevertheless, the current study was conducted in 2013, prior to the publication of Japanese guidelines indicating that HRT can be considered after treatment of EC. Even after allowing for the fact that most of the current respondents were gynecologic oncologists, results suggested that approval for HRT after treatment of EC is gaining ground in Japan. Since a case presented as high-risk EC is not a stage IIIA in a new FIGO classification system, interpretation of the result may require caution.
In a Belgian survey, 67 % of physicians responded that they would prescribe HRT for survivors of early EC with climacteric symptoms [18]. In Greece, 30.4 % of physicians responded that they would probably prescribe HRT to survivors of EC, and 69.6 % indicated that HRT would be contraindicated because of the risk of recurrence [19]. In South Korea, 82.7 % of physicians responded that they had previously administered HRT to survivors of EC; 61.9 % would prescribe tibolone, an estrogen alternative, while 18.5 % would only prescribe estrogen [20]. In contrast, about 80 % of Japanese gynecologists would prescribe estrogen as the therapy of choice for women who have undergone surgery for EC before menopause (sugical menopause). The response by 80 % of Japanese gynecologists that they would prescribe HRT is a conspicuously high percentage in comparison with previous studies, even if one takes into account differences in the year when the study was conducted and differences in study samples.
The fact that surgical menopause adversely impacts women’s health is clear. In a cohort study of patients who underwent a prophylactic oophorectomy, Rocca et al. [21] reported that removal of the ovaries of women under age 45 resulted in a significantly reduced survival rate. They also reported that patients who had not yet received estrogen in particular had a high mortality rate [21]. In addition, surgical menopause is a risk factor for dyslipidemia and cardiovascular disease [22, 23]. In terms of bone turnover, bone mass is known to decrease by as much as 6.7 % a year after an oophorectomy [22]. A hysterectomy and bilateral oophorectomy is one of the standard procedures for treatment of EC. The incidence of EC in premenopausal women is increasing. Given this fact, whether or not postoperative HRT increases the risk of recurrence needs to be determined once again. Prospective trials also need to observe whether HRT offers an advantage in terms of QOL, bone mass, lipid profile, blood pressure, and climacteric symptoms over the long term.
There were 363 responses to the current survey, and most were from gynecologic oncologists. Thus, one could probably contend that this survey reflects the attitudes of only some of Japan’s gynecologists. Nevertheless, gynecologic oncologists are responsible for the long-term management of the health of female cancer survivors after treatment, so a survey of their attitudes is important. Results of this survey revealed that many Japanese gynecologists (primarily gynecologic oncologists) had a favorable attitude toward HRT after the conclusion of treatment for EC. Results also revealed that those gynecologists were willing to prescribe HRT for a prolonged period relatively early on. These findings suggest that there is a basis for designing a phase III trial in Japan to examine the risks and benefits of HRT after surgery for EC. In addition, because it is not clear in this survey that to what extent HRT is remediable for EC survivors with high-risk factors, a prospective trial incorporating an early stopping rule or defining high-risk factors may be necessary.
References
Amant F, Moerman P, Neven P et al (2005) Endometrial cancer. Lancet 366(9484):491–505
Aoki D (2014) Annual report of Gynecologic Oncology Committee, Japan Society of Obstetrics and Gynecology, 2013. J Obstet Gynaecol Res 40(2):338–348
Decruze SB, Green JA (2007) Hormone therapy in advanced and recurrent endometrial cancer: a systematic review. Int J Gynecol Cancer 17(5):964–978
Kilic S, Yilmaz N, Erdogan G et al (2010) Effect of non-oral estrogen on risk markers for metabolic syndrome in early surgically menopausal women. Climacteric 13(1):55–62
Creasman WT, Henderson D, Hinshaw W et al (1986) Estrogen replacement therapy in the patient treated for endometrial cancer. Obstet Gynecol 67(3):326–330
Lee RB, Burke TW, Park RC (1990) Estrogen replacement therapy following treatment for stage I endometrial carcinoma. Gynecol Oncol 36(2):189–191
Chapman JA, DiSaia PJ, Osann K et al (1996) Estrogen replacement in surgical stage I and II endometrial cancer survivors. Am J Obstet Gynecol 175(5):1195–1200
Suriano KA, McHale M, McLaren CE et al (2001) Estrogen replacement therapy in endometrial cancer patients: a matched control study. Obstet Gynecol 97(4):555–560
Ayhan A, Taskiran C, Simsek S et al (2006) Does immediate hormone replacement therapy affect the oncologic outcome in endometrial cancer survivors? Int J Gynecol Cancer 16(2):805–808
Barakat RR, Bundy BN, Spirtos NM et al (2006) Randomized double-blind trial of estrogen replacement therapy versus placebo in stage I or II endometrial cancer: a Gynecologic Oncology Group Study. J Clin Oncol 24(4):587–592
Hancke K, Foeldi M, Zahradnik HP et al (2010) Estrogen replacement therapy after endometrial cancer: a survey of physicians’ prescribing practice. Climacteric 13(3):271–277
Committee on Gynecologic Practice, The American College of Obstetricians and Gynecologists (2001) ACOG committee opinion: tamoxifen and endometrial cancer. Int J Gynecol Obstet 73(1):77–79
Querleu D, Planchamp F, Narducci F, Institut National du Cancer; Societe Francaise d’Oncologie Gynecologique et al (2011) Clinical practice guidelines for the management of patients with endometrial cancer in France: recommendations of the Institut National du Cancer and the Société Française d’Oncologie Gynécologique. Int J Gynecol Cancer 21(5):945–950
Japan Society of Gynecologic Oncology (2013) Endometrial cancer treatment guidelines. Kanehara & CO., LTD
Rossouw JE, Anderson GL, Prentice RL, Writing Group for the Women’s Health Initiative Investigators et al (2002) Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA 288(3):321–333
Holinka CF, Hata H, Gravanis A et al (1986) Effects of estradiol on proliferation of endometrial adenocarcinoma cells (Ishikawa line). J Steroid Biochem 25(58):781–786
Farnell YZ, Ing NH (2003) The effects of estradiol and selective estrogen receptor modulators on gene expression and messenger RNA stability in immortalized sheep endometrial stromal cells and human endometrial adenocarcinoma cells. J Steroid Biochem Mol Biol 84(4):453–461
Rozenberg S, Vasquez JB (2000) Estrogen replacement therapy in patients with endometrial cancer: prescription attitude of Belgian gynecologists. Maturitas 35(2):125–128
Vavilis D, Tsolakidis D, Goulis DG et al (2011) Hormone therapy for postmenopausal endometrial cancer survivors: a survey among Greek obstetricians-gynaecologists. Eur J Gynaecol Oncol 32(1):81–83
Lee SJ, Yeo SG, Kang SB et al (2013) Attitudes and practices of Korean gynecologists towards hormone replacement therapy in endometrial cancer survivors. Eur J Gynaecol Oncol 34(6):513–517
Rocca WA, Grossardt BR, de Andrade M et al (2006) Survival patterns after oophorectomy in premenopausal women: a population-based cohort study. Lancet Oncol 7(10):821–828
Yoshida T, Takahashi K, Yamatani H et al (2011) Impact of surgical menopause on lipid and bone metabolism. Climacteric 14(4):445–452
Atsma F, Bartelink ML, Grobbee DE et al (2006) Postmenopausal status and early menopause as independent risk factors for cardiovascular disease: a meta-analysis. Menopause 13(2):265–279
Acknowledgments
We thank all members who participated in this JGOG survey. We also thank Mr. Shigeru Honda and Ms. Aya Noguchi of the JGOG secretariat for their kind assistance.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Consortia
Corresponding author
About this article
Cite this article
Yokoyama, Y., Ito, K., Takamatsu, K. et al. How do Japanese gynecologists view hormone replacement therapy for survivors of endometrial cancer? Japanese Gynecologic Oncology Group (JGOG) survey. Int J Clin Oncol 20, 997–1004 (2015). https://doi.org/10.1007/s10147-015-0808-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-015-0808-5