Abstract
Background
Patients with urinary bladder urothelial carcinoma (UC) with variant histology have features of more advanced disease and a likelihood of poorer survival than those with pure UC. We investigated the impact of variant histology on disease aggressiveness and clinical outcome after radical nephroureterectomy (RNU) in Japanese patients with upper tract UC (UTUC). Information on variant histology might guide appropriate patient selection for adjuvant therapy after RNU.
Methods
We enrolled 502 UTUC patients treated with RNU in this retrospective cohort study, and analyzed associations of variant histology with clinicopathological variables and disease-specific survival.
Results
The median follow-up was 41.4 months. A total of 60 (12.0 %) UTUC patients had variant histology. UTUC with variant histology was significantly associated with advanced pathological T stage (pT ≥ 3), higher tumor grade (G3), and more lymphovascular invasion (P < 0.0001). Variant histology in all patients was significantly associated with worse disease-specific survival after RNU on univariate analysis (P = 0.0004), but this effect did not remain significant on multivariate analysis. However, variant histology was a significantly independent predictor for disease-specific survival in patients with pT ≥ 3 tumors (P = 0.0095).
Conclusions
UTUC with variant histology might be a phenotype of high-grade, locally aggressive advanced tumors rather than of systemic disease. Variant histology may be useful for selection of patients with pT ≥ 3 UTUC for adjuvant therapy. Prospective studies in a larger number of patients with a centralized pathological review are needed to confirm our results.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Upper tract urothelial carcinoma (UTUC) is a rare but potentially lethal disease, accounting for ~5 % of urothelial malignancies and <10 % of renal tumors [1]. In patients with a normal contralateral kidney, radical nephroureterectomy (RNU) is the gold standard treatment for UTUC [1–3]. After RNU in clinically non-metastatic disease, a significant proportion of patients experienced disease recurrence and subsequently died from metastatic UTUC [2–4]. Pathological T (pT) stage, tumor grade, lymph node involvement, and lymphovascular invasion (LVI) are thought to be prognostic factors for UTUC [5]. This information could help select appropriate patients for additional therapeutic modalities, such as adjuvant chemotherapy.
Urinary bladder UC patients with variant histology have features of more advanced disease and likelihood of poorer survival than those with pure UC [6–8]. Upper urinary tract (UUT) carcinomas are histologically similar to bladder carcinomas and the majority of UUT carcinomas are also UC; some of them include variant histology, like bladder carcinomas. The conclusions of studies on UTUC with variant histology have been limited by small sample sizes and types of analyses [9, 10]. However, Rink et al. [11] recently reported in a large international cohort of >1,600 patients that ~25 % of patients with UTUC treated with RNU harbored histological variants, and that variant histology was associated with features of biologically aggressive UTUC. On the other hand, major differences have been reported in the clinicopathological characteristics of UTUC between Caucasian and Japanese patients [12].
In the present study, we therefore investigated the impact of variant histology on disease aggressiveness and outcome after RNU specifically in Japanese patients with UTUC. Information on variant histology might guide selection of Japanese patients with UTUC for adjuvant therapy after RNU, as well as for counseling and inclusion in relevant clinical trials.
Patients and methods
Patients
Twenty-one hospitals in or near Yamaguchi Prefecture, Japan, participated in this multicenter, retrospective cohort study, and the investigators in each hospital reviewed original medical records to collect the data. The study included consecutive patients with UUT carcinoma who underwent RNU between 1995 and 2009. RNU was performed via an open or laparoscopic approach. Hilar or regional lymphadenectomy was performed in patients with suspicious lymph nodes on preoperative imaging or with suspicious intraoperative findings. Lymphadenectomy extent was at the discretion of the surgeon. We excluded patients with distant metastasis or unresectable disease; prior or concurrent contralateral UUT carcinoma; neoadjuvant chemotherapy or radiotherapy; and those with <3 months follow-up. After exclusion, 510 patients were enrolled in the study. Eight (1.6 %) of the patients had pure non-UCs that were excluded, comprising four squamous cell carcinomas, three adenocarcinomas, and one carcinosarcoma. Finally, 502 UTUC patients were included in the analysis. Adjuvant chemotherapy and radiotherapy regimens were administered to 164 (33.3 %) and 48 (9.7 %) patients, respectively. Patients were followed postoperatively with cystoscopy and urine cytology every 3 months for 2 years and every 6 months thereafter. We also performed computed tomography scans every 6 months for 5 years and annually thereafter.
Clinicopathological data
The clinicopathological data comprised patient age at RNU; sex; Eastern Cooperative Oncology Group performance status (ECOG-PS); previous bladder cancer; tumor location (renal pelvis, ureter, or both); number of tumors (single or multiple); pT stage (<3 or ≥3); pathological tumor grade (G1/2 or G3); histological UC variants; concomitant carcinoma in situ (CIS); and LVI.
All surgical specimens were processed according to standard pathological procedures at each institution and pathologists examined all specimens according to standardized criteria [11]. Multiple tumors were defined as the synchronous presence of ≥2 pathologically confirmed tumors at any site. Tumors were staged according to the Tumor–Node–Metastasis classification of the American Joint Committee on Cancer (7th ed., 2010). The tumor grade was classified according to the World Health Organization system (1973) [13]. LVI was defined as the intraluminal presence of tumor cells within the endothelial linings of either vascular channels or lymphatic channels [5, 14]. The clinicopathological characteristics of the patients are summarized in Table 1.
Statistical analysis
Associations of histological variants with clinicopathological variables were assessed using the χ2 or Fisher exact test. The primary endpoint of this study was disease-specific survival defined as the time from the date of RNU to the date of death from UTUC or last follow-up. Disease-specific survival was analyzed by plotting Kaplan–Meier curves and the survival probability distributions were compared using the log-rank test. Categorical variables influencing disease-specific survival were compared using Cox proportional hazards regression models. Variables with P < 0.05 in univariate analysis were also assessed for their relationship with disease-specific survival in multivariate analysis. All data were analyzed using JMP software (SAS Institute, Cary, NC, USA), with P < 0.05 (two-sided) indicating statistical significance.
Results
The median patient age at RNU was 72 years (range 32–93 years), and the median follow-up was 41.4 months (range 3–200 months). During the follow-up period, 102 (20.3 %) of the 502 patients died of UTUC. Five- and 10-year disease-specific survival rates in all patients were 77.1 % and 68.5 %, respectively. Of the 502 UTUC patients, 442 (88.0 %) had pure UC and 60 (12.0 %) had UC with variant histology. Squamous cell differentiation was the most common histological variant (45 patients), followed by glandular differentiation (10 patients), sarcomatoid differentiation (2 patients), and multiple differentiation (3 patients). UTUC with variant histology was significantly associated with advanced pT stage (≥3), higher tumor grade (G3), and more LVI (P < 0.0001; Table 1). The patients with UTUC histological variants were more likely to receive adjuvant chemotherapy (P = 0.0001).
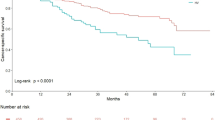
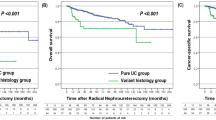
On univariate Cox proportional hazards regression analysis in all UTUC patients, number of tumors, pT stage, pathological grade, variant histology, LVI, adjuvant chemotherapy, and adjuvant radiotherapy had a significant influence on disease-specific survival (P < 0.05; Table 2). Disease-specific survival was plotted for UTUC patients with variant histology compared with those with pure UC using Kaplan–Meier survival curves (Fig. 1). The variant histology was associated with worse disease-specific survival (P < 0.0001). Multivariate analysis revealed that pT stage [hazard ratio (HR): 2.57; 95 % confidence interval (CI): 1.43–4.71; P = 0.0015], pathological tumor grade (HR: 2.16; 95 % CI: 1.29–3.70; P = 0.0032), and LVI (HR: 3.14; 95 % CI: 1.81–5.57; P < 0.0001) were independent predictors for disease-specific survival (Table 2). The variant UTUC histology was not an independent predictor of disease-specific survival on multivariate analysis adjusted for the effects of standard pathological characteristics.
In patients with stage pT ≥ 3 UTUC, univariate Cox proportional hazards regression analysis revealed that pathological tumor grade, variant histology, concomitant CIS, and LVI had a significant influence on disease-specific survival (P < 0.05; Table 3). Disease-specific survival was plotted for pT ≥ 3 patients with variant histology compared with those with pure UC using Kaplan–Meier survival curves (Fig. 2). Variant histology was associated with worse disease-specific survival (P = 0.0015). On multivariate analysis in patients with stage pT ≥ 3 tumors, pathological grade (HR: 2.38; 95 % CI: 1.26–4.84; P = 0.0066), variant histology (HR: 2.16; 95 % CI: 1.21–3.71; P = 0.0095), and LVI (HR: 2.11; 95 % CI: 1.13–4.19; P = 0.017) were independent predictors for disease-specific survival (Table 3).
Discussion
Black et al. [6] focused on the implications of aberrant differentiation on the management of patients with UC of the bladder. They concluded that variant histology in a bladder tumor increased the likelihood of locally advanced disease and metastasis, and that there was an increased risk of clinical under-staging and occult metastatic disease. In a study of 1,013 bladder UC patients who underwent radical cystectomy, patients with squamous and/or glandular differentiation were more likely to have extra vesical tumors and node-positive disease at cystectomy [15]. However, there was no significant association of mixed histological features with local tumor recurrence or the risk of death from bladder cancer after controlling for clinicopathological disease features. A mixed histological component may be a phenotype of dedifferentiated high-grade, locally aggressive advanced tumors rather than of systemic disease [15]. Concerning a Japanese retrospective study, a concomitant squamous cell carcinoma component in the specimen was an independent predictor of local recurrence in muscle-invasive bladder cancer patients treated with radical cystectomy [8].
In the current study, Japanese UTUC patients with variant histology harbored features of biologically aggressive disease, such as advanced tumor stage, higher grade, and LVI, similar to previous studies [9–11], as well as in bladder UC [6, 7, 15]. Additionally, the patients with variant UTUC histology were at higher risk for disease-specific mortality after RNU than were patients with pure UTUC. However, after controlling for the effects of standard clinical and pathological features, variant histology was not an independent predictor of clinical outcome after RNU in all patients. These findings are in accordance with a previous study [11]. UTUC with histological variants might be a phenotype of high-grade, locally aggressive advanced tumors rather than of systemic disease.
Our study showed that variant histology is a significant predictor for disease-specific survival in UTUC patients with pT ≥ 3 tumors, independent of pathological grade and LVI. Patients with pT ≥ 3 vary in their prognosis after RNU, and therefore it is meaningful to predict their outcome [14]. However, analysis results of the predictive value of variant histology in the subgroup of patients with pT ≥ 3 tumors are controversial because the power of the analysis was limited by the number of patients and heterogeneity of this subgroup (i.e., some variant histological subtypes were analyzed altogether). We hypothesize that patients with deeply invasive (pT ≥ 3) UTUC with variant histology are more likely to have local recurrence after RNU than are those without variant histology. Therefore, variant histology might be an independent predictor of survival in patients with pT ≥ 3 tumors. Jenkins et al. [16] revealed that the presence of squamous differentiation predicted a greater extent of extravesical extension in a study of patients with muscle-invasive bladder cancer who had undergone radical cystectomy. Additionally, Honma et al. [8] reported that a concomitant squamous cell carcinoma component was an independent predictor of local recurrence in Japanese patients with muscle-invasive bladder cancer after radical cystectomy, as described above. Conversely, LVI in UTUC might be associated with distant lymphogenous or hematogenous metastasis. In the current study, however, we could not analyze the exact association between variant histology and local recurrence because of incomplete data on local recurrence or distant metastasis in each patient and the relatively short follow-up period.
Concerning bladder cancer, although squamous cell carcinoma is no more radiosensitive than UC, it is less chemosensitive, and thus there is no good alternative to adjuvant or neoadjuvant therapy [6]. This feature, in combination with the fact that most patients die of local progression, has led some centers to recommend neoadjuvant or adjuvant radiotherapy for squamous cell carcinoma. For UTUC, adjuvant radiotherapy may improve local control of the disease [1]. When given in combination with cisplatinum, it may result in longer disease-free and overall survival [17]. Thus, adjuvant chemoradiotherapy after RNU might improve outcome in patients with locally advanced UTUC with variant histology.
Our study had several limitations. This was a retrospective study with a limited number of patients and follow-up period, and the patients in this multicenter cohort study underwent RNU by several surgeons over a long time period. Additionally, we did not re-review all specimens but rather relied on the pathologist to identify and report variant histology according to standard pathological procedures at each institution [11]. The frequency with which variant histology is diagnosed will depend on how extensively the tumor is pathologically sampled and how attentive the pathologist is to detecting small foci [6]. Furthermore, we did not perform a centralized pathological review, which could have led to misinterpretations of pathological specimens and under-reporting [18]. Finally, although the population of UTUC with variant histology was a very heterogeneous population that included squamous, glandular, and sarcomatoid differentiation, we analyzed this heterogeneous population as one group.
In conclusion, the Japanese UTUC patients with variant histology harbored features of biologically aggressive disease. Although variant histology in all patients was associated with worse disease-specific survival after RNU on univariate analysis, this effect did not remain significant on multivariate analysis. UTUC with histological variants might be a phenotype of high-grade, locally aggressive advanced tumors rather than of systemic disease. However, variant histology was an independent predictor for disease-specific survival in patients with pT ≥ 3 tumors. Variant histology may help select patients with pT ≥ 3 UTUC for adjuvant therapy.
References
Roupret M, Zigeuner R, Palou J et al (2011) European guidelines for the diagnosis and management of upper urinary tract urothelial cell carcinomas: 2011 update. Eur Urol 59:584–594
Zigeuner R, Pummer K (2008) Urothelial carcinoma of the upper urinary tract: surgical approach and prognostic factors. Eur Urol 53:720–731
Margulis V, Shariat SF, Matin SF et al (2009) Outcomes of radical nephroureterectomy: a series from the upper tract urothelial carcinoma collaboration. Cancer 115:1224–1233
Sakano S, Matsuyama H, Kamiryo Y et al (2013) Risk group stratification based on preoperative factors to predict survival after nephroureterectomy in patients with upper urinary tract urothelial carcinoma. Ann Surg Oncol 20:4389–4396
Kikuchi E, Margulis V, Karakiewicz PI et al (2009) Lymphovascular invasion predicts clinical outcomes in patients with node-negative upper tract urothelial carcinoma. J Clin Oncol 27:612–618
Black PC, Brown GA, Dinney CP (2009) The impact of variant histology on the outcome of bladder cancer treated with curative intent. Urol Oncol 27:3–7
Pons F, Orsola A, Morote J et al (2011) Variant forms of bladder cancer: basic considerations on treatment approaches. Curr Oncol Rep 13:216–221
Honma I, Masumori N, Sato E et al (2004) Local recurrence after radical cystectomy for invasive bladder cancer: an analysis of predictive factors. Urology 64:744–748
Perez-Montiel D, Wakely PE, Hes O et al (2006) High-grade urothelial carcinoma of the renal pelvis: clinicopathologic study of 108 cases with emphasis on unusual morphologic variants. Mod Pathol 19:494–503
Holmang S, Lele SM, Johansson SL (2007) Squamous cell carcinoma of the renal pelvis and ureter: incidence, symptoms, treatment and outcome. J Urol 178:51–56
Rink M, Robinson BD, Green DA et al (2012) Impact of histological variants on clinical outcomes of patients with upper urinary tract urothelial carcinoma. J Urol 188:398–404
Matsumoto K, Novara G, Gupta A et al (2011) Racial differences in the outcome of patients with urothelial carcinoma of the upper urinary tract: an international study. BJU Int 108:E304–E309
Mostofi FK, Sorbin LH, Torloni H (1973) Histological typing of urinary bladder tumours. International classification of tumours, vol 19. World Health Organization, Geneva
Akao J, Matsuyama H, Yamamoto Y et al (2008) Clinical significance of lymphovascular invasion in upper urinary tract urothelial cancer. BJU Int 102:572–575
Kim SP, Frank I, Cheville JC et al (2012) The impact of squamous and glandular differentiation on survival after radical cystectomy for urothelial carcinoma. J Urol 188:405–409
Jenkins P, Anjarwalla S, Gilbert H et al (2009) Defining the clinical target volume for bladder cancer radiotherapy treatment planning. Int J Radiat Oncol Biol Phys 75:1379–1384
Czito B, Zietman A, Kaufman D et al (2004) Adjuvant radiotherapy with and without concurrent chemotherapy for locally advanced transitional cell carcinoma of the renal pelvis and ureter. J Urol 172:1271–1275
Chromecki TF, Cha EK, Fajkovic H et al (2012) The impact of tumor multifocality on outcomes in patients treated with radical nephroureterectomy. Eur Urol 61:245–253
Acknowledgments
This study was supported, in part, by a Grant-in-Aid for Scientific Research (C) (24592393) from the Japan Society for the Promotion of Science. The authors wish to thank the members of the Yamaguchi Uro-Oncology Group for important contributions to this study.
Conflict of interest
No author has any conflict of interest.
Author information
Authors and Affiliations
Consortia
Corresponding author
About this article
Cite this article
Sakano, S., Matsuyama, H., Kamiryo, Y. et al. Impact of variant histology on disease aggressiveness and outcome after nephroureterectomy in Japanese patients with upper tract urothelial carcinoma. Int J Clin Oncol 20, 362–368 (2015). https://doi.org/10.1007/s10147-014-0721-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-014-0721-3