Abstract
Background
We evaluated the association between subclinical interstitial lung disease (ILD) and fatal radiation pneumonitis (RP) in patients with thoracic tumors treated with thoracic radiotherapy (RT).
Methods
Sixty-two consecutive patients with thoracic tumors treated with thoracic RT were retrospectively analyzed. According to our protocols, patients with subclinical ILD (untreated and asymptomatic) were considered to be indicated for thoracic RT, while patients with clinical ILD (post- or during treatment) were not considered candidates for thoracic RT. The presence, extent and distribution of subclinical ILD on CT findings at pre-thoracic RT were reviewed and scored by two chest radiologists. The relationships between RP and clinical factors, including subclinical ILD, were investigated.
Results
Subclinical ILD was recognized in 11 (18 %) of the 62 patients. Grade 2–5 RP was recognized in eight (13 %) of the 62 patients, with Grade 5 in three patients and Grade 2 in five patients. Grade 2–5 RP was observed in four (36 %) of the 11 patients with subclinical ILD. Subclinical ILD was found to be a significant factor influencing the development of Grade 2–5 RP (p = 0.0274). Subclinical ILD tended to be significant for the occurrence of Grade 5 RP (p = 0.0785). Regarding the CT score, more extensive ILD (bilateral fibrosis in multiple lobes) was recognized in two of the three patients with Grade 5 RP.
Conclusions
In this study, fatal RP tended to be more common in the patients with subclinical ILD. In particular, the presence of extensive fibrosis on CT may be a contraindication for thoracic RT.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Radiation pneumonitis (RP) is one of the most common toxicities in patients with thoracic malignancies treated with radiation therapy (RT). The risk for RP appears to be related to the total dose, fractionation schedule and irradiated lung volume [1, 2]. Moreover, various clinical factors (e.g., age, smoking history, performance status) and treatment-related factors (e.g., chemotherapy regimen and schedule) have been proposed, as well as the radiation dosimetric factors [2–6].
A group of non-infectious, acute or chronic, diffuse parenchymal lung disorders are classified as interstitial lung disease (ILD). More than 150 clinical conditions and/or causes are associated with ILD [7]. The COPDGene Study group previously demonstrated both chest computed tomography (CT) and pathological evidence of subclinical ILD in asymptomatic subjects, and of the 2416 screening high-resolution CT scans for smokers evaluated, 194 (8 %) showed interstitial lung abnormalities. Idiopathic pulmonary fibrosis (IPF), which is the most common form of ILD, is found significantly more often in lung cancer patients than in the general population [8–10]. IPF was recognized in 7.5 % of lung cancer patients treated with surgical resection [11]. Recently, Hubbard et al. reported that IPF is a risk factor for lung carcinogenesis [10]. IPF is characterized as a slowly progressive respiratory insufficiency. However, an acute exacerbation of ILD often results in respiratory failure and death, with new lung opacities and pathological lesions of diffuse alveolar damage. Acute exacerbation of ILD was first proposed in Japan, and has now been recognized globally, although racial differences in the frequency of acute exacerbation were assumed between Mongolians (including Japanese) and whites [12, 13]. Minegishi et al. [14] reported that the incidence of acute exacerbation related to chemotherapy was recognized in 20 % of the patients with both lung cancer and idiopathic interstitial pneumonias. Similarly, acute exacerbation of subclinical ILD triggered by surgery in patients with lung tumors has been demonstrated [15].
There is no current consensus on whether thoracic RT in patients with subclinical ILD is safe in view of the risk of RP, including acute exacerbations of ILD. To our knowledge, there have been only a few case series evaluating thoracic RT in patients with subclinical ILD [16, 17]. Beginning in May 2006, at our institution, the use of thoracic RT to treat patients with thoracic tumors was initiated according to our own protocols. Patients with subclinical (untreated and oxygen-free) ILD were treated with thoracic RT, while those with clinical ILD were not. The purpose of the present study was to evaluate the rate of occurrence of fatal RP and grade of RP after thoracic RT in patients with subclinical ILD and to investigate whether subclinical ILD is a predictor of RP.
Materials and methods
Patients
From May 2006 to May 2012, 93 consecutive patients with thoracic tumors were treated by thoracic RT at our institution. Patients followed up for <3 months were excluded from this study. Patients with RP were included in the study, even those with a follow-up period of <3 months. Consequently, the 13 excluded patients with a shorter follow-up period did not have RP. Eighteen patients treated with palliative RT were also excluded. Therefore, a total of 62 patients were retrospectively analyzed. According to our protocols for thoracic RT during the same period, patients with subclinical ILD, which was defined as the presence of untreated and asymptomatic ILD on CT, were considered to be indicated for thoracic RT. However, thoracic RT was not performed in patients with clinical ILD, which was defined as a status of symptomatic disease post- or during treatment. The pretreatment evaluation included a complete history, physical examination, complete blood cell count, body CT scans, and, in some cases, 18F-FDG positron emission tomography/CT and/or magnetic resonance imaging and/or bone scintigraphy were also used. Written informed consent for radiation therapy was obtained from all patients. This retrospective study was approved by the Institutional Review Board of our institution.
The characteristics of the patients are shown in Table 1. The tumor/node/metastasis (TNM) stages (International Union Against Cancer TNM classification, 6th edition) were evaluated. A total of 40 patients had lung cancer and 19 patients had esophageal cancer. The remaining cases included two patients with thymic cancer (T1N0M0 in one patient and T2N0M0 in one patient) and one patient with cancer of an unknown primary site.
Although there was no specific chemotherapy protocol, 45 (73 %) of the 62 patients underwent chemotherapy. Thirteen patients received chemotherapy before thoracic RT. Twenty-four patients underwent concurrent chemotherapy during thoracic RT: 5-fluorouracil was used in combination with cisplatin in nine patients, vinorelbine in combination with cisplatin in five patients, paclitaxel in combination with carboplatin in three patients, etoposide in combination with cisplatin in two patients, oral S-1 in two patients, 5-fluorouracil in combination with docetaxel in one patient, vinorelbine in one patient and docetaxel in one patient. Thirty-one patients received adjuvant chemotherapy after thoracic RT.
Radiotherapy
All treated patients underwent CT simulation and were treated with 4 and 10 MV. The simulation CT images were taken in 5-mm increments over the region of interest. Three-dimensional conformal RT was planned using the Xio (CMS Japan, Tokyo, Japan) treatment planning system. The median total dose was 60 Gy (range 32–72 Gy). The daily dose was 1.8–3.0 Gy (median 2.0 Gy): 2.0 Gy in 56 patients, 1.8 Gy in four patients, 2.4 Gy in one patient and 3.0 Gy in one patient. Patients treated with stereotactic body RT were not included in this study.
During typical definitive thoracic RT, the gross tumor volume (GTV) was defined as the volume of a primary tumor demonstrated by a CT scan, as well as metastatic lymph nodes that measured ≥1.0 cm in the short axis. The clinical target volume (CTV) for the primary tumor was created to add a 0.5–1.0-cm margin to the GTV and to include elective regional lymph nodes. The planning target volume (PTV) was defined by adding margins at the discretion of the radiation oncologists (typically 0.5–1.0 cm for lateral margins and 1.0–2.0 cm for cranio-caudal margins, depending on the respiratory motion and patient fixation). The lungs were considered together as a single paired organ. Lung contours were obtained automatically by the CT threshold, the trachea and bronchi were excluded manually, and the GTV within the lung was subtracted automatically.
Regarding the 59 patients with esophageal cancer or lung cancer, a typical RT field consisted of two opposing fields for the administration of 40 Gy in 20 fractions (1.8–2.0 Gy per fraction, five fractions per week), followed by the use of off-cord oblique fields in 26 patients or multiple (3–7) fields in the remaining 33 patients as a boost of 20–30 Gy to the GTV (or CTV of the tumor bed in postoperative cases). The contralateral hilum was excluded from the initial two opposing fields. However, the contralateral hilum received a low dose of <20 Gy, as the multiple (3–7) field technique was selected as a boost to the GTV, after the use of two opposing fields, in order to reduce the heart dose. Each dose was delivered to an isocenter. Dose calculation was performed using a superposition algorithm. The treatment was delivered using a Toshiba PRIMUS linear accelerator equipped with standard multileaf collimators. The dose–volume histogram (DVH) of the lungs was analyzed for each patient, and V5, V10, V15, V20 and V25 were obtained as the percentages of the pulmonary volume irradiated exceeding 5, 10, 15, 20 and 25 Gy, respectively. The mean lung dose (MLD) was calculated at the same time. Complete DVH data were available for 44 patients treated between July 2007 and May 2012. The DVH data in the remaining 18 patients treated between May 2006 and July 2007 were not available due to incomplete dosimetric data on the old treatment planning systems, which could not be accessed using the current computer planning systems.
Diagnoses of subclinical ILD
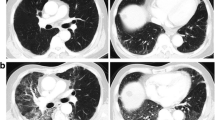
The presence, extent and distribution of the CT criteria for ILD were determined on the basis of a previous study [18, 19]; pre-thoracic RT CT findings were reviewed by two chest radiologists as no evidence of ILD (score 0), slight ILD (score 1), mild ILD (score 2) or moderate ILD (score 3) (Fig. 1). Slight ILD was defined as focal or unilateral ground-glass attenuation, focal or unilateral reticulation and patchy ground-glass abnormalities (<5 % of the lung). Mild ILD was defined as follows: non-dependent ground-glass abnormality affecting more than 5 % of any lung zone, non-dependent reticular abnormality, diffuse centrilobular nodularity with ground-glass abnormality, honeycombing, traction bronchiectasis, non-emphysematous cysts and architectural distortion. Moderate ILD was defined as bilateral fibrosis in multiple lobes associated with honeycombing and traction bronchiectasis with a sub-pleural distribution.
Typical CT images used to determine the ILD score. Score 1 predominant peripheral reticular abnormalities with a small amount of ground-glass opacity without honeycombing (a). Score 2 predominant peripheral reticular abnormalities with both ground glass opacity and a small amount of honeycombing (b). Score 3 predominant peripheral and basal reticular abnormalities. Including traction bronchiectasis, the CT findings of a score of 3 are more remarkable than those of a score of 2 (c)
Follow-up
All patients were monitored for RP on an outpatient basis with chest X-ray examinations. Additionally, a CT scan was requested if they were suspected to have RP. Otherwise, a CT scan was basically planned at 1 month after radiotherapy and every 3–6 months thereafter [20]. RP was graded according to the Common Terminology Criteria for Adverse Events, version 3.0 (CTCAE ver. 3.0). For this study, the onset of RP was recorded by radiologists based on CT findings taken either as planned or upon request.
Statistical analysis
The relationships between Grade 2–5 RP and the clinical factors were investigated using Fisher’s exact probability test. The relationships between the ILD score and RP Grade were assessed using Spearman’s correlation test. The relationships between the dosimetric factors and Grade 2–5 RP were analyzed using the Mann–Whitney U test. Multivariate logistic regression analyses were performed to evaluate the data for the association between the clinical/dosimetric factors and RP.
Results
The median follow-up period was 11.8 months for all patients. Subclinical ILD was recognized in 11 (18 %) of the 62 patients. A total of 51 patients had no evidence of ILD (score 0),while there were no patients with slight ILD (score 1), three patients with mild ILD (score 2) and eight patients with moderate ILD (score 3).
Grade 2–5 RP was recognized in eight (13 %) of the 62 patients: Grade 5 in three patients and Grade 2 in five patients. RP occurred between 1.8 and 6.4 months (median 4.7 month) after the start of thoracic RT. Table 2 shows the relationships between the clinical factors and Grade 2–5 RP in all patients. Subclinical ILD was a significant factor predicting the occurrence of Grade 2–5 RP (p = 0.0274). Grade 2–5 RP was observed in four (36 %) of the 11 patients with subclinical ILD. Subclinical ILD tended to be a significant factor associated with the occurrence of Grade 5 RP (p = 0.0785); two patients with subclinical ILD, one patient without ILD and two of the three patients with Grade 5 RP had an ILD score of 3 (Table 3; Fig. 2). All of the patients with Grade 5 RP exhibited extensive RP beyond the irradiated field, including the contralateral lung. Analyses of the correlation between the ILD score on CT and the RP grade using Spearman’s correlation coefficient showed these parameters to be significantly related (Table 4; r = 0.253, p = 0.048).
A case with Grade 5 RP (case 1 in Table 4). a, b CT images prior to thoracic RT. A score of 3 for subclinical ILD was recognized. c CT with dose distribution. Red, light blue, yellow, green and blue lines are 50.4, 45.4, 25.2, 15.1 and 2.0 Gy, respectively. d A CT image taken 1.8 months after the completion of thoracic RT showed extensive ground-glass abnormalities and focal consolidations
Table 5 shows the relationships between the dosimetric factors and Grade 2–5 RP in the 44 assessable patients. The lung doses of V5, V10 and MLD tended to be significantly associated with the occurrence of Grade 2–5 RP.
Discussion
The current study demonstrated that the presence of subclinical ILD is significantly associated with the development of symptomatic RP in patients treated with thoracic RT. As mentioned in the introduction, acute exacerbations of ILD related to cancer treatment have often been reported [14, 15]. Hanibuchi et al. [21] reported that acute exacerbation of idiopathic interstitial pneumonia after anti-cancer treatment occurred in seven (16 %) of 45 patients with both lung cancer and idiopathic interstitial pneumonia who received chemotherapy, RT, chemoradiotherapy or surgical resection. Chida et al. [22] analyzed the relationships between thoracotomy and acute exacerbation of subclinical ILD in lung cancer patients; postoperative acute respiratory distress syndrome (ARDS) occurred in 8.8 % of subclinical ILD-positive patients, and in 0.4 % of subclinical ILD-negative patients (p < 0.001), and they concluded that some components of postoperative ARDS are actually an acute exacerbation of subclinical IPF.
Previously reported clinical results for thoracic RT in patients with ILD have been limited [16, 23, 24]. Recently, Sanuki et al. [16] reported associations of pre-existing interstitial changes with RP for patients treated with thoracic RT; the incidence of severe RP (≥Grade 3) was significantly increased from 3 to 26 %. In our series, the rate of fatal RP tended to be significant in the patients with subclinical ILD. Our results also implied that fatal RP might be an acute exacerbation of subclinical ILD triggered by thoracic RT, so the risk of fatal RP should be considered in patients with subclinical ILD, and careful observation and management of RP must be provided after thoracic RT in such patients. In particular, an ILD score of 3 (moderate ILD) on CT may be a contraindication for thoracic RT. Grade 5 RP was recognized in two of the eight patients with a score of 3 or more, indicating extensive ILD (bilateral fibrosis in multiple lobes), on CT performed prior to thoracic RT.
The classical RP changes in the lungs are considered to be confined to the site of irradiation. However, there have been several reports in the early literature of extensive RP occurring beyond the irradiated field [25, 26]. Morgan et al. [27] indicated that sporadic pneumonitis, including extensive RP, appears to be an entirely different disease process involving immune modulation and genetic factors, as opposed to classical RP, which is characterized by the inflammatory consequences of direct radiation-related injury to pulmonary tissue. Roberts et al. demonstrated that lymphocytic alveolitis develops in both lung fields after strictly unilateral thoracic irradiation, and is more pronounced in patients who develop clinical pneumonitis. They concluded that RT may cause generalized lymphocyte-mediated hypersensitivity reactions [28]. In the current study, the contribution of the non-classical disease process described above was suggested in the patients with Grade 5 RP involving the contralateral lung, because the mean irradiation dose given to the contralateral lung was relatively low.
The relationships between the dosimetric factors of the lungs and the occurrence of symptomatic RP have been investigated in patients treated with thoracic RT [29–31]. The published quantitative analysis of normal tissue effects in the clinic (QUANTEC) review recommended a lung V20 of ≤30–35 % and a MLD of ≤20–23 Gy (with conventional fractionation) to limit the risk of RP to ≤20 % in definitively treated patients with non–small cell lung cancer [31]. Radiation-induced heart disease has been reported in patients with esophageal cancer treated with CCRT using simple two opposing fields according to the AP/PA technique [32]. Therefore, in the current study, the use of multiple (3–7) fields as a boost to the GTV after a typical RT field consisting of two opposing fields was selected in order to reduce the heart dose in the patients with GTV in close vicinity to the heart. On the other hand, when the multiple fields technique is used to reduce the heart dose, the lung volume receiving low-dose irradiation tends to broaden. Recently, several studies have demonstrated that the administration of a large distribution of low-dose radiation to the normal lung resulted in a higher risk of lung toxicity [33, 34]. These findings are supported by the results of the current study, as the total lung V5 and V10 tended to be significant factors in the relationships between the dosimetric factors and Grade 2–5 RP (Table 5). The administration of low-dose radiation to the normal lung may be associated with RP. Further studies are needed to determine the relationship between the administration of low-dose radiation to the normal lung, particularly the contralateral hilum, and the incidence of RP and subclinical ILD.
There was one major limitation associated with this study, which was the fact that the current study was a retrospective series, so the possibility of selection bias with regard to the predictive factors cannot be ruled out. A formal prospective study is needed to determine the toxicity, efficacy and prognostic factors associated with thoracic RT in patients with subclinical ILD.
In summary, the current study of Japanese patients demonstrated evidence of subclinical ILD on CT performed prior to thoracic RT in 18 % of the patients with thoracic tumors and that subclinical ILD was a significant factor related to the occurrence of Grade 2–5 RP. In addition, subclinical ILD tended to be significant for the occurrence of Grade 5 RP. In particular, the presence of extensive fibrosis on CT may therefore be a contraindication for thoracic RT, as more extensive ILD (bilateral fibrosis in multiple lobes) on CT performed prior to thoracic RT was recognized in two of the three patients with Grade 5 RP. The risk of fatal RP should be considered in patients with subclinical ILD, and the careful observation and management of RP must be provided after thoracic RT in such patients.
References
Seppenwoolde Y, Lebesque JV (2001) Partial irradiation of the lung. Semin Radiat Oncol 11:247–258
Rodrigues G, Lock M, D’Souza D et al (2004) Prediction of radiation pneumonitis by dose–volume histogram parameters in lung cancer—a systematic review. Radiother Oncol 71:127–138
Tsujino K, Hirota S, Endo M et al (2003) Predictive value of dose–volume histogram parameters for predicting radiation pneumonitis after concurrent chemoradiation for lung cancer. Int J Radiat Oncol Biol Phys 55:110–115
Mehta V (2005) Radiation pneumonitis and pulmonary fibrosis in non-small-cell lung cancer: pulmonary function, prediction, and prevention. Int J Radiat Oncol Biol Phys 63:5–24
Oh D, Ahn YC, Park HC et al (2009) Prediction of radiation pneumonitis following high-dose thoracic radiation therapy by 3 Gy/fraction for non-small cell lung cancer: analysis of clinical and dosimetric factors. Jpn J Clin Oncol 39:151–157
Kim DS, Park JH, Park BK et al (2006) Acute exacerbation of idiopathic pulmonary fibrosis: frequency and clinical features. Eur Respir J 27:143–150
Raghu G, Mageto YN, Lockhart D et al (1999) The accuracy of the clinical diagnosis of new-onset idiopathic pulmonary fibrosis and other interstitial lung disease: a prospective study. Chest 116:1168–1174
Park J, Kim DS, Shim TS et al (2001) Lung cancer in patients with idiopathic pulmonary fibrosis. Eur Respir J 17:1216–1219
Turner-Warwick M, Lebowitz M, Burrows B et al (1980) Cryptogenic fibrosing alveolitis and lung cancer. Thorax 35:496–499
Panos RJ, Mortenson RL, Niccoli SA et al (1990) Clinical deterioration in patients with idiopathic pulmonary fibrosis: causes and assessment. Am J Med 88:396–404
Kawasaki H, Nagai K, Yokose T et al (2001) Clinicopathological characteristics of surgically resected lung cancer associated with idiopathic pulmonary fibrosis. J Surg Oncol 76:53–57
Kondoh Y, Taniguchi H, Kawabata Y et al (1993) Acute exacerbation in idiopathic pulmonary fibrosis. Analysis of clinical and pathologic findings in three cases. Chest 103:1808–1812
Akira M, Hamada H, Sakatani M et al (1997) CT findings during phase of accelerated deterioration in patients with idiopathic pulmonary fibrosis. AJR Am J Roentgenol 168:79–83
Minegishi Y, Takenaka K, Mizutani H et al (2009) Exacerbation of idiopathic interstitial pneumonias associated with lung cancer therapy. Intern Med 48:665–672
Kawabata Y, Fukushima K, Uchiyama T et al (2001) A focal usual interstitial pneumonia lesion: an important risk factor in diffuse alveolar damage—acute exacerbation of a focal usual interstitial pneumonia patient. Nihon Kokyuki Gakkai Zasshi 39:316–321
Sanuki N, Ono A, Komatsu E et al (2012) Association of computed tomography-detected pulmonary interstitial changes with severe radiation pneumonitis for patients treated with thoracic radiotherapy. J Radiat Res 53:110–116
Takeda A, Enomoto T, Sanuki N et al (2008) Acute exacerbation of subclinical idiopathic pulmonary fibrosis triggered by hypofractionated stereotactic body radiotherapy in a patient with primary lung cancer and slightly focal honeycombing. Radiat Med 26:504–507
Washko GR, Lynch DA, Matsuoka S et al (2010) Identification of early interstitial lung disease in smokers from the COPDGene Study. Acad Radiol 17:48–53
Washko GR, Hunninghake GM, Fernandez IE et al (2011) Lung volumes and emphysema in smokers with interstitial lung abnormalities. N Engl J Med 364:897–906
Kyas I, Hof H, Debus J et al (2007) Prediction of radiation-induced changes in the lung after stereotactic body radiation therapy of non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 67:768–774
Hanibuchi M, Yamaguchi T, Okada T et al (2001) Clinical examination of acute exacerbation of idiopathic interstitial pneumonia (IIP) combined with lung cancer after anti-cancer treatment. Jpn J Lung Cancer 41:281–286
Chida M, Ono S, Hoshikawa Y et al (2008) Subclinical idiopathic pulmonary fibrosis is also a risk factor of postoperative acute respiratory distress syndrome following thoracic surgery. Eur J Cardiothorac Surg 34:878–881
Takenaka N, Mine T, Ikeda E et al (1988) Acute epidural hematoma of the posterior fossa in a case of von Willebrand’s disease. No Shinkei Geka 16:529–533
Baba Y, Inoue H, Sasaki M et al (2000) Percutaneous mechanical thrombectomy for thrombosed vessels with a hydrolyser (hydrodynamic thrombectomy catheter): clinical experience. Nihon Igaku Hoshasen Gakkai Zasshi 60:23–27
Gibson PG, Bryant DH, Morgan GW et al (1988) Radiation-induced lung injury: a hypersensitivity pneumonitis? Ann Intern Med 109:288–291
Smith JC (1964) Radiation pneumonitis. case report of bilateral reaction after unilaternal irradiation. Am Rev Respir Dis 89:264–269
Morgan GW, Breit SN (1995) Radiation and the lung: a reevaluation of the mechanisms mediating pulmonary injury. Int J Radiat Oncol Biol Phys 31:361–369
Roberts CM, Foulcher E, Zaunders JJ et al (1993) Radiation pneumonitis: a possible lymphocyte-mediated hypersensitivity reaction. Ann Intern Med 118:696–700
Matsuo Y, Shibuya K, Nakamura M et al (2012) Dose–volume metrics associated with radiation pneumonitis after stereotactic body radiation therapy for lung cancer. Int J Radiat Oncol Biol Phys 83:e545–e549
Tsujino K, Hirota S, Kotani Y et al (2006) Radiation pneumonitis following concurrent accelerated hyperfractionated radiotherapy and chemotherapy for limited-stage small-cell lung cancer: dose–volume histogram analysis and comparison with conventional chemoradiation. Int J Radiat Oncol Biol Phys 64:1100–1105
Marks LB, Bentzen SM, Deasy JO et al (2010) Radiation dose–volume effects in the lung. Int J Radiat Oncol Biol Phys 76:S70–S76
Ishikura S, Nihei K, Ohtsu A et al (2003) Long-term toxicity after definitive chemoradiotherapy for squamous cell carcinoma of the thoracic esophagus. J Clin Oncol 21:2697–2702
Wang SL, Liao Z, Vaporciyan AA et al (2006) Investigation of clinical and dosimetric factors associated with postoperative pulmonary complications in esophageal cancer patients treated with concurrent chemoradiotherapy followed by surgery. Int J Radiat Oncol Biol Phys 64:692–699
Tanabe S, Myojin M, Shimizu S et al (2013) Dose–volume analysis for respiratory toxicity in intrathoracic esophageal cancer patients treated with definitive chemoradiotherapy using extended fields. J Radiat Res 54:1085–1094
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Yamaguchi, S., Ohguri, T., Matsuki, Y. et al. Radiotherapy for thoracic tumors: association between subclinical interstitial lung disease and fatal radiation pneumonitis. Int J Clin Oncol 20, 45–52 (2015). https://doi.org/10.1007/s10147-014-0679-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-014-0679-1