Abstract
Background
Sarcomatous intrahepatic cholangiocarcinoma (ICC) is a rare histological variant of ICC. The prognosis of sarcomatous ICC is poorly understood.
Methods
We analyzed the prognosis of sarcomatous ICC by reviewing the previous reports and our own case.
Results
Only 15 cases of sarcomatous ICC have been reported in the English-language literature so far. Median survival time of patients with sarcomatous ICC with and without surgery was 11 and 3 months, respectively. Survival rate of patients operated on for sarcomatous ICC was similar to that of patients with ordinary ICC without surgery in the early postoperative period. In the long-term view, however, the prognosis for the patients with sarcomatous ICC receiving surgery was better than that for the patients with ordinary ICC without surgery.
Conclusion
Although the prognosis for the patients with sarcomatous ICC was poor even after curative resection, surgery would be justified as the primary treatment for sarcomatous ICC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sarcomatous intrahepatic cholangiocarcinoma (ICC) is a rare variant of ICC, and is composed of both adenocarcinoma (ICC component) and sarcomatous components. The sarcomatous component of the tumor microscopically resembles sarcoma, but the expression of both epithelial and mesenchymal features is characteristic [1]. Therefore, sarcomatous ICC should be strictly distinguished from ICC with sarcomatoid transformation or carcinosarcoma.
However, due to the rarity of this disease, it is very hard to know the accurate prognosis of the patients from single institutional experiences. We therefore analyzed the prognosis for sarcomatous ICC using the data obtained by reviewing the published case reports in the English-language literature as well as our own case.
Materials and methods
Case search
We searched PubMed to identify the published case reports of sarcomatous ICC in the English-language literature. We used the search terms “liver”, “sarcomatous”, “sarcomatoid” and “cholangiocarcinoma”, and limited our search to reports published between January 1, 1990, and June 20, 2012. We reviewed the cases with sarcomatous ICC according to the definition [1]: (1) coexistence of both ICC (adenocarcinoma) and sarcomatous components in the tumor morphologically, and (2) expression of not only molecular features of mesenchyme (e.g. vimentin) but also molecular features of epithelium (e.g. cytokeratin) in the sarcomatous component. Although ICC with sarcomatoid transformation and carcinosarcoma are morphologically composed of both carcinomatous and sarcomatous components, we carefully distinguished sarcomatous ICC from ICC with sarcomatoid transformation, in which only molecular features of epithelium were expressed in the sarcomatous lesion, or from carcinosarcoma, in which only molecular features of mesenchyme were expressed in the sarcomatous lesion (Table 1).
Our own case
Histopathology of our own case was confirmed by immunohistochemical staining, performed on formalin-fixed, paraffin-embedded sections using the EnVision + system (Dako Cytomation, Glostrup, Denmark). Deparaffinized sections were microwaved in 10 mM Tris buffer and 1 mM EDTA, pH 9.0 for 5 min. Monoclonal antibodies for cytokeratin (clone AE1/AE3; 1:50 dilution; Dako) and vimentin (clone V9; 1:50 dilution; Dako) were used.
Case–control study
As the control, patients with the ordinary type of ICC (ordinary ICC) admitted to the Department of Gastroenterological Surgery, Akita University Hospital between 1990 and 2010 were used in this study.
Statistical analysis
The data are shown as mean ± SD. Mann–Whitney U test, Fisher’s exact probability test and log-rank test were used for the statistical analysis. All statistical calculations were performed with the software SPSS Ver.20.0 (IBM, Armonk, NY, USA). In the analyses, a probability of P < 0.05 was taken to indicate statistical significance.
Results
Prognosis analysis of sarcomatous ICC
Our search in PubMed identified a total of 221 reports. Of these, 74 case reports described a case with a sarcomatous component in the liver. These included ICC with sarcomatoid transformation (2 reports), carcinosarcoma (7 reports), hepatocellular carcinoma (HCC) with sarcomatoid transformation (27 reports), combined HCC/ICC with sarcomatoid transformation (6 reports), undifferentiated sarcomatous cancer (10 reports) and primary liver sarcoma (5 reports). Consequently, only 15 cases in 11 papers [2–12] were identified as true sarcomatous ICC. Fourteen cases were diagnosed by immunohistochemical staining, and in one case the epithelial feature in the sarcomatous component was proven by electron microscopy [11]. Table 2 is the list of reported sarcomatous ICC including our case. Out of 15 patients, 11 patients underwent surgery.
Clinical characteristics of these reported sarcomatous ICC were compared with ordinary ICC in our series. The numbers of patients with ordinary ICC treated with and without surgery in our series were 27 and 29, respectively. All the surgical patients with ordinary ICC were histopathologically proven, but the patients with ordinary ICC without surgery were diagnosed primarily by radiological findings without histopathological examination. Therefore, we thought that it was more appropriate to compare clinical characteristics of operated sarcomatous ICC with those of operated (histopathologically proven) ordinary ICC in our series. Clinical characteristics of the surgical patients with sarcomatous ICC in the literature and ordinary ICC in our series were compared, and the results are shown in Table 3. There were no significant differences in clinical variables between sarcomatous ICC and ordinary ICC.
Figure 1 illustrates the survival curve of 11 sarcomatous ICCs and 6 carcinosarcomas after surgical resection. Of 9 carcinosarcomas, 7 patients underwent surgery (one case of carcinosarcoma was excluded from this analysis because of lack of prognostic data). There was no surgical case of ICC with sarcomatoid transformation. Prognosis of both sarcomatous ICC and carcinosarcoma was very dismal.
Survival rates of operated sarcomatous ICC and carcinosarcoma. There were 11 cases of sarcomatous ICC and 7 cases of carcinosarcoma (one case of carcinosarcoma was excluded from this analysis because of lack of prognostic data). Survival rates, calculated by the Kaplan–Meier method, of sarcomatous ICC (Sarcomatous, black solid line) and carcinosarcoma (black broken line) are shown
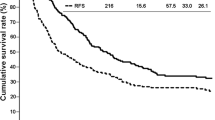
Survival rates of the patients with sarcomatous ICC and ordinary ICC were analyzed in the presence or absence of surgical resection. In comparison to the patients with ordinary ICC, survival rates of the patients with sarcomatous ICC treated with and without surgery were significantly poorer (log-rank test, P = 0.018 and P = 0.011, respectively) (Fig. 2). Median survival time (MST) of the surgical patients of sarcomatous ICC was 11 months, and the survival rate was similar to that of patients with ordinary ICC without surgery in the early postoperative period. However, the prognosis for the patients with sarcomatous ICC who survived more than 1 year after surgery was relatively favorable in comparison to the patients with ordinary ICC without surgery, and the 4-year survival rate was 20 %. In contrast, the prognosis for the patients with sarcomatous ICC without surgery was very dismal, with a MST of 3 months, and none of the patients survived more than 4 months.
Survival rates of sarcomatous ICC and ordinary ICC. Survival rates were calculated by the Kaplan–Meier method. Survival rates of the cases of sarcomatous ICC (Sarcomatous, black line) and ordinary ICC (Ordinary, gray line) were separately analyzed in the presence (Op+, solid line) or absence (Op−, broken line) of surgical operation. One case of sarcomatous ICC was excluded from this analysis because of lack of prognostic data
Details of our patient
A 62-year-old man was referred to our hospital with liver tumor and jaundice. On admission, a hyperechoic tumor was detected on abdominal ultrasonography (US) (Fig. 3a). An abdominal computed tomography (CT) scan showed that a low-density liver tumor was located right at the hepatic hilum and was causing an obstruction of the intrahepatic bile duct bilaterally. The tumor was 3.9 cm in diameter, and the periphery of the tumor was enhanced by contrast medium in the arterial phase (Fig. 3b) and the portal phase (Fig. 3c). Results of relevant laboratory data (with the normal range) were as follows: AST 174 U/L (13–33); ALT 356 U/L (8–42); ALP 2115 U/L (115–359); LDH 199 U/L (119–229); γ-GTP 405 U/L (11–47); total bilirubin 7.3 mg/dL (0.2–1.2); albumin 3.2 g/dL (4.0–5.0); carcinoembryonic antigen (CEA) 1.4 ng/mL (<4.9); carbohydrate antigen (CA) 19-9 1109.9 U/mL (<37.0); prothrombin time 85.5 %. Hepatitis B virus surface antigen and hepatitis C virus antibody were negative. The tumor was clinically diagnosed as ICC. He was planned to undergo extended right hemihepatectomy, and percutaneous transhepatic biliary drainage was performed for reducing jaundice. Four weeks after biliary drainage, serum total bilirubin level decreased to the normal range. CT showed that the tumor had increased in size to 4.5 cm in diameter and some intrahepatic metastases had appeared around the tumor. Because the distribution of the tumors was limited to the right lobe of the liver although they were multiple, extended right hemihepatectomy, resection of the extrahepatic bile duct and cholangiojejunostomy were performed. Lymph nodes including hilar, hepatic, portal, peripancreatic and a part of para-aortic lymph nodes were dissected.
Macroscopically, the surgical specimen showed a white-reddish, demarcated, solid tumor, 5.0 cm in diameter, and intrahepatic metastases (Fig. 4a). Microscopically, the tumors were mostly composed of pleomorphic cells and spindle cells with high-grade dysplastic nuclei (sarcomatous component), and moderately to poorly differentiated adenocarcinoma (ICC component) was scattered in the tumor (Fig. 4b, c). Vascular invasion to the right portal vein and the intraparenchymal hepatic artery, and lymph node metastasis into the hilar and hepatic lymph nodes were detected. There was much collagen fiber in the sarcomatous component on Elastica–Masson staining (Fig. 4d). With immunohistochemical staining, only cytokeratin was positive in the ICC component. However, the cells—especially pleomorphic cells—in the sarcomatous component stained positively for both cytokeratin (Fig. 4e) and vimentin (Fig. 4f). The tumor was diagnosed pathologically as sarcomatous ICC.
Macroscopic and microscopic findings of sarcomatous ICC. Macroscopic appearance of surgical specimen (a), and microscopic findings on H&E staining (b ×40, c ×200), Elastica–Masson staining (d ×200), and immunohistochemical staining for cytokeratin (e ×200) and vimentin (f ×200) are shown. Arrowhead indicates intrahepatic metastasis, and asterisks indicate the identical adenocarcinoma lesion
The postoperative course was uneventful. Because sarcomatous ICC had been reported to show poor prognosis with early recurrence, we recommended him to receive adjuvant chemotherapy using gemcitabine (1000 mg/m2 of body-surface area, on days 1 and 8 every 3 weeks) although there was no evidence that adjuvant chemotherapy for ICC improved the survival. With his consent, adjuvant chemotherapy was performed for 6 months, starting from 1 month after the operation. During the adjuvant chemotherapy, there was no adverse effect, and no apparent recurrence was observed at the completion of adjuvant chemotherapy (Fig. 5a, b).
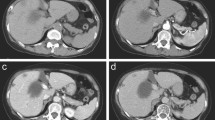
Images at the completion of adjuvant chemotherapy and at recurrence. MR images with contrast-enhanced vascular phase (a) and Kupffer phase (b) at the completion of adjuvant chemotherapy are shown. Images on abdominal contrast-enhanced CT in axial view (c) and coronal view (d) at the recurrence are shown. Arrow indicates the point of complete obstruction of the portal vein
Three months after the cessation of the adjuvant chemotherapy (10 months after surgery), he presented with abdominal distension. Abdominal CT showed multiple liver metastases in the remnant liver and massive ascites due to complete obstruction of the portal vein by the metastases (Fig. 5c, d). His poor physical condition did not allow further chemotherapy and resulted in his death 11 months after surgery.
Discussion
The prevalence of sarcomatous ICC is unknown although ICC with sarcomatoid transformation was reportedly 4.5 % of all ICC [4]. The etiology of sarcomatous ICC remains uncertain, and it is suspected that the tumor origin or precursor lesion of sarcomatous ICC might be different from ICC with sarcomatoid transformation and carcinosarcoma. Reviewing the published case reports demonstrated that survival rates of sarcomatous ICC and carcinosarcoma after surgery were similarly discouraging, and the prognosis of ICC with sarcomatoid transformation after surgery was unknown because none of the cases underwent surgery.
As for diagnosis of sarcomatous ICC, it might be almost impossible to differentiate sarcomatous ICC from ordinary ICC without histopathological examination. In the present study, we showed that there were no significant differences in clinical variables between them. Shimada et al. [7] reported that serum alkaline phosphatase (ALP) level in sarcomatous ICC was significantly lower than that in ordinary ICC, although they did not discuss the clinical value. We also compared serum ALP level in sarcomatous ICC with that in ordinary ICC, but there was no difference in serum ALP level (P = 0.08). Serum ALP level might not be a good indicator because, in contrast to the previous report, serum ALP level in sarcomatous ICC was even higher than that in ordinary ICC in the present study.
Radiological images might also be difficult for distinguishing sarcomatous ICC from ordinary ICC. Hypoechoic tumor on US, low-density mass with enhancement in the periphery by contrast medium on CT, hypointensity on T1-weighted magnetic resonance imaging (MRI), and hyperintensity on T2-weighted MRI were reportedly dominant features [8, 10], but these findings are also common in ordinary ICC. The gold standard of the diagnosis is histopathological examination. Although needle biopsy of the tumor was described as being good for diagnosis [10], indiscreet biopsy should be avoided because of the possibility of peritoneal dissemination, especially in patients planned for curative operation.
In the present study, we showed that the prognosis for patients with sarcomatous ICC was much poorer than ordinary ICC. Shimada et al. [7] reported that the prognosis for 4 operated patients with sarcomatous ICC in their institution was similar to that of ordinary ICC in the early postoperative period. However, all their cases resulted in death by 29 months and long-term survival was not found. Kaibori et al. [8] showed that the prognosis for sarcomatous ICC treated with hepatectomy was better than that without hepatectomy. However, some cases of carcinosarcoma might be included in their study because the findings of immunohistochemical staining of the cases were not compatible with sarcomatous ICC but rather with carcinosarcoma. To the best of our knowledge, our report is the most comprehensive review of sarcomatous ICC so far. Although there was analytical limitation in the details of tumor extension due to literature review, we showed that the prognoses for patients with sarcomatous ICC with or without surgery were significantly worse than those for the corresponding ordinary ICC. Furthermore, MST of sarcomatous ICC with surgery (11 months) was comparable with that of ordinary ICC without surgery (8 months), indicating the poor prognosis of sarcomatous ICC. However, the prognosis for the patients with sarcomatous ICC who survived more than 1 year after surgery was more promising than that of ordinary ICC without surgery. This fact would partly support the relevancy of selecting surgery as the primary treatment.
Multidisciplinary treatment including chemotherapy might be necessary to obtain better prognosis. Malhotra et al. [11] showed that patients with recurrent sarcomatous ICC after surgery achieved sustained partial remission for more than 2 years with combination chemotherapy using gemcitabine and cisplatin [13]. However, the effect of adjuvant chemotherapy for sarcomatous ICC has not so far been reported. As far as we know, the present case is the first patient who underwent adjuvant chemotherapy for sarcomatous ICC. Gemcitabine was used for adjuvant chemotherapy because even monotherapy with gemcitabine had shown a 30–36 % response rate for patients with inoperable cholangiocarcinoma and gallbladder cancer [14]. Cisplatin was not used, to avoid its adverse effects. The duration of the adjuvant chemotherapy was scheduled for 6 months, according to the period of adjuvant chemotherapy for pancreatic cancer [15] and colorectal cancer [16, 17]. We thought that adjuvant chemotherapy using gemcitabine was presumably effective for this patient because apparent recurrence was not observed during the period of adjuvant chemotherapy, but liver metastases grew rapidly after the cessation of gemcitabine administration. Therefore, continuation or a longer period of adjuvant chemotherapy might be recommended to improve patient prognosis after surgery in sarcomatous ICC. Because none of the cases of sarcomatous ICC were treated with either neoadjuvant chemotherapy or radiation therapy, the effects of these therapies on the prognosis were unknown.
Finally, the limitation of this study was the difficulty of analyzing prognostic factors of sarcomatous ICC due to a shortage of available parameters including extent of lymph node dissection and basic laboratory examination in the published case reports. For rare diseases, it is usually hard to know the prognostic expectation, but such information is rather requested in the setting of clinical practice. Although case reports are the only alternative source of prognostic information, it is often difficult to get an overview of variables of interest. In this study, we could compare the survival rate, but not the significance of lymph node dissection. Cancer case reports should ideally include fundamental information on cancer treatment from the perspective of future scientific integration.
In summary, we reviewed case reports of sarcomatous ICC, and showed the clinical characteristics and prognosis of sarcomatous ICC. The prognosis for the patients with sarcomatous ICC was very poor even after curative resection, with a MST of 11 months. However, surgery would be justified as the primary treatment for three reasons: (1) preoperative differential diagnosis from ordinary ICC is difficult even with modern diagnostic tools, (2) there are some populations who achieved 4-year survival through surgery, and (3) intensive adjuvant chemotherapy may contribute to the improvement of the patient’s prognosis after surgery.
References
Kwon JH, Kang YN, Kang KJ (2007) Carcinosarcoma of the liver: a case report. Korean J Radiol 8:343–347
Sasaki M, Nakanuma Y, Nagai Y et al (1991) Intrahepatic cholangiocarcinoma with sarcomatous transformation: an autopsy case. J Clin Gastroenterol 13:220–225
Haratake J, Yamada H, Horie A et al (1992) Giant cell tumor-like cholangiocarcinoma associated with systemic cholelithiasis. Cancer 69:2444–2448
Nakajima T, Tajima Y, Sugano I et al (1993) Intrahepatic cholangiocarcinoma with sarcomatous change. Clinicopathologic and immunohistochemical evaluation of seven cases. Cancer 72:1872–1877
Honda M, Enjoji M, Sakai H et al (1996) Case report: intrahepatic cholangiocarcinoma with rhabdoid transformation. J Gastroenterol Hepatol 11:771–774
Itamoto T, Asahara T, Katayama K et al (1999) Double cancer—hepatocellular carcinoma and intrahepatic cholangiocarcinoma with a spindle-cell variant. J Hepatobiliary Pancreat Surg 6:422–426
Shimada M, Takenaka K, Rikimaru T et al (2000) Characteristics of sarcomatous cholangiocarcinoma of the liver. Hepatogastroenterology 47:956–961
Kaibori M, Kawaguchi Y, Yokoigawa N et al (2003) Intrahepatic sarcomatoid cholangiocarcinoma. J Gastroenterol 38:1097–1101
Sato K, Murai H, Ueda Y et al (2006) Intrahepatic sarcomatoid cholangiocarcinoma of round cell variant: a case report and immunohistochemical studies. Virchows Arch 449:585–590
Tsou YK, Wu RC, Hung CF et al (2008) Intrahepatic sarcomatoid cholangiocarcinoma: clinical analysis of seven cases during a 15-year period. Chang Gung Med J 31:599–605
Malhotra S, Wood J, Mansy T et al (2010) Intrahepatic sarcomatoid cholangiocarcinoma. J Oncol 2010:1–4
Inoue Y, Lefor AT, Yasuda Y (2012) Intrahepatic cholangiocarcinoma with sarcomatous changes. Case Rep Gastroenterol 6:1–4
Valle J, Wasan H, Palmer DH et al (2010) Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 362:1273–1281
Dingle BH, Rumble RB, Brouwers MC (2005) The role of gemcitabine in the treatment of cholangiocarcinoma and gallbladder cancer: a systematic review. Can J Gastroenterol 19:711–716
Oettle H, Post S, Neuhaus P et al (2007) Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA 297:267–277
Kuebler JP, Wieand HS, O’Connell MJ et al (2007) Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: results from NSABP C-07. J Clin Oncol 25:2198–2204
Andre T, Boni C, Mounedji-Boudiaf L et al (2004) Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med 350:2343–2351
Conflict of interest
The authors have no potential conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Watanabe, G., Uchinami, H., Yoshioka, M. et al. Prognosis analysis of sarcomatous intrahepatic cholangiocarcinoma from a review of the literature. Int J Clin Oncol 19, 490–496 (2014). https://doi.org/10.1007/s10147-013-0586-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-013-0586-x