Abstract
Objective
Lymph node metastasis (LNM) is known to be the most important prognostic factor in cervical cancer. We analyzed the number of positive lymph nodes and other clinicopathological factors as prognostic factors for survival in node-positive patients with cervical cancer.
Methods
Node-positive cervical cancer patients (n = 108) who underwent radical hysterectomy and systematic lymphadenectomy in Hokkaido University Hospital from 1982 to 2002 were enrolled. Clinicopathological data including age, stage, histologic subtype, and the number of LNM sites were collected. The main outcome was the overall survival (OS) rate for Stage Ib–IIb patients treated with surgery and postoperative radiotherapy.
Results
The 5-year OS rate of patients with 1 positive node was 93.3%, that for 2 nodes was 77.3%, for 3 nodes it was 33.3%, and for 4 or more it was 13.8%. The OS rate of patients with 1 or 2 LNM sites was significantly better than that for patients with more than 2 LNM sites. The OS rate of patients with adenocarcinoma (Ad) (28.6%) was significantly lower than that for patients with other histologic subtypes (squamous cell carcinoma; 66.7%, adenosquamous carcinoma; 75.0%, p = 0.0003). Multivariate analysis revealed that >2 LNM sites and Ad were independent prognostic factors for survival. The 5-year OS rate of patients with 1 or 2 LNM sites was 86.8%, a more favorable prognosis than the OS rates in other reports.
Conclusion
More than two LNM sites and adenocarcinoma were independent prognostic factors for node-positive patients with cervical cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many studies have reported that pathological type, bulky tumor, deep stromal invasion, lymph vascular space involvement and lymph node metastasis (LNM) are prognostic factors for recurrence in Stage Ib–IIb cervical cancer patients [1–3]. In particular, LNM is reported to be the most important prognostic factor for invasive cervical cancer. Node-positive patients usually receive adjuvant radiotherapy (RT) after surgery [4]. However, there is no clear evidence that RT contributes to improving the overall survival of node-positive patients [5, 6]. The aim of this study was to identify clinicopathological risk factors (including the number of LNM sites) for recurrence, and to stratify survival according to these risk factors for node-positive patients.
Patients and methods
Patients
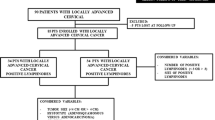
We enrolled 108 node-positive cervical cancer patients among 425 total cervical cancer patients who underwent radical hysterectomy and systematic pelvic lymphadenectomy in our institution from 1982 to 2002. The patients’ characteristics are shown in Table 1. The median age was 50 years (range 28–71). Among these patients, 19 had clinical Stage Ib, 6 had Stage IIa, and 83 had Stage IIb. The numbers of patients with squamous cell carcinoma (SCC), adenocarcinoma (Ad), and adenosquamous carcinoma (Ad-Sq) were 90, 14 and 4, respectively. All patients received whole-pelvic irradiation (50 Gy/25 fr) as postsurgical adjuvant therapy. The median follow-up period was 54 months (range 4–171).
LNM and pathological factors
We counted the number of pelvic lymph nodes positive for metastasis separately on the left and right sides. We also analyzed pathological factors, including deep (≥2/3) stromal invasion (DSI, n = 63), parametrial invasion (PI, n = 63), bulky tumor (BT, n = 50), corpus invasion (n = 43), and vaginal invasion (n = 25) (Table 2).
Statistical analysis
Categorical variables were analyzed using the chi-square test or Fisher’s exact test. We used the Kaplan–Meier method, the log-rank test for survival analysis, and the Cox hazard method for prognostic analysis. A result was considered significant when the p value was <0.05.
Results
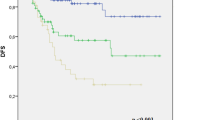
The median number of pelvic lymph nodes removed was 65 (range 23–117). The 5-year OS of node-positive patients was 62.0%, which was significantly worse than that of 310 node-negative patients (94.8%) (p < 0.0001, Fig. 1). The survival rate differed according to the number of positive lymph node sites: 1 site, 93.3%; 2 sites, 77.3%; 3 sites, 33.3%; ≥4 sites, 13.8%. The survival of patients with >2 sites was significantly worse than the survival of patients with 1 or 2 sites (p < 0.0001, Fig. 2). There was no significant difference among the survivals for each clinical stage (78.9% for Stage Ib, 66.7% for IIa, and 57.8% for IIb) (Ib vs. IIa, p = 0.59; Ib vs. IIb, p = 0.14; IIa vs. IIb, p = 0.73). We grouped Stage Ib and IIa into one group for univariate analysis because the number of patients for Stage IIa was small.
Five-year overall survival of patients with lymph node metastasis according to the number of lymph node metastasis sites (1 site, 93.3%; 2 sites, 77.3%; 3 sites, 33.3%; ≥4 sites: 13.8%). The survival of patients with >2 sites was significantly worse than the survival of patients with 1 or 2 sites (p < 0.0001) (1 site vs. 2 sites, p = 0.06; 2 sites vs. 3 sites, p = 0.008; 3 sites vs. ≥4 sites, p = 0.24; 1 site vs. 3 sites, p < 0.0001; 1 site vs. ≥4 sites, p < 0.0001; 2 site vs. ≥4 sites, p < 0.0001; <2 sites vs. >3 sites, p < 0.0001)
Survivals according to histologic subtype were 66.7% for SCC, 75.0% for Ad-Sq, and 28.6% for Ad. The survival of patients with Ad was significantly worse than that for patients with other histologic types (p = 0.0003). Univariate analysis revealed that a histologic subtype of Ad (p = 0.0006), PI (p = 0.01), BT (p = 0.006), and DSI (p = 0.047), and having >2 LNM sites (p < 0.0001) were statistically significant. Multivariate analysis showed that Ad (p = 0.0005) and >2 LNM sites (p < 0.0001) were independent prognostic factors for survival (Table 2).
The survival of node-positive patients was stratified by combining these independent prognostic factors into three groups (Fig. 3): group A, 1 or 2 LNM sites irrespective of histologic subtype (n = 68); group B, >2 LNM sites with SCC or Ad-Sq (n = 32); and group C, >2 LNM sites with Ad (n = 8). The survivals of each group were 86.8, 25.0, and 0% for groups A, B, and C, respectively (A vs. B, p < 0.0001; B vs. C, p < 0.0001; A vs. C, p < 0.0001).
Discussion
The 5-year survival of patients with 1 or 2 LNM sites was significantly better than that of patients with >2 LNM sites. There have been some reports that the 5-year OS reduces by as much as 50–60% depending on the number of LNM sites in cervical cancer; >1 site has a significantly poorer prognosis than 1 site [2, 7]. Tsai et al. reported that the 5-year OS rate of patients with 1 positive node site (84%) was statistically different from that of patients with >1 site (61%) [8]. The survival of patients with 1 or 2 LNM sites (86.8%) in our institution seems favorable compared with previous reports in the literature, and we speculate that the reason is at least in part due to the radicality of our surgery. We have performed type IV radical hysterectomy (Piver classification)—a more radical method than type II or III radical surgery [9, 10]. In addition, we removed more lymph nodes than in other reports [11] (Table 3). A therapeutic effect of lymphadenectomy was not clear for cervical cancer, but several reports have shown that extensive lymphadenectomy increases survival. Pieterse et al. [12] reported that the 5-year disease-free survival of node-positive patients with ≥18 removed lymph nodes was about 20% higher than that of patients with <18 removed lymph nodes. Kenter et al. [13] reported that in 63 patients with positive nodes, those with complete lymphadenectomy had significantly fewer recurrences (25%) than those with incomplete lymphadenectomy (56%). Recently, sentinel lymph node biopsy has become common, and when these lymph nodes are negative for metastasis, many gynecologists omit the systematic lymphadenectomy. There are many benefits of this method, including low invasiveness and a low rate of complications [14, 15], but Marchiole et al. [16] reported that the sentinel lymph node biopsy method resulted in 14.3% false negatives, and Lentz et al. [17] described that 15% of the negative sentinel nodes had micrometastasis. Therefore, a complete lymphadenectomy may be able to elevate the detection rate of LNM and may improve the prognosis in cases with 1 or 2 LNM sites.
We administered whole pelvic irradiation as the adjuvant therapy to node-positive patients postoperation. Unfortunately, RT did not contribute to improving the survival of patients with >2 LNM sites (Fig. 2). Some reports indicate that adjuvant concurrent chemoradiotherapy (CCRT) with cisplatin is superior to RT alone for both OS and progression-free survival of node-positive patients [18, 19]. We need to further investigate the effectiveness of adjuvant CCRT for patients with multiple LNM sites in the future.
Adenocarcinoma was an independent prognostic factor for node-positive cervical cancer. However, there was no significant difference between the survivals of patients with Ad and those with SCC but without LNM in our study. Ad cases with bulky tumors seemed to tend to have many LNM sites, but no statistically significant difference between these two sets of patients was found (Table 4). Berek et al. [20] and Irie et al. [21] reported that the 5-year survival for node-positive patients with Stage Ib–IIb cervical adenocarcinoma was 10–48%. This was probably caused by the biological aggressiveness and resistance to adjuvant therapy of Ad compared to SCC [1]. However, the 5-year survival for Ad patients with 1 or 2 LNM sites was 100% in our institution—apparently not inferior to the survival for SCC/Ad-Sq patients. Unfortunately, the survival of patients with both Ad and >2 LNM sites was quite poor. Peters et al. [19] reported the effect of GOG109 on CCRT using cisplatin and 5-fluorouracil versus RT alone as an adjuvant therapy after radical surgery for Stage Ia–IIa.
The 5-year OS of the group undergoing CCRT for Ad was 80%, significantly higher than that of the group undergoing RT alone for Ad (40%). Some reports have described the responses of advanced or relapsed patients to CT; the response of Ad to paclitaxel was 31%, that of non-SCC to topotecan and cisplatin was 27%, and that of non-SCC to docetaxel and carboplatin was 86% [22, 23]. We therefore need to compare the survival effect of adjuvant CT to those of RT alone or CCRT for Ad cases in a clinical trial setting. Furthermore, we should investigate the synergistic effects of molecular targeting drugs. To find good target molecules for cervical cancer, we need to perform basic and/or clinical research on Ad to improve survival. Watari et al. [24] recently reported that positive clusterin expression with multiple positive nodes gave a significantly poorer prognosis than negative expression, and suggested a potential combination of clusterin-targeting drugs to improve survival in such cases. Since this report targeted a small number of patients, further data evaluation for a large population is needed to establish optimal therapies for Ad and multiple LNM.
References
Kodama J, Seki N, Ojima Y et al (2006) Prognostic factors in node-positive patients with Stage IB–IIB cervical cancer treated by radical hysterectomy and pelvic lymphadenectomy. Int J Gynaecol Obstet 93:130–135
Takeda N, Sakuragi N, Takeda M et al (2002) Multivariate analysis of histopathologic prognostic factors for invasive cervical cancer treated with radical hysterectomy and systematic retroperitoneal lymphadenectomy. Acta Obstet Gynecol Scand 81:1144–1151
Trattner M, Graf AH, Lax S et al (2001) Prognostic factors in surgically treated Stage Ib–IIb cervical carcinomas with special emphasis on the importance of tumor volume. Gynecol Oncol 82:11–16
Burghardt E, Pickel H, Haas J et al (1987) Prognostic factors and operative treatment of Stages IB to IIB cervical cancer. Am J Obstet Gynecol 156:988–996
Chatani M, Nose T, Masaki N et al (1998) Adjuvant radiotherapy after radical hysterectomy of the cervical cancer. Prognostic factors and complications. Strahlenther Onkol 174:504–509
Uno T, Isobe K, Yamamoto S et al (2006) Postoperative radiation therapy for carcinoma of the uterine cervix. Radiat Med 24:91–97
Inoue T, Morita K (1990) The prognostic significance of number of positive nodes in cervical carcinoma Stages IB, IIA, and IIB. Cancer 65:1923–1927
Tsai CS, Lai CH, Wang CC et al (1999) The prognostic factors for patients with early cervical cancer treated by radical hysterectomy and postoperative radiotherapy. Gynecol Oncol 75:328–333
Sakuragi N, Todo Y, Kudo M et al (2005) A systematic nerve-sparing radical hysterectomy technique in invasive cervical cancer for preserving postsurgical bladder function. Int J Gynecol Cancer 15:389–397
Piver MS, Rutledge F, Smith JP (1974) Five classes of extended hysterectomy for women with cervical cancer. Obstet Gynecol 44:265–272
Ramirez PT, Slomovitz BM, Soliman PT et al (2006) Total laparoscopic radical hysterectomy and lymphadenectomy: the M.D. Anderson Cancer Center experience. Gynecol Oncol 102:252–255
Pieterse QD, Kenter GG, Gaarenstroom KN et al (2007) The number of pelvic lymph nodes in the quality control and prognosis of radical hysterectomy for the treatment of cervical cancer. Eur J Surg Oncol 33:216–221
Kenter GG, Hellebrekers BW, Zwinderman KH et al (2000) The case for completing the lymphadenectomy when positive lymph nodes are found during radical hysterectomy for cervical carcinoma. Acta Obstet Gynecol Scand 79:72–76
Wydra D, Sawicki S, Wojtylak S (2006) Sentinel node identification in cervical cancer patients undergoing transperitoneal radical hysterectomy: a study of 100 cases. Int J Gynecol Cancer 16:649–654
Daraï E, Lavoué V, Rouzier R et al (2007) Contribution of the sentinel node procedure to tailoring the radicality of hysterectomy for cervical cancer. Gynecol Oncol 106:251–256
Marchiolè P, Buénerd A, Scoazec JY et al (2004) Sentinel lymph node biopsy is not accurate in predicting lymph node status for patients with cervical carcinoma. Cancer 100:2154–2159
Lentz SE, Muderspach LI, Felix JC et al (2004) Identification of micrometastases in histologically negative lymph nodes of early-stage cervical cancer patients. Obstet Gynecol 103:1204–1210
Ryu HS, Chun M, Chang KH et al (2005) Postoperative adjuvant concurrent chemoradiotherapy improves survival rates for high-risk, early stage cervical cancer patients. Gynecol Oncol 96:490–495
Peters WA 3rd, Liu PY, Barrett RJ 2nd et al (2000) Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol 18:1606–1613
Berek JS, Hacker NF, Fu YS et al (1985) Adenocarcinoma of the uterine cervix: histologic variables associated with lymph node metastasis and survival. Obstet Gynecol 65:46–52
Irie T, Kigawa J, Minagawa Y et al (2000) Prognosis and clinicopathological characteristics of Ib–IIb adenocarcinoma of the uterine cervix in patients who have had radical hysterectomy. Eur J Surg Oncol 26:464–467
Kim SM, Choi HS, Byun JS (2000) Overall 5-year survival rate and prognostic factors in patients with Stage IB and IIA cervical cancer treated by radical hysterectomy and pelvic lymph node dissection. Int J Gynecol Cancer 10:305–312
Cheng X, Cai S, Li Z et al (2004) The prognosis of women with Stage IB1–IIB node-positive cervical carcinoma after radical surgery. World J Surg Oncol 2:47
Watari H, Ohta Y, Hassan MK et al (2008) Clusterin expression predicts survival of invasive cervical cancer patients treated with radical hysterectomy and systematic lymphadenectomy. Gynecol Oncol 108:527–532
Acknowledgments
We thank our colleagues (Department of Gynecology, Hokkaido University Graduate School of Medicine) for their assistance with the collection and analysis data.
Conflict of interest
No author has any conflict of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Hosaka, M., Watari, H., Mitamura, T. et al. Survival and prognosticators of node-positive cervical cancer patients treated with radical hysterectomy and systematic lymphadenectomy. Int J Clin Oncol 16, 33–38 (2011). https://doi.org/10.1007/s10147-010-0123-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-010-0123-0