Abstract
A 62-year-old Japanese man presented with a 1-month history of inter-digestive epigastralgia. His family history included a sister with gastric cancer. Gastroendoscopy and gastrography demonstrated a type-2 tumor in the upper region of the stomach. CT scan and fluorodeoxyglucose–positron emission tomography (FDG–PET) scan demonstrated gastric cancer and its metastatic lymph nodes. The patient underwent total gastrectomy with splenectomy and extended lymph node dissection. Although postoperative adjuvant chemotherapy by S-1 was started, the deteriorating condition of the patient prevented drug administration and even eating meals. On the 19th postoperative day (POD), FDG–PET scan of the body demonstrated new uptake in the liver and lymph node around the aorta. Without any sign of infection, leukocytosis developed around the 30th POD. On the 49th POD, remarkable uptake in the whole upper abdomen was detected on FDG–PET scan. Finally, leukocyte count increased to 125,200 and granulocyte colony stimulating factor (G-CSF) was elevated to 28 pg/ml on the 54th POD. The patient died of multiple liver metastases and carcinomatous peritonitis only 56 days after surgery. G-CSF-producing tumor is a rare but aggressive disease, particularly as recurrent tumor. If leukocytosis is detected in relation to a non-lympho hematopoietic malignant tumor, G-CSF-producing tumor should be considered and FDG–PET scan is recommended for early detection. Chemotherapy for G-CSF-producing tumor must be conducted as soon as possible.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Granulocyte-colony-stimulating factor (G-CSF)-producing tumor causes remarkable peripheral leukocytosis and neutrophil production in bone marrow. G-CSF-producing tumor was first described by Robinson [1] in 1974, and has been described in lung cancer and gastroenterological tumors. G-CSF-producing tumor is a rare condition but its aggressive growth results in very poor prognosis. Autopsy study described the prognosis as within 6 months in 4 cases [2]. Therefore, detection and treatment of the tumor should be as early as possible.
The first G-CSF-producing gastric cancer was reported by Obara et al. [3] in 1985. Although 37 case reports of G-CSF-producing gastric cancer had been described by 2008 [4–8], the rarity of the disease makes its features unknown.
In this report, aggressive recurrence of gastric cancer after curative resection as G-CSF-producing tumor will be described. The recurrent tumors and their rapid progression were apparent on fluorodeoxyglucose (FDG)–positron emission tomography (PET) scan. Additionally, G-CSF-producing gastric cancer will be reviewed.
Case report
A 62-year-old Japanese man presented with a history of inter-digestive epigastralgia. His family history included a sister with gastric cancer. Serum levels of carcinoembryonic antigen (CEA) and carbohydrate antigen (CA) 19-9 were elevated, at 5.6 ng/ml (normal range <5 ng/ml) and 479 U/ml (normal range <37 U/ml), respectively.
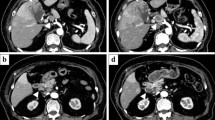
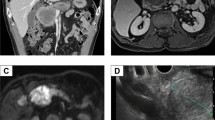
Gastroendoscopy (Fig. 1) and gastrography (Fig. 2) demonstrated a type-2 tumor in the upper region of the stomach (Fig. 2). The initial diagnosis was poorly differentiated adenocarcinoma on biopsy, and was not a G-CSF-producing tumor at that time. CT scan demonstrated wall thickening of the stomach and lymph node swelling around the stomach. FDG–PET scan demonstrated uptake in the stomach and surrounding lymph nodes (Fig. 3a).
a Fluorodeoxyglucose (FDG)–positron emission tomography (PET) scan demonstrated uptake in the gastric cancer and lymph node. b On the 19th postoperative day (POD), recurrence of the cancer in the liver and lymph nodes around the aorta was demonstrated. c On the 49th POD, aggressive recurrence occupied the upper abdominal cavity
The patient underwent total gastrectomy with splenectomy and extended lymph node dissection (D2). At surgery, there were no abnormalities in the liver, common bile duct, or peritoneum.
Macroscopic examination showed a type-2 gastric cancer measuring 8.0 cm × 8.2 cm (Fig. 4). Microscopic examination demonstrated the tumor to be a poorly differentiated adenocarcinoma without excessive infiltration of neutrophils (Fig. 5). Immunohistological examination for G-CSF was negative. According to the TNM classification, the stage of the cancer was T3N2M0 (Stage IIIB), and curability B was obtained.
The clinical course with leukocyte count and serum level of C-reactive protein (CRP) are shown in Fig. 6. Postoperatively, CEA was within the normal range, at 1.6 ng/ml and CA19-9 was decreased but remained above the normal range, at 61 U/ml. Although postoperative adjuvant chemotherapy by S-1 (TS-1, Taiho Pharmaceutical, Tokyo) 100 mg/day was started, the deteriorating condition of the patient prevented drug administration and even having meals. On the 19th postoperative day (POD), to confirm resection of the cancer, FDG–PET scanning of the whole body was performed. Surprisingly, new areas of uptake in the liver and lymph nodes around the aorta were detected (Fig. 3b). Without any signs of infection, leukocytosis with a leukocyte count of 54,300/mm3 was observed (Fig. 6), and tumor markers on the 47th POD were: CEA <1 ng/ml, CA19-9 67 U/ml, alpha feto protein (AFP) 4 ng/ml (normal range <10 ng/ml), and CA125 105 U/ml (normal range <35 U/ml). On the 49th POD, FDG–PET scan detected remarkable uptake in the whole upper abdomen (Fig. 3c). Finally, the leukocyte count reached 125,200/mm3 and G-CSF was elevated to 28 pg/ml (normal range <18.1 pg/ml) on the 54th POD. The patient died of multiple liver metastases and carcinomatosis peritonitis only 56 days after surgery. Permission for necropsy was not obtained.
Postoperative course showing changes in the leukocyte count and serum level of C-reactive protein (CRP). Leukocyte counts are plotted as a logarithmic graph. The additional line shows exponential regression. Serum levels of CRP are plotted as a bar chart. PET fluorodeoxyglucose–positron emission tomography
Discussion
Remarkable leukocytosis accompanying malignant tumor without inflammatory disease suggests G-CSF-producing tumor, even after the resection of a non-G-CSF-producing tumor. In a study of 439 autopsy cases, 1.2% of non-lympho hematopoietic malignant tumors with leukocytosis showed a G-CSF or granulocyte macrophage colony-stimulating-factor-producing tumor [2].
Granulocyte colony-stimulating-factor-producing gastric cancer is rare. Sporadically reports are available, and most of the reports are written by Japanese, however, it may not mean difference of racial or geographic prevalence but of recognition among society. To our knowledge, 37 cases of G-CSF-producing gastric cancer have been reported [4–8]. Among these, men:women was 31:6 and median age was 68 (45–92). Among 36 cases described histologically, while 17 of the cases of G-CSF-producing gastric cancer showed poorly differentiated adenocarcinoma, another 3 cases showed a poorly differentiated component within adenocarcinoma. Three cases showed undifferentiated adenocarcinoma, 3 showed moderately differentiated adenocarcinoma, and 1 showed well differentiated adenocarcinoma. Adenosquamous cell carcinoma was shown in 5 cases and the prevalence of adenosquamous cell carcinoma of the stomach is relatively high [6]. Eto et al. [4] described double G-CSF-producing gastric cancer. Prognosis of G-CSF-producing gastric cancer is very poor and the median survival was 14 months (95% CI 8–16 months) among 30 cases with described prognosis. A few cases has been reported with no recurrence after surgery [9, 10], then early detection and curative resection possibly resulted in cure of G-CSF-producing gastric cancer.
To diagnose G-CSF-producing tumor, histological confirmation of leukocytosis is needed but our case was not confirmed by histological examination. Although histological examination of the gastric cancer of this case did not show G-CSF-producing tumor, explosive leukocytosis and elevation of G-CSF with normal CRP level were observed at the early postoperative period (Fig. 6). Recurrence of the tumors and their rapid progression were detected by FDG–PET. Therefore, we concluded that the recurrent tumor was a G-CSF-producing tumor.
In this case, G-CSF-producing character was acquired after surgery, because the start of leukocytosis seemed to be about 30 days after surgery (Fig. 6) and the CEA and CA19-9 were decreased and not elevated when leukocytosis developed after surgery. Such a change in the tumor behavior suggests a change in the nature of the tumor cells or of the cell populations. Recurrence as a G-CSF-producing tumor has been reported in gastric cancer [2, 11]. However, the trigger causing the change to G-CSF-producing tumor is not yet known. To elucidate such a phenomenon, further studies are needed.
Fluorodeoxyglucose–positron emission tomography scan apparently demonstrated recurrence and rapid progression of the G-CSF-producing tumor. Whereas G-CSF interferes with FDG uptake in bone marrow [12], accumulation of FDG in G-CSF-producing tumor occurs not only in the tumor cells but also in aggregating neutrophils [13]. In this case, FDG–PET scan detected the recurrent tumor before onset of leukocytosis (Figs. 3b, 6). Furthermore, FDG–PET scan can search the whole body for the tumor whether it is primary or metastatic. Therefore, FDG–PET scan is useful for detection and evaluation of the progression of G-CSF-producing tumor.
Although CT scan and ultrasonography (US) are often used for surveillance of recurrent tumor after resection, they are not as useful as FDG–PET scan for detection of recurrent G-CSF-producing tumor in its early phase. As in our case, recurrent G-CSF-producing tumor is mainly local recurrence and liver metastasis [4–8]. CT and US are useful to detect anatomical change or to compare with a previous study, therefore they are not good for local recurrence after surgery or diffuse infiltration of the tumor, especially in the early phase.
After detection of the G-CSF-producing tumor postoperatively, additional chemotherapy should be conducted. In our case, burst leukocytosis was started about 30 days after surgery, despite the start of S-1 therapy (Fig. 6). Thereafter, the patient died within 26 days of the onset of leukocytosis. The aggressive recurrence and poor prognosis of G-CSF-producing tumor has been described previously [7, 11, 14]. Therefore, therapy for G-CSF-producing tumor is urgently needed. However, definitive chemotherapy for G-CSF-producing tumor has not yet been established, because the rarity and aggressive progression of G-CSF-producing tumor has not allowed sufficient clinical research. Although S-1 is established as an adjuvant therapy for gastric cancer [15], it was not effective in this case, as in the report by Endo et al. [8]. They reported irinotecan followed by paclitaxel provided a comparatively stable period of about a year and a half [8]. Although that was a single case report, it may be worth trying such a regimen for G-CSF-producing tumor.
In conclusion, G-CSF-producing tumor is a rare but aggressive disease, particularly as a recurrent tumor. If leukocytosis is detected related to non-lympho hematopoietic malignant tumor, G-CSF-producing tumor should be considered, and FDG–PET scan is recommended for early detection. Then, chemotherapy for G-CSF-producing tumor must be conducted as soon as possible.
References
Robinson WA (1974) Granulocytosis in neoplasia. Ann N Y Acad Sci 230:212–218
Kojima K, Nakashima F, Boku A et al (2002) Clinicopathological study of involvement of granulocyte colony stimulating factor and granulocyte–macrophage colony stimulating factor in non-lymphohematopoietic malignant tumors accompanied by leukocytosis. Histol Histopathol 17:1005–1016
Obara T, Ito Y, Kodama T et al (1985) A case of gastric carcinoma associated with excessive granulocytosis. Production of a colony-stimulating factor by the tumor. Cancer 56:782–788
Eto T, Kuroda S, Takahashi M et al (2006) A case of granulocyte-colony stimulating factor producing multiple gastric carcinoma (in Japanese). Jpn J Gastroenterol Surg 39:457–463
Uji Y, Kusano T, Iida H et al (2006) A case of granulocyte-colony stimulating factor producing gastric carcinoma (in Japanese). Jpn J Gastroenterol Surg 39:653–659
Sato T, Yamada R, Yamamoto N et al (2007) A case of granulocyte-colony stimulating factor producing gastric cancer (in Japanese). Jpn J Gastroenterol Surg 40:169–174
Yokoyama T, Hyodo M, Hosoya Y et al (2005) Aggressive G-CSF-producing gastric cancer complicated by lung and brain abscesses, mimicking metastases. Gastric Cancer 8:198–201
Endo K, Kohnoe S, Okamura T et al (2005) Gastric adenosquamous carcinoma producing granulocyte-colony stimulating factor. Gastric Cancer 8:173–177
Katsuda M, Tabuse Y, Oka M et al (2004) Chouki seizon wo eta G-CSF sansei-igan no ichirei (in Japanese). Jpn J Gastroenterol Surg 37:1224
Nasu M, Maekawa H, Sato K et al (2004) Two cases of gastric cancer with marked elevation of serum granulocyte-colony stimulating factor (in Japanese). Nihon Rinsho Geka-igakkai Gakkai Zasshi 65:1823–1827
Yamano T, Morii E, Ikeda J et al (2007) Granulocyte colony-stimulating factor production and rapid progression of gastric cancer after histological change in the tumor. Jpn J Clin Oncol 37:793–796
Mayer D, Bednarczyk EM (2002) Interaction of colony-stimulating factors and fluorodeoxyglucose f(18) positron emission tomography. Ann Pharmacother 36:1796–1799
Morooka M, Kubota K, Murata Y et al (2008) (18)F-FDG-PET/CT findings of granulocyte colony stimulating factor (G-CSF)-producing lung tumors. Ann Nucl Med 22:635–639
Takami K, Miura K, Takeuchi H et al (2008) Granulocyte-colony stimulating factor-producing pancreatic cancer: report of a case. Surg Today 38:453–457
Sakuramoto S, Sasako M, Yamaguchi T et al (2007) Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med 357:1810–1820
Conflict of interest statement
No author has any conflict of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Kawaguchi, M., Asada, Y., Terada, T. et al. Aggressive recurrence of gastric cancer as a granulocyte-colony-stimulating factor-producing tumor. Int J Clin Oncol 15, 191–195 (2010). https://doi.org/10.1007/s10147-010-0023-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-010-0023-3