Abstract
The spatial structure of populations determines the relative importance of reproduction, survival and movement on population dynamics. However, the mechanisms by which local individuals and immigrants interact and the subsequent effects of immigrants on productivity are poorly known. We developed an integrated population model (IPM) to study the extent and consequences of immigration on the dynamics of a neotropical migrant (American redstart, Setophaga ruticilla) over an 11-year period in Ontario, Canada. New immigrants represented the majority of the study population each year with higher immigration rates for males than females and for first-year breeders than breeders in their second year or older. Immigration was negatively density dependent, with immigrants replacing previously established breeders in a compensatory manner following their death or emigration. Because of the tradeoff between immigration and apparent survival, neither had a strong influence on population growth and reproductive output was most strongly correlated with a change in abundance between years. However, if immigration ceased, the study population would become locally extinct within 7 years and thus immigrants were essential for local population persistence. We found no evidence for reduced breeding success when immigrants represented a higher proportion of the study population. Our research highlights the importance of movement in the stability of open populations and the strong correlation between the fates of local breeders and the number of immigrants entering the population. We recommend the use of IPMs to address the spatial scale over which immigration occurs and how different scales influence its contribution to population dynamics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The extent to which population dynamics are determined by within-site processes (reproduction, survival) versus among-site processes (immigration, emigration) should differ depending on where a population lies along a continuum of spatial structure (Hanski and Gilpin 1997; Thomas and Kunin 1999; Bowne and Bowers 2004). This continuum ranges from isolated populations with low immigration through a series of patchy populations with varying connectivity to a highly interconnected network with frequent movement among patches within a larger population (Harrison and Taylor 1997; Thomas and Kunin 1999). The ability of a species to disperse relative to the heterogeneity of the habitat in the ecosystem will largely influence where a population falls along this continuum (Addicott et al. 1987; Bowne and Bowers 2004). For a given species and ecosystem, the relative influence of movement on population dynamics will also depend on the spatial scale at which a study is conducted (Camus and Lima 2002; Bowler and Benton 2005). In particular, as the spatial scale increases, immigrants will typically comprise a lower proportion of the individuals in a patch and thus within-patch processes become relatively more influential.
Early studies on the importance of movement for populations focused mainly on the influence of immigration in systems with a clear distinction between habitat patches and an inhospitable intervening matrix (Brown and Kodric-Brown 1977; Fahrig and Merriam 1985; Hanski 1998). Numerous animal species inhabit environments with such a spatial structure either naturally (e.g., freshwater ponds) or due to anthropogenic causes (e.g., forest patches in agricultural landscapes). In these types of environments, immigration can provide an essential rescue effect allowing for population persistence even when immigration rates are relatively low (Stacey and Taper 1992; Vilá et al. 2003; Sanz-Aguilar et al. 2016). Less well known is how local demographic processes (i.e., reproduction and survival) and movement interact to drive patch-level population dynamics when there is a high degree of connectivity among patches of a larger population. Many species occupy landscapes where it can be difficult to distinguish between habitat and non-habitat, and individuals may easily move among preferred patches (Thomas and Kunin 1999; Franklin et al. 2002). Empirical studies based in patches of large, open populations support the high extent and influence of movement on patch population dynamics (Schaub et al. 2012, 2013) but in general few studies have examined the role of within vs. among patch processes in these situations.
There are several potential mechanisms by which local individuals and immigrants might interact to influence local abundance within a patch. One possibility is that immigration is negatively density-dependent (Massot et al. 1992; Sæther et al. 1999) with higher immigration following a greater loss of local residents due to mortality or emigration. Negative density-dependent immigration has been observed in a range of terrestrial and aquatic taxa (Massot et al. 1992; Gundersson et al. 2002; Greathouse et al. 2005; Wilson and Arcese 2008; Schaub et al. 2010; Turgeon and Kramer 2012). Because immigrants are at least partially compensating for the loss of local individuals, this process limits declines in abundance within the patch (Brown and Kodric-Brown 1977; Stacey and Taper 1992; Püttker et al. 2011) and can help to stabilize the broader population system (Sæther et al. 1999; Bowler and Benton 2005). Immigration may also be positively density dependent and increase following years of higher local productivity or survival. Positive density-dependent immigration may occur if individuals use conspecific presence as an indicator of patch quality (Stamps and Krishnan 2005; Betts et al. 2008) but can be destabilizing if immigration declines with decreasing densities leading to local patch extinction (Sæther et al. 1999). Immigration may also be unrelated to local demographic processes, for instance with populations that occupy ephemeral habitats where there is a high degree of inter-annual movement in the system and little correlation between within and among patch processes.
Our limited knowledge on how movement affects the dynamics of open populations with high patch connectivity is due in part to the difficulty in estimating immigration rate, defined as the proportion of the recipient population represented by immigrants. A recently developed technique, integrated population modeling (IPM, Brooks et al. 2004; Schaub et al. 2007; Abadi et al. 2010), allows for the estimation of immigration rate by combining data on abundance and demographic rates under a single modeling framework. The estimate of apparent survival from live encounter mark-recapture techniques incorporates mortality and permanent emigration (Lebreton et al. 1992). With additional data on breeding productivity, the only unknown demographic parameter that remains is immigration, but this can be estimated because the abundance data in an IPM represents the sum of all demographic processes. This technique not only allows for an unbiased estimate of immigration rate but also the potential to test hypotheses on how immigration influences population dynamics (Abadi et al. 2010; Schaub et al. 2012).
We developed an IPM to examine the extent of immigration and how it influenced the population dynamics and population-level productivity of a Neotropical migratory bird, the American redstart (Setophaga ruticilla). This species has served as an important model for the study of population limitation and regulation in migratory animals, with previous research focusing primarily on factors that influence reproduction and survival (McKellar et al. 2014, 2015; Marra et al. 2015; Sherry et al. 2015). American redstarts are distributed across boreal and eastern hardwood ecosystems of North America and select early to mid-successional deciduous and mixed forests (Sherry and Holmes 1997). Like many other forest songbirds in these ecosystems, American redstarts are not localized in discrete habitat patches but are more broadly distributed across forest ecosystems containing different successional stages. Therefore, they provide an excellent model species to understand the extent and influence of movement on the dynamics of large, open populations.
We first used the IPM to quantify annual variability in the rates of immigration by age and sex, and examined the contributions of immigration, reproduction and apparent survival to annual population growth. We then tested whether immigration patterns were negatively or positively density dependent as described by the hypotheses above. Finally, we examined whether average breeding success declined when immigrants represented a higher proportion of the study population. While the addition of immigrants can provide numerical stability to a population, it is possible that average breeding success declines when immigrants represent a larger fraction of the population. Several factors might contribute to lower productivity in this situation, including increased agonistic interactions among immigrants and residents as new territories are established (Smith and Ivins 1983; Hayes et al. 2004), lower familiarity of immigrants to the local environment (Stamps 1987), and the possibility that immigrants are lower quality individuals that were only able to secure territories after vacancies arose (McKellar et al. 2013).
Methods
Study area and sampling methods
We conducted field work for this project at the Queen’s University Biological Station (QUBS), near Chaffey’s Lock, ON, Canada (44°34′N, 76°19′W) from May–July 2001–2011. American redstarts were monitored in a mixed-deciduous forest consisting primarily of sugar maple (Acer accharum) and Eastern hop hornbeam (Ostrya virginiana). The approximately 100 ha study area consists of a 25 ha campground and a largely undisturbed 75 ha forest separated by a two lane country road (for a detailed description see McKellar et al. 2015). The study site is nested within a broader area of heterogeneous habitat consisting of forest, wetlands, lakes, agriculture and urban areas. Hereafter we refer to the individuals in the 100 ha study area as the ‘study population’. American redstarts exhibit protandry, with males arriving on the breeding grounds several days before females. Upon arrival, males begin singing and establish territory boundaries. From May 1–31, we surveyed the study population from 0600 to 1200, mapping territory boundaries of males, recording whether females were present on territories and identifying any previously banded individuals that returned. The abundance of males and females provided our annual count estimates that were used in the IPM. We also recorded pairing dates and the location of the nest, which we monitored to record laying date, number of eggs laid, hatching success, and fledging success (see McKellar et al. 2014).
Birds were captured as soon as possible upon arrival using mist nets and simulated territorial intrusions consisting of song playback and a decoy or fledgling distress calls. All captured individuals were banded with a single Canadian Wildlife Service-issued aluminum leg band and a unique combination of 2–3 colour bands. Nestlings were banded with a single aluminum band, generally between days 5 and 8 after hatching. Age classes for males were determined based on plumage coloration. Males exhibit delayed plumage maturation, with second-year (SY) males exhibiting female-like grey and yellow plumage and after-second-year (ASY) males exhibiting black plumage with orange patches on the wings, tail, and flanks, and a white underside. Female age can only be reliably determined in the hand and was based on retrix coloration and wear, and molt limits when individuals were captured (Pyle 1997).
Integrated population model
The IPM combined three data sets of demographic information: (1) annual breeding counts of females and males in the study population, (2) annual number of fledged young produced by a sample of breeding-age females and (3) an encounter history of colour-banded individuals to estimate apparent survival. The core of the IPM is a state-space model with a state process that projected the true but unknown development of the population over time as a function of the demographic rates and an observation process that linked the observed counts to the true size.
We used a pre-breeding count with separate projections of population size for females and males. We assumed that females bred for the first time at 1 year of age (i.e., as an SY) (Sherry and Holmes 1997). As noted above, females are not easily distinguished by age, and we previously found similar estimates of apparent survival among SY and ASY females at this site (McKellar et al. 2015). Therefore, our annual population counts included breeding-age females (C f ) and the demographic information allowed us to estimate the annual number of female fledglings (FL f ), local SY female recruits (REC f ), local ASY returning females (RET f ) and female immigrants in at least their second year (I f ). Because male American redstarts exhibit delayed plumage maturation, we were able to distinguish annual male counts between second-year (C sym ) and after-second-year (C asym ) individuals (Sherry and Holmes 1997). This distinction allowed us to estimate additional male age classes: annual number of male fledglings (FL m ), local SY male recruits (REC sym ), SY male immigrants (I sym ), returning males that bred at the site in the previous year (RET asym ) and ASY male immigrants in at least their second breeding year (I asym ).
Fledgling production was estimated as FL tot,t ~ Poisson (frate t · N f,t ) where FL tot,t is the total number of fledglings in year t, frate t is the number of fledglings per breeding female in year t and N f,t is the number of breeding females in year t. The number of female fledglings (FL f,t ) in year t was then estimated as a binomial process assuming a 50% probability of being female. Male fledgling number (FL m,t ) in year t was estimated as the difference between total and female fledglings.
For each sex, the abundance of local SY recruits was estimated as a binomial process with the number of recruits in year t + 1 dependent on the number of fledglings produced in year t, a first year apparent survival probability of juveniles that was assumed to be the same for both sexes due to low return rates (ϕ juv, t ) and the probability that a returning juvenile was a male (pMale). The encounter history for juveniles was based only on marked nestlings that were known to have fledged. The number of returning individuals of each sex in year t + 1 was also estimated as a binomial process based on the number of SY males, ASY males and females in year t and the apparent survival probabilities for each age-sex class from year t to t + 1 (ϕ sym,t , ϕ asym,t , and ϕ f,t respectively). The total number of individuals in year t also included immigrants that were assumed to enter the population just prior to breeding in year t and contributed to reproduction in that year. Immigration was assumed to follow a Poisson process with I a,s,t ~ Poisson (ω a,s,t ) with ω a,s,t equal to the expected number of immigrants entering in year t for age class a and sex s. We chose to estimate the number of immigrants rather than an immigration rate because precision tends to be higher for the former (Schaub and Fletcher 2015). Immigration rate was subsequently measured as a derived parameter (see below). The total number of individuals for each sex at the start of each year t was then the sum of local recruits, returning local breeders and immigrants, which in the case of males could be further distinguished between immigrants born in the prior year (i.e., SY) and those in at least their second breeding year (i.e., ASY). The observation model describes the relationship between the count of females, SY males, and ASY males, and the true population size. We assumed a Poisson distribution C a,s,t ~ Poisson (N a,s,t ) where N refers to the estimated annual population size of each age and sex class from the state process model.
We also used the IPM to measure two derived parameters (1) population growth rate based on breeding females, λ f,t = N f,t+1/N f,t and breeding males λ m,t = N m,t+1/N m,t and (2) the rates of female, SY male, and ASY male immigration, where the rate is estimated as the number of immigrants for the respective age-sex class divided by the total number of individuals in that class at time t. This definition is based on our expectation that immigrants will enter the study population just prior to breeding at the start of time interval t and is different from similar studies that estimated immigration rate based on the number of individuals entering the population in year t + 1 relative to the number of individuals that were present in year t (e.g., Schaub et al. 2013; Duarte et al. 2016). We also estimated female λ* without immigration using the following estimation as a derived parameter within the IPM:
Estimation of reproductive output and apparent survival
The time-varying demographic parameters were specified with a hierarchical structure where annual estimates of each parameter were assumed to originate from a random process with a common mean and temporal variance. For these estimates we used logit link functions for apparent survival and a log link for fledge rate. Each year we had complete reproductive fates for a sample of all territories in the study area. The nesting attempts from this sample were used to determine the annual fledging rate (fledge t ) ~ Poisson (frate t · F rep,t ) where the number of fledged young in the sample of territories (fledge t ) was the product of the fledge rate (frate t ) and the number of females monitored for reproduction (F rep,t ).
Age and sex-specific survival was estimated with a state-space likelihood and a multi-state representation (Lebreton et al. 1999, 2009) for juveniles (ϕ juv ), adult females (ϕ f ), SY males (ϕ sym ) and ASY males (ϕ asym ). With the multi-state representation for apparent survival, the state equation represents the state (i.e., age-sex class) of the individual:
where i is an individual, t is a time period and (Ω Zi,t−1, i, t, 1…S ) is a matrix of survival and transition probabilities from each state at time t − 1 to each state at time t with S representing the number of true states. Initially, an individual can move from any state to any other state but constraints are applied to the matrix probabilities such that biologically unreasonable transitions are not allowed (e.g., probability SY male to female = 0). The observation equation represents the observation of individual i at time t given its state at time t:
with O equal to the number of observed states. If an individual survives and returns to the study area it may be detected with re-sighting probability p. We used the earlier findings from McKellar et al. (2015) to inform variation in re-sighting probability and apparent survival by age and sex in this analysis. Re-sight probability does not differ between age classes but does differ between the sexes as males are more easily detected. Therefore, annual estimates of re-sight probability were estimated with a mean (μ) and temporal residual (ε) for each sex:
We previously found that apparent survival differs between the sexes and between SY males and ASY males but not between the two female age classes (McKellar et al. 2015). Therefore, apparent survival for the IPM was estimated as:
where annual survival for each age class a (males only) and sex s is estimated with a mean and temporal residual.
Model implementation
We fit the IPM using a Bayesian analysis with Markov Chain Monte Carlo Sampling in OpenBUGS version 3.2.3 (Lunn et al. 2000) implemented through R (version 3.2.3) using the package R2OpenBUGS (R Development Core Team 2004; Sturtz et al. 2005, model code provided in Electronic Supplementary Material [ESM] S2). With this approach, inference is based on sampling from the posterior distribution, which is proportional to the prior distribution and the likelihood from the data. We used non-informative priors for all model parameters except starting population size where the priors were weakly informative. We ran two chains for 25,000 iterations, discarded the first 15,000 as a burn-in and used a thinning rate of 2 to give 10,000 samples from the posterior distribution for inference. We assessed model convergence through the parameter history plots and R-hat convergence diagnostics (Sturtz et al. 2005). There are currently no goodness-of-fit tests for integrated population models (Schaub and Abadi 2011). To evaluate the fit of the mark-recapture model we used a bootstrap goodness of fit test in program MARK on a model with apparent survival varying by age, sex and time, and re-sighting probability varying by sex and time. The estimated \(\hat{c}\) from this model was 1.08 indicating no evidence for a lack of fit (Lebreton et al. 1992).
Assessing relationships between abundance, demographic rates and population growth
We used an approach similar to that employed by Schaub et al. (2012, 2013) to assess relationships among demographic rates and between demographic rates and population growth. For all samples from the posterior distributions we estimated the correlation between the two variables of interest thus incorporating sampling uncertainty in the estimation. This approach was used for two specific sets of tests. First, we examined the relationship between each rate at time t and population growth from time t to t + 1. Second, we tested for the influence of density dependence on immigration with a correlation between immigration rate in year t + 1 and the apparent survival probability of local males and females between years t and t + 1. Because a change in apparent survival would also influence the number of previous breeders returning to the study population in the following year, we also tested the relationship between the immigration rate in year t + 1 and the number of local individuals returning in year t + 1. Posterior distributions for the correlation coefficients are typically skewed and we present the posterior median of the correlation coefficient and the percent of the coefficient posterior mass above 0. For correlations of demographic rates and population growth, values near 1 reflect a high probability that a positive change in the demographic rate is correlated with positive population growth. For density dependent relationships, values near 1 and 0 indicate a high probability of positively density-dependent and negatively density-dependent relationships respectively.
Finally, we tested whether the proportion of immigrant males and females in year t had a negative influence on population level productivity (mean fledge rates) in year t. Because reproductive output at the population level is also influenced by density (McKellar et al. 2014), we included male abundance from the IPM as a covariate in this analysis. We used a similar approach as described above and used the simulations from the posterior distribution in a linear model with fledging rate as the response variable and, immigration rate and male abundance as predictors. This effect was tested for both female and male immigration rate and we report the 95% CI for the beta coefficient in each case.
Results
Population growth and demographic rates
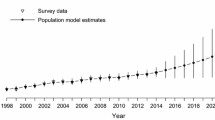
Mean population growth (λ) in the QUBS study population was 1.02 for females [95% CI (0.98, 1.05)] and 1.01 for males [95% CI (0.97, 1.06)] (Fig. 1). The estimated total population size varied from a low of 73 individuals to a high of 94 (Table S1 in ESM). The fledging rate averaged 1.68 [95% CI (1.17, 2.37)] fledglings per female and varied from a high of 2.92 in 2001 to a low of 0.68 in 2011. The apparent survival probability of juveniles was low, averaging only 0.06 [95% CI (0.03, 0.10)], while the probability that a returning juvenile that was detected in the study area was a male was 0.68 [95% CI (0.42, 0.90)]. Of 464 banded nestlings that survived to fledge, only 16 returned to the study population and were detected in a subsequent year with an average of 2.3 local recruits for each sex (range 1–4) per year. Average apparent annual survival for SY and ASY females combined was 0.38 [95% CI (0.25, 0.53)] and averaged higher than probabilities for males where the mean was 0.34 [95% CI (0.21, 0.48)] for ASY males and only 0.20 for SY males [95% CI (0.11, 0.37)]. Re-sighting probabilities were higher for males at 0.74 [95% CI (0.47, 0.92)] than for females at 0.34 [95% CI (0.17, 0.56)]. Estimates of temporal process variance for all demographic parameters are provided in Table S2 in ESM.
For females, the estimated average immigration rate (immigrants/total females) was 0.57 [95% CI (0.44, 0.69), Fig. 1] with both SY and ASY females combined. Across years, the female immigration rate varied from a low of 0.51 to a high of 0.66 (Fig. 1; Table S3 in ESM). Average immigration rates were similar for ASY males at 0.60 [95% CI (0.49, 0.69)] but higher for SY males at 0.78 [95% CI (0.59, 0.91)]. Thus, in all years the majority of breeding-age individuals for both sexes came from outside the study population (Fig. 1). The estimated mean lambda for females based only on within population rates of reproduction and survival was 0.42 [95% CI (0.30, 0.54)] and with this estimate, an initial population size of 40 females would drop to less than one in only 7 years if immigration ceased. Moreover, the highest estimate of lambda without immigration for any year was 0.49 [95% CI (0.32, 0.75)] in 2005 and thus the study population required immigrants to prevent a decline (i.e., to maintain λ ≥ 1) in all years.
Contributions of demographic rates to population growth
Annual fledge rate in year t had the strongest contribution to population growth of females (r = 0.40) and males (r = 0.44) with probabilities of a positive correlation for 93 and 95 percent of simulations (Table 1). The correlation between observed population growth and adult apparent survival was only slightly lower with r = 0.38 for both sexes and probabilities of a positive correlation for 83 and 87 percent of simulations for females and males respectively. There was no evidence for correlations with population growth for apparent survival of juveniles, SY males or immigration rate for any age and sex class (all r < 0.20 and probability of a positive correlation < 0.70). Thus, despite being essential for maintaining the size of the QUBS study population, immigration did not consistently contribute to higher population growth for either sex (Table 1).
Density-dependent effects on immigration
To test our hypotheses for density-dependent immigration, we examined whether the immigration rate for each age and sex class at the start of year t + 1 was correlated with the survival of local breeders from year t to t + 1 and whether immigration rate was correlated with the number of local breeders at the start of year t + 1. We found evidence for negative density-dependent effects in both cases. The median correlation between apparent survival from year t to t + 1 and immigration rate in year t + 1 for ASY males was r = −0.60 (P (r > 0) = 0.05, Fig. 2) and the correlation between ASY male immigration rate and the number of local males returning was r = −0.84 (P (r > 0) = 0.0007). For females the correlation between apparent survival and immigration rate was −0.61 (P (r > 0) = 0.09) and between the immigration rate and the number of local females was −0.89 (P (r > 0) = 0.0001). Because immigration rate is defined as a proportion of total individuals, the strong correlation between immigration rate and returning local individuals is to be expected if the number of immigrants remains constant or increases as the number of returning local individuals declines. In contrast to these patterns, the immigration rate of SY males was not correlated with apparent survival of SY (r = −0.08, P (r > 0) = 0.40) or ASY males (r = −0.04, P (r > 0) = 0.45) and only weakly correlated with the number of returning local males (r = −0.34, P (r > 0) = 0.16).
Posterior distribution histogram plots for the correlation of immigration rate with annual apparent survival and local abundance for ASY male and female American redstarts at Queen’s University Biological Station, 2001–2011. Estimates of the correlation are based on 10,000 replicates from the posterior distributions. The dashed line in each panel represents the median correlation coefficient
Effects of immigration rate on population level productivity
We hypothesized that productivity would be lower in years when a higher proportion of the population was comprised of immigrants but found no support for this hypothesis. The beta coefficient for the effect of female immigration rate on fledging rate was \(\hat \beta \) = 0.59 [95% CI (−2.55, 3.62)] and for the effect of male immigration rate was \(\hat \beta \) = 0.16 [95% CI (−2.71, 2.64)].
Discussion
Immigration has been one of the most difficult demographic rates to study, particularly in open populations where there is a high degree of connectivity among patches of suitable habitat. Using an integrated population model, we estimated annual rates of immigration and tested hypotheses on how immigration and within-patch processes (reproduction, apparent survival) interact to influence patch dynamics for a Neotropical migratory bird breeding in eastern North America. Immigration rates were high with immigrants representing on average 57% of females, 60% of after-second-year males and 78% of second-year males annually. We found strong support for the hypothesis that immigration was negatively density dependent with immigrants replacing previously established breeders in a compensatory manner following their death or emigration. Because of this relationship, immigration rate was not correlated with the growth of the study population. However, if immigration were to cease, the study population would become locally extinct in 7 years and thus immigration provided an essential rescue effect (Brown and Kodric-Brown 1977) allowing for stability around a carrying capacity despite low juvenile apparent survival and strong annual variability in adult apparent survival. American redstarts exhibit conspecific attraction (Hahn and Silverman 2006) and we have shown previously that small scale movements within the study area are common with individuals more likely to move towards areas with higher density (McKellar et al. 2015). However, at the entire patch scale there was no support for positive density-dependent immigration. While individuals may select breeding sites in closer proximity to one another, our results seem to indicate that there is still a broader patch scale carrying capacity that regulates immigration rate even if breeders are not uniformly distributed throughout the patch.
Our findings are similar to those from studies on other territorial species showing negative density-dependent immigration into a population. Schaub et al. (2013) found high rates of immigration for females (0.56 ± 0.02, mean ± SD) and males (0.43 ± 0.02, mean ± SD) in an open population of red-backed shrikes (Lanius collurio) with immigration rates negatively correlated with population density. Wilson and Arcese (2008) showed that male immigration rate in a song sparrow (Melospiza melodia) island metapopulation declined with increasing population size on the recipient islands. Experimental approaches have also been used to test the influence of density on immigration rate for non-avian taxa. Turgeon and Kramer (2012) found strong evidence for compensatory immigration in response to localized mortality in two damselfish species (Stegastes spp.) in Barbados. They also found that for one species, compensatory immigration was related to a perceived increase in habitat quality for immigrants and that it had an effect on the size of immigrant source populations. Gundersson et al. (2002) manipulated patch densities of root voles (Microtus oeconomus) and showed that immigration was negatively density-dependent and that individuals most likely to survive after immigration were those that had previously dispersed and successfully established in other patches. Other experimental examples of an increase in immigration following declines in local density include common lizards (Lacerta vivipara) in France (Massot et al. 1992) and freshwater fish in streams in Virginia, USA (Albanese et al. 2009).
Our study population is a strong sink that is dependent on immigration for persistence (Pulliam 1988) but how we interpret the reliance on immigration depends on the extent to which low apparent survival was driven by mortality or permanent emigration of local breeders (Runge et al. 2006). Our estimates of annual apparent survival are low relative to previous estimates for this species from other populations. Male apparent survival in our study population averaged only 0.20 and 0.34 for SY and ASY males respectively with substantial annual variation from 0.20 to 0.55 for the latter. Annual apparent survival for male American redstarts is more frequently in the 0.50–0.60 range (Johnson et al. 2006; Marra et al. 2015). Our estimate of female apparent survival was 0.38, which is more similar to, yet still lower than, estimates from these other studies at 0.40–0.50. Although poorly studied, average apparent juvenile survival is often in the 15–35% range for migratory passerines (e.g., Gardali et al. 2003; Duarte et al. 2014), but averaged only 6% in our study population. The low estimates of apparent survival for all age and sex classes suggest that emigration was likely high and especially so for males and juveniles. The causes and extent of inter-annual variability in emigration are poorly known but other studies have shown that emigration rates vary with prior reproductive success (Cline et al. 2013), environmental conditions on the breeding grounds (Rushing et al. 2015; Wilson et al. 2016) and population density (Kim et al. 2009).
We do not know the source of immigrants that filled vacancies in the habitat following mortality or permanent emigration of local breeders and recruits. Within the study population, the majority of inter-annual movements for established breeders that switched territories was less than 100 m but for movements greater than 100 m there was more variability in distance moved out to the limit of monitoring at 1.4 km (McKellar et al. 2015). This finding might suggest that most immigrants are from sites near the study area. Experimental studies of this species on the breeding and wintering grounds have shown that individuals will upgrade to higher quality habitat following the disappearance of territorial birds (Studds and Marra 2005; McKellar et al. 2013). After controlling for density-dependent effects on reproductive output we also found no evidence that years with a higher proportion of these immigrants led to a reduction in productivity at the population level. This appears to indicate that immigrants are able to quickly adapt to the new habitat and that they are not necessarily lower quality individuals than the local individuals they replaced. Additional study on reproductive success of immigrants and local birds at the individual level would be a useful complement to our study here at the population level.
Conclusions
Understanding the extent and scale of immigration and emigration has important implications for conservation because it affects the spatial scale at which monitoring and habitat protection needs to be considered for a population (Schaub et al. 2012; Duarte et al. 2016). If regular natal and breeding movement occurs among patches then we must not only consider management needs for a local patch where individuals breed but also for the broader landscape over which dispersal of juveniles and adults regularly occurs. The extent to which populations are synchronized over broader scales is related to the immigration rate per generation. In general, populations within patches are not independent if, on average, individuals occupy more than one habitat patch over their lifetime (Harrison 1991; Harrison and Taylor 1997). The movement rates in our study exceeded this level and thus not only would immigration have a strong influence on our study population but would also be expected to synchronize the dynamics of the broader population system (Hastings 1993).
Because our study took place in a single patch covering approximately 1 km2 we were not able to estimate how the strength and influence of within vs among-patch processes changes with spatial scale. This would be an interesting and important area for future study and integrated population models provide a methodology for this approach without having to track the movements of individuals across a large area. Collecting demographic data over large scales is difficult but it would be practical to use methods that account for detection probability to estimate abundance (Buckland et al. 2001; Royle 2004) along with a sample of data on reproduction and apparent survival over several years and with increasing spatial scale increments (e.g., 0.5, 1.5, 2.5 km2). This approach would only require a study area covering the largest scale but with sufficient sample sizes to estimate rates separately at the smaller scales. An IPM could then be used to estimate immigration rate and the contributions of immigration, reproduction and apparent survival to population growth at each scale, and thus allow us to estimate how movement and the relative importance of within vs among-patch processes changes as the spatial scale increases.
References
Abadi F, Gimenez O, Ullrich B, Arlettaz R, Schaub M (2010) Estimation of immigration rate using integrated üopulation models. J Appl Ecol 47:393–400
Addicott JF, Aho JM, Antolin MF, Padilla DK, Richardson JS, Soluk DA (1987) Ecological neighborhoods: scaling environmental patterns. Oikos 49:340–346
Albanese B, Angermeier PL, Peterson JT (2009) Does mobility explain variation in colonisation and population recovery among stream fishes? Freshw Biol 54:1444–1460
Betts MG, Hadley AS, Rodenhouse N, Nocera JJ (2008) Social information trumps vegetation structure in breeding-site selection by a migrant songbird. Proc R Soc Lond B 275:2257–2263
Bowler DE, Benton TG (2005) Causes and consequences of animal dispersal strategies: relating individual behaviour to spatial dynamics. Biol Rev 80:205–225
Bowne DR, Bowers MA (2004) Interpatch movements in spatially structured populations: a literature review. Landsc Ecol 19:1–20
Brooks SP, King R, Morgan BJT (2004) A Bayesian approach to combining animal abundance and demographic data. Animal. Biodivers Conserv 27:515–529
Brown JH, Kodric-Brown A (1977) Turnover rates in insular biogeography: effect of immigration on extinction. Ecology 58:445–449
Buckland ST, Anderson DR, Burnham KP, Laake JL, Borchers DL, Thomas L (2001) Introduction to distance sampling. Oxford University Press, Oxford
Camus PA, Lima M (2002) Populations, metapopulations and the open-closed dilemma: the conflict between operational and natural concepts. Oikos 97:433–438
Cline MH, Strong AM, Sillett TS, Rodenhouse NL, Holmes RT (2013) Correlates and consequences of breeding dispersal in a migratory songbird. Auk 130:742–752
Duarte A, Hines JE, Nichols JD, Hatfield JS, Weckerly FW (2014) Age-specific survival of male golden-cheeked warblers on the Fort Hood Military Reservation, Texas. Avian Conserv Ecol 9:4
Duarte A, Weckerly FW, Schaub M, Hatfield JS (2016) Estimating golden-cheeked warbler immigration: implications for the spatial scale of conservation. Anim Conserv 19:65–74
Fahrig L, Merriam G (1985) Habitat patch connectivity and population survival. Ecology 66:1762–1768
Franklin AB, Noon BR, George TL (2002) What is habitat fragmentation? Stud Avian Biol 25:20–29
Gardali T, Barton DC, White JD, Geupel GR (2003) Juvenile and adult survival of Swainson’s Thrush (Catharus ustulatus) in coastal California: annual estimates using capture-recapture analyses. Auk 120:1188–1194
Greathouse EA, March JG, Pringle CM (2005) Recovery of a tropical stream after a harvest-related chlorine poisoning event. Freshw Biol 50:603–615
Gunderssen G, Andreassen HP, Ims RA (2002) Individual and population level determinants of immigration success on local habitat patches: an experimental approach. Ecol Lett 5:294–301
Hahn BA, Silverman ED (2006) Social cues facilitate habitat selection: American redstarts establish breeding territories in response to song. Biol Lett 11:337–340
Hanski I (1998) Metapopulation dynamics. Nature 396:41–49
Hanski I, Gilpin ME (1997) Metapopulation biology: ecology, genetics and evolution. Academic Press, San Diego
Harrison S (1991) Local extinction in a metapopulation context: an empirical evaluation. Biol J Linnean Soc 42:73–88
Harrison S, Taylor AD (1997) Empirical evidence for metapopulation dynamics. In: Hanski I, Gilpin ME (eds) Metapopulation biology: ecology, genetics and evolution. Academic Press, San Diego, pp 27–42
Hastings A (1993) Coupled interactions between dispersal and dynamics: lessons from coupled logistic equations. Ecology 72:896–903
Hayes LD, Lin YK, Solomon NG (2004) The effect of female prairie vole (Microtus ochrogaster) immigrants on space use of conspecific female residents. Am Midl Nat 151:88–92
Johnson MD, Sherry TW, Holmes RT, Marra PP (2006) Assessing habitat quality for a wintering songbird in natural and agricultural areas. Conserv Biol 20:1433–1444
Kim SY, Torres R, Drummond H (2009) Simultaneous positive and negative density-dependent dispersal in a colonial bird species. Ecology 90:230–239
Lebreton JD, Burnham KP, Clobert J, Anderson DR (1992) Modelling survival and testing biological hypotheses using marked animals: a unified approach with case studies. Ecol Monogr 62:67–118
Lebreton JD, Almeras T, Pradel R (1999) Competing events, mixtures of information and multistratum recapture models. Bird Study 46:S39–S46
Lebreton JD, Nichols JD, Barker RJ, Pradel R, Spendelow JA (2009) Modeling individual animal histories with multistate capture-recapture models. Adv Ecol Res 41:87–173
Lunn DJ, Thomas A, Best N, Spiegelhalter D (2000) WinBUGS—a Bayesian modeling framework: concepts, structure, and extensibility. Stat Comput 10:25–37
Marra PP, Studds CE, Wilson S, Sillett TS, Sherry TW, Holmes RT (2015) Non-breeding season habitat quality mediates the strength of density dependence for a migratory bird. Proc R Soc Lond B 282:20150624
Massot M, Clobert J, Pilorge T, Lecomte J, Barbault R (1992) Density dependence in the common lizard: demographic consequences of a density manipulation. Ecology 73:1742–1756
McKellar AE, Marra PP, Ratcliffe LM (2013) Starting over: experimental effects of breeding delay on reproductive success in early-arriving male American redstarts. J Avian Biol 44:495–503
McKellar AE, Marra PP, Boag PT, Ratcliffe LM (2014) Form, function and consequences of density dependence in a long-distance migratory bird. Oikos 123:356–364
McKellar AE, Reudink MW, Marra PP, Ratcliffe LM, Wilson S (2015) Climate and density influence annual survival and movement in a migratory songbird. Ecol Evol 5:5892–5904
Pulliam HR (1988) Sources, sinks and population regulation. Am Nat 132:652–661
Püttker T, Bueno AA, Dos Santos de Barros C, Sommer S, Pardini R (2011) Immigration rates in fragmented landscapes—empirical evidence for the importance of habitat amount for species persistence. PLoS One 6(11):e27963
Pyle P (1997) Identification guide to North American birds. Part 1. Slate Creek Press, Bolinas
R Development Core Team (2004) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. http://www.r-project.org. Accessed 20 Jan 2017
Royle JA (2004) N-mixture models for estimating population size from spatially replicated counts. Biometrics 60:108–115
Runge JP, Runge MC, Nichols JD (2006) The role of local populations within a landscape context: defining and classifying sources and sinks. Am Nat 167:925–938
Rushing CS, Dudash MR, Studds CE, Marra PP (2015) Annual variation in long-distance dispersal driven by breeding and non-breeding season climatic conditions in a migratory bird. Ecography 38:1006–1014
Sæther B-E, Engen S, Lande R (1999) Finite metapopulation models with density-dependent migration and stochastic local dynamics. Proc R Soc Lond B 266:113–118
Sanz-Aguilar A, Igual J, Tavecchia G, Genovart M, Oro D (2016) When immigration masks threats: the rescue effect of a Scopoli’s shearwater colony in the Western Mediterranean as a case study. Biol Conserv 198:33–36
Schaub M, Abadi F (2011) Integrated population models: a novel analysis framework for deeper insights into population dynamics. J Ornithol 152:S227–S237
Schaub M, Fletcher D (2015) Estimating immigration using a Bayesian integrated population model: choice of parameterization and priors. Environ Ecol Stat 22:535–549
Schaub M, Gimenez O, Sierro A, Arlettaz R (2007) Use of integrated modeling to enhance estimates of population dynamics obtained from limited data. Conserv Biol 21:945–955
Schaub M, Aebischer A, Gimenez O, Berger S, Arlettaz R (2010) Massive immigration balances high human induced mortality in a stable eagle owl population. Biol Conserv 143:1911–1918
Schaub M, Reichlin TS, Abadi F, Kéry M, Jenni L, Arlettaz R (2012) The demographic drivers of local population dynamics in two rare migratory birds. Oecologia 168:97–108
Schaub M, Jakober H, Stauber W (2013) Strong contribution of immigration to local population regulation: evidence from a migratory passerine. Ecology 94:1828–1838
Sherry TW, Holmes RT (1997) American Redstart (Setophaga ruticilla). In: Poole A (ed) The birds of North America online. Cornell Lab of Ornithology, Ithaca. Retrieved from the Birds of North America Online: http://bna.birds.cornell.edu/bna/species/277. Accessed 20 Jan 2017
Sherry TW, Wilson S, Hunter S, Holmes RT (2015) Impacts of nest predators and weather on reproductive success and population limitation in a long-distance migratory songbird. J Avian Biol 46:559–569
Smith AT, Ivins BL (1983) Colonization in a pika population: Dispersal vs. philopatry. Behav Ecol Sociobiol 13:37–47
Stacey PB, Taper M (1992) Environmental variation and the persistence of small populations. Ecol Appl 2:18–29
Stamps JA (1987) The effect of familiarity with a neighborhood on territory acquisition. Behav Ecol Sociobiol 21:273–277
Stamps J, Krishnan VV (2005) Nonintuitive cue use in habitat selection. Ecology 86:2860–2867
Studds CE, Marra PP (2005) Nonbreeding habitat occupancy and population processes: an upgrade experiment with a migratory bird. Ecology 86:2380–2385
Sturtz S, Ligges U, Gelman A (2005) R2WinBUGS: A package for running WinBUGS from R. J Stat Softw 12:1–16
Thomas CD, Kunin WE (1999) The spatial structure of populations. J Anim Ecol 68:647–657
Turgeon K, Kramer DL (2012) Compensatory immigration depends on adjacent population size and habitat quality but not on landscape connectivity. J Anim Ecol 81:1161–1170
Vilá C, Sundqvist AK, Flagstad Ø, Seddon J, Björnerfeldt S, Kojola I, Casuli A, Sand H, Wabakken P, Ellegren H (2003) Rescue of a severely bottlenecked wolf (Canis lupus) population by a single immigrant. Proc R Soc Lond B 270:91–97
Wilson AG, Arcese P (2008) Influential factors for natal dispersal in an avian island metapopulation. J Avian Biol 39:341–347
Wilson S, Alisauskas RT, Kellett DK (2016) Environmental and population effects on permanent emigration of Ross’s and Snow Geese from an Arctic breeding area. J Wildl Manag 80:117–126
Acknowledgements
We thank the many field assistants, technicians, and students who worked on this project over the years and an anonymous reviewer for providing valuable comments on an earlier version of this manuscript. Funding was provided by the National Science Foundation (PPM) and Natural Sciences and Engineering Research Council of Canada (LMR and students).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wilson, S., McKellar, A.E., Reudink, M.W. et al. Density-dependent immigration promotes population stability in a long-distance migratory bird. Popul Ecol 59, 169–178 (2017). https://doi.org/10.1007/s10144-017-0582-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10144-017-0582-5