Abstract
Radiotelemetry and unmarked occupancy modeling have been used to estimate animal population growth, but have not been compared for ungulates. We compared white-tailed deer (Odocoileus virginianus) population growth estimates from radiomarked individuals and occupancy modeling of unmarked individuals and evaluated advantages and disadvantages of each method. Estimates of population growth were obtained using remote camera (N = 54/year) detection/non-detection occupancy surveys of unmarked deer and from survival and recruitment data of radiomarked adult females (N = 87) and neonate fawns (N = 127) in a predominantly forested region of the Upper Peninsula of Michigan, USA, 2009–2011. We hypothesized that occupancy models and radiotelemetry data would have similar population growth trends because both methods sampled the same temporally closed population. Percent changes in camera trap data generally reflected finite population growth (λ) of radiomarked deer which increased (λ = 1.10 ± 0.01) from 2009 to 2010, but decreased (λ = 0.87 ± 0.02) from 2010 to 2011. Also, unmarked adult female abundance and fawn:adult female ratios generally reflected trends in radiomarked deer survival and recruitment. Royle–Nichols occupancy model abundance estimates had wide confidence intervals, which may preclude using this method from accurately estimating deer population growth. Radiotelemetry provided more precise population growth estimates, while allowing collection of vital rates and location data. However, the Royle–Nichols occupancy model may be preferred to radiotelemetry because it reflected yearly variation in population growth with reduced labor and no invasive marking. Researchers should consider the objectives and logistics of their study when choosing a specific method.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wildlife management and conservation commonly depend on monitoring population growth and demography (McCullough 1994; Bender 2006). For this reason researchers regularly develop and assess the accuracy, precision, and applicability of new or existing estimators of population growth and demography. Population growth is intrinsically related to animal abundance, which is the culmination of past and present survival, productivity, and migratory processes (Skalski et al. 2005). However, abundance is often difficult to reliably estimate due to species rarity, uneven distribution, or poor detectability (DeCesare et al. 2012). In lieu of abundance, demographic models incorporating individual estimates of vital rates (i.e., survival and recruitment) have been used as alternative estimators of population growth (e.g., Hatter and Bergerud 1991). Additionally, estimates of individual vital rates assist in interpreting population growth, particularly as their variation (e.g., age-specific survival; DelGiudice et al. 2006) can lead to changes in age structure (Skalski et al. 2005).
Age ratios are a fundamental component of population demography and provide information on productivity and mortality rates (Caughley 1974; Harris et al. 2008). For example, ungulate young:adult ratios are frequently used to index recruitment (DeCesare et al. 2012; Ikeda et al. 2013), which is the product of fecundity and survival of young (Gaillard et al. 2000). Researchers have argued that age ratios alone may be of limited use in interpreting population growth because multiple underlying vital rates influence ratios (Caughley 1974; McCullough 1994). Also, estimates of adult female survival are crucial to interpreting young:adult female ratios because adult female abundance is used as the denominator in the ratio. Further, using age ratios to interpret population growth depends on understanding the age structure of breeding and non-breeding females (DeCesare et al. 2012), which is often difficult to attain with recruitment estimation methods such as aerial surveys. Age-specific fecundity rates are therefore often sought to estimate the number of reproductive females (DelGiudice et al. 2007; Duquette et al. 2012) to include in ratios. Although limitations exist, age ratio estimates can be useful for understanding ungulate recruitment and population growth rates (Harris et al. 2008; DeCesare et al. 2012).
Ungulate population growth and structure are often difficult to estimate because ungulates exhibit behaviors (e.g., secretive or migratory) and use habitats (e.g., coniferous forest) which hinder many estimation methods (e.g., aerial counts; Storm et al. 2011). Numerous indices including trail counts (McCaffery 1976), browsing pressure (Morellet et al. 2001), pellet counts (Fuller 1991), and spotlight counts (Collier et al. 2007) have been used to monitor population trends and demographics because they are relatively easy and inexpensive to collect. However, indices are plagued with bias (e.g., defecation rates; Millspaugh et al. 2002) and have unknown relationships with population growth (Skalski et al. 2005). Therefore, numerous indexes have been developed which use marked and/or unmarked individuals to estimate population growth and provide correction factors for behavioral (e.g., imperfect detection) or survey (e.g., number of sampling units) limitations, allowing estimates to be converted to absolute population numbers. For example, indexes including genetic mark-recapture (Ebert et al. 2012), distance sampling (Anderson et al. 2013), aerial infrared camera surveys (Naugle et al. 1996; White et al. 2001; Haroldson et al. 2003; DeCesare et al. 2012), and remote camera surveys (Jacobson et al. 1997; Koerth and Kroll 2000; Roberts et al. 2006; Watts et al. 2008; Dougherty and Bowman 2012) have provided advancements in estimating ungulate population dynamics. However, these indexes often have assumptions that are commonly difficult to meet (e.g., perfect detection; Weckel et al. 2011), are expensive (e.g., aerial counts; Storm et al. 2011), difficult to apply over extensive areas (e.g., animal movements; Rowcliffe et al. 2009), or do not provide independent estimates of vital rates (e.g., camera surveys; Jacobson et al. 1997). For these reasons, radiotelemetry studies of marked animals have commonly been used to estimate population growth from key vital rates (e.g., DeCesare et al. 2012).
Advantages of using radiomarked animals to estimate population growth include estimates of population level survival (DelGiudice et al. 2006) and reproductive rates (Grund and Woolf 2004; Duquette et al. 2012) which may differ among animal ages. Survival and recruitment rates can then be incorporated into matrix models (e.g., Leslie matrix model; Leslie 1945) to estimate potential finite population growth rate (λ; Skalski et al. 2005). Additionally, radiotelemetry can provide locations of marked animals and determine whether they remain in the study area (i.e., geographic closure of population). However, capturing, marking, and monitoring animals can be labor intensive and expensive, often limiting the use of radiotelemetry. Additionally, several assumptions must be met to estimate population growth with radiotelemetry, including marking a random sample of the target population, independence of monitoring sessions of marked animals, working radiotransmitters are always located and do not impact survival (Millspaugh and Marzluff 2001). Due to these constraints, researchers often seek alternative methods which do not require radiomarking and monitoring animals to estimate population growth.
Occupancy modeling has provided a practicable method of estimating species abundance for many taxa (MacKenzie et al. 2006), using marked and/or unmarked individuals (e.g., Wibisono et al. (2011). These models are particularly useful because they incorporate detection/non-detection data of species which can be recorded by identifying physical sign (e.g., scat; Karanth et al. 2011). Surveying unmarked individuals can be particularly advantageous because observers are not always able to uniquely recognize individuals visiting multiple sites (Fiske and Chandler 2011). Unmarked animal abundance can be estimated with an occupancy model that accounts for imperfect detection (MacKenzie et al. 2006) and links the heterogeneity in detectability among sites with variation in site abundance (Royle and Nichols 2003; Fiske and Chandler 2011). Remote cameras have been a popular and useful tool to estimate population abundance (e.g., Watts et al. 2008) or structure (Ikeda et al. 2013) of unmarked individuals because factors such as thick canopy cover, expense, or species rarity preclude using methods such as mark-resight or mark-recapture methods. Further, the Royle–Nichols occupancy model (Royle and Nichols 2003) using detection/non-detection data can be equally or more useful than mark-recapture data for monitoring population trends (Tempel and Gutiérrez 2013). Despite the common use of radiotelemetry and occupancy modeling, to our knowledge no research has compared abundance estimates of ungulates from radiotelemetry data and the Royle–Nichols occupancy models using remote cameras.
Our overall goal was to compare white-tailed deer (Odocoileus virginianus) population growth estimates from radiomarked individuals and occupancy modeling of unmarked individuals and evaluate advantages and disadvantages of each method. Our specific objectives were to: (1) estimate fawn:adult female ratios and abundance of unmarked deer using remote camera surveys, (2) estimate finite rate of deer population growth using estimates of survival and fecundity from radiomarked adult females and recruitment of radiomarked fawns, and (3) compare adult female and fawn percent changes in abundance estimated with unmarked individuals with population growth rate of radiomarked deer. We hypothesized percent changes in camera trap data would reflect yearly variation in radiomarked deer population growth because we applied both methods to the same temporally closed population (Tempel and Gutiérrez 2013). We also predicted trends in age ratios estimated from camera trap data would follow trends in radiomarked adult female survival and fawn recruitment as similarly shown by previous studies (Roth and Amrhein 2010; Tempel and Gutiérrez 2013).
Methods
Study area
We conducted our study within a 248.9 km2 area of the south-central Upper Peninsula of Michigan (45°43′47″N, 87°4′48″W). Mean elevation was 185 m above sea level and topography was flat. Lowland forest was the prominent land cover and was mainly coniferous with typical winter cover or browse species including eastern white cedar (Thuja occidentalis), eastern hemlock (Tsuga canadensis), and balsam fir (Abies balsamea). Upland forest was a mixture of coniferous and deciduous stands, including pine (Pinus spp.), aspen (Populus spp.), maple (Acer spp.), and birch (Betula spp.). Grassland and shrubland were typically mixed and sparse across the study area. The western portion of the study area was interspersed with pasture and cropland. Road density was about 1.68 km/km2 and permanent water (i.e., rivers and stream) density was about 1.05 km/km2. Mean daily snow depth during the study was 9.60 cm (SE = 0.51) from January through March 2009–2011 based on data collected from a weather station sensor (Ultrasonic Depth Sensor, Judd Communications LLC, Salt Lake City, UT) we placed in the center of the study area. Mean monthly temperature from January through March 2009–2011 was −5.69 °C (range = −12.44–2.50) and from September through October was 7.03 °C (range = −17.80–16.10) based on data collected from a weather station sensor (model 107-L, Campbell Scientific Inc., Logan UT).
Deer capture and monitoring
We opportunistically captured female white-tailed deer (age ≥ 1.5 year, n = 101) in baited collapsible Clover traps (Clover 1956) or air-powered cannon nets from January to March 2009–2011. We manually restrained deer by collapsing the traps and hand-injected deer intramuscularly with a 3:1 (4 ml dose) or 4:1 (5 ml dose) combination of 100 mg/ml ketamine (Ketaset®; Fort Dodge Laboratories, Inc., Fort Dodge, IA) and 100 mg/ml xylazine (X-Ject E™; Butler Schein Animal Health, Dublin, OH; Duquette et al. 2013). We fitted pregnant deer (Duquette et al. 2012) with very high frequency radiocollars (Model 500, Telonics, Mesa, AZ, USA; Model 2610B, Advanced Telemetry Systems Inc., Isanti, MN) and vaginal implant transmitters (model 3930, Advanced Telemetry Systems Inc., Isanti, MN). Radiocollars were equipped with motion-sensitive mortality switches that indicated a collar was stationary for ≥8 h (e.g., possible mortality) and precise event transmitters that provided an estimate of the length of time the radiocollar was stationary. We extracted a lower canine for age estimation (Nelson 2001) conducted by the Michigan Department of Natural Resources, Wildlife Disease Laboratory. We categorized adult deer into age classes including yearlings (1.5 years old), prime-aged (2.5–6.5 years old), or late-aged (7.5–15.5 years old) because of potential survival (DelGiudice et al. 2006) and reproduction (Verme 1969) differences among these age classes. We administered 1.5 ml (10 mg/ml) or 2.2–7 ml (2 mg/ml) of yohimbine (Hospira©; Forest Lake, IL) intravenously or intramuscularly to antagonize effects of xylazine (Kreeger and Arnemo 2007). We released all deer at respective capture sites. We assessed variation in deer age structure among years using a Kruskal–Wallis test (Zar 1999) to evaluate potential survival and fecundity bias from age structure variation among years.
We captured neonatal fawns (≤15 days old) opportunistically or with vaginal implant transmitter searches (Carstensen et al. 2003) from May to July 2009–2011. We fitted fawns with expandable radiocollars (model 4210, Advanced Telemetry Systems Inc., Isanti, MN) with motion-sensitive mortality switches that indicated the collar was stationary for ≥8 h and precise event transmitters that recorded timing of the mortality event switch. We attached white ear tags (model agpf#1, Allflex®, DFW Airport, TX), identified sex, and estimated age and birth date based on new hoof growth (Carstensen et al. 2009). The Mississippi State University Institutional Animal Care and Use Committee (#09-004) approved all capture and handling procedures.
Each year we relocated radiomarked adult females ≥1 time weekly from capture to the last week of April, and adult females and fawns ≥5 times weekly from 1 May–31 August and ≥1 time weekly September through March, using truck-mounted and aerial radiotelemetry. We estimated adult female locations using ≥3 bearings collected within 20 min (Millspaugh and Marzluff 2001) and Location of a Signal 4.0 software (Ecological Software Solutions LLC). We excluded locations with error ellipses larger than the mean error (4,230 m2) of telemetry locations from all individuals conducting aerial and ground-based telemetry. We located adult and fawn radiocollars within 24 h (88 % of mortalities ≤6 h) of detecting a mortality signal and recorded if the signal was due to deer mortality or other causes (e.g., slipped collar). We censored deer with radiocollars that failed or were slipped before 52 weeks post-capture and excluded adult deer mortalities that occurred ≤14 days after capture as possible capture myopathy (Beringer et al. 1996).
Abundance
We used ArcMap 10.0 (Environmental Systems Research Institute, Inc 2010) and R package ks (Duong 2007) with unconstrained plug-in smoothing parameter to estimate the core area (50 % fixed kernel isopleth) used by radiomarked adult females with ≥30 radiolocations (Millspaugh and Marzluff 2001) from date of capture through 24 July 2009. We created a minimum convex polygon encompassing all telemetry locations of radiomarked adult females (n = 27) through 24 July 2009 to define the study area (248.9 km2) within which the population was considered temporally closed. We created a non-overlapping hexagonal grid across the study area where each hexagon equaled the mean 50 % fixed kernel home range size (1.58 km2) of deer. We considered each grid cell a camera sampling area. We developed our sampling grid using deer captured in 2009 because we wanted to base our grid on radiomarked deer space use and only had 2009 data available for the initial survey, but needed to maintain the same grid across years for comparison and occupancy modeling (MacKenzie et al. 2006). Also, we assumed that using the mean space use of adult females would provide a biologically relevant camera sampling area size which would reduce deer visiting >1 camera and allow us to better meet the model assumption that site occupancy is constant throughout the survey (Fiske and Chandler 2011). We used a generalized random-tessellation stratified design (Stevens and Olsen 2004) to assign half the cameras to randomly selected cells with no radiomarked doe use and remaining cameras to cells with known radiomarked deer use. We used this design to spatially balance our cameras across the study area and permit replacement of sample quadrats which were lost due to navigation hazards or non-applicable cells (e.g., lake). Also, cameras placed into cells with known deer use were intended to evaluate detection rates of radiomarked deer for future surveys.
Within each selected cell, we chose sites that had recent evidence of deer use (e.g., fecal pellets) and in vegetation (e.g., early successional forest) we believed would maximize the number of deer images. We pre-baited sites with 11.3 kg of corn 10 days before setting cameras and rebaited ad libitum during surveys. Each year we deployed 54 Cuddeback® Excite infrared cameras (Non Typical Inc., De Pere, WI) for 10 days at sites using a 5-min delay between images, which we considered adequate sampling time with continuous bait (Dougherty and Bowman 2012). We attached cameras to trees 75 cm above ground to approximate the mid-chest height of radiomarked females. We conducted annual surveys in September, but included the first week of October in 2011, and divided surveys into 2 or 3 consecutive 10-day periods due to logistical constraints. We recorded the number of fawns and adult females observed on images to estimate sex and age ratios. We converted adult female and fawn data, irrespective of marking (i.e., presence of ear tags or radiocollar), to detection/non-detection for each sample day to model abundance.
We used package unmarked (Fiske and Chandler 2011) for R 3.0.0 software (R Core Team 2013) to estimate adult female and fawn abundance with the occuRN function and Poisson distribution to characterize site abundance. The occuRN function models population abundance using detection/non-detection data of unmarked individuals by linking heterogeneity in detection probability to differences in site abundance (Royle and Nichols 2003). The Royle–Nichols model assumes that: (1) animal detections are independent, (2) detection probability of a single animal is assumed to be constant across time, and (3) occupancy state at a site remains constant throughout the season. We evaluated detection covariates including total area of deciduous, coniferous, or lowland forest in models because we assumed vegetative composition could influence deer behavior (e.g., foraging) around camera sites. To obtain covariates we clipped 2006 national landcover data (30 m pixels; United States Geological Survey 2011) within each camera grid cell. We combined mixed forest with deciduous forest into a deciduous forest classification and woody wetlands with emergent herbaceous wetland into a lowland forest classification using ArcMap 10.0 (Environmental Systems Research Institute, Inc 2010) to reduce over-parameterizing models. We used class metric analyses in program FRAGSTATS 3.4 (McGarigal and Marks 1995) to obtain landcover covariate estimates. We used a Spearman-rank test to evaluate covariates for collinearity prior to fitting models. We used daily detection/non-detection of adult females or fawns over the 10-day survey periods to develop encounter histories. We then used encounter histories within 4 abundance models to estimate density and abundance of adult females or fawns each year during 2009–2011, including 3 vegetation covariate models and a null model. We evaluated model fit using a parametric bootstrap (n = 100) method based on a Chi-square test statistic and ranked models using Akaike’s Information Criterion corrected for small sample size (AICc; Burnham and Anderson 1998). We retained deer density estimates only from the top model or model averaged estimates for models ≤2 AICc of the top model (Burnham and Anderson 1998). We estimated annual deer abundance by extrapolating our deer density site estimates to the study area and obtained a pooled estimate by summing adult female and fawn relative abundances in each year. We calculated percent change in abundance of adult females or fawns by subtracting annual abundance from previous year abundance and dividing by previous year abundance.
Reproduction and recruitment
We estimated annual fecundity of captured adult females using pregnancy-specific protein B (Duquette et al. 2012). We used the Pradel survival and recruitment model (Pradel 1996) in program MARK (White and Burnham 1999) to estimate annual radiomarked fawn recruitment from estimated birth date to 52 weeks. The Pradel model is a temporal symmetry model that uses a forward-time model for survival and a reverse-time model for recruitment that directly estimates λ from the temporal encounter histories of the age class (Pradel 1996). We used the Pradel model because probability of fawn relocation was <100 % and also did not include fawns that were censored before 52 weeks due to collar failure. We used a staggered entry design and categorized fawns into groups using 7-day birth periods with each annual start date equal to the earliest fawn birth date in that year. Encounter histories were developed using alive or dead status of fawns at 7-day intervals following their respective start dates. Within each year, we combined groups with analogous encounter histories into frequency sets. We estimated combined male and female fawn recruitment to facilitate comparisons with the camera trap data, which did not allow us to differentiate male and female fawns. We estimated annual fawn:adult female ratios using abundance estimates from remote camera surveys by dividing annual fawn abundance by adult female abundance. We presumed detectability of adult females and fawns was equal because surveys took place after fawns were completely mobile and functional ruminants (Verme 1989), allowing them to independently be captured by cameras. Additionally, we assumed fawn:adult female ratios observed during the survey were representative of ratios at time of annual recruitment because we observed minimal winter mortality rates for radiomarked fawns after completion of the camera surveys (Bender 2006).
Survival
We used Kaplan–Meier models in the survival package (Therneau 2012) in R 3.0.0 software to estimate annual radiomarked adult female survival over weekly intervals from capture to 52 weeks post capture. We used a staggered entry design and categorized deer into groups based on 7-day capture periods with each annual starting date equal to the earliest capture date. We used adult female age class as the predictor (time) variable and presence or absence of mortality as the response (status) variable. We used Cox proportional hazards models to assess capture week (i.e., group) as a covariate of survival and evaluated its influence using the likelihood ratio and Wald test, with α = 0.05.
Population growth rate
We developed a Leslie matrix (Leslie 1945) and used Poptools (Version 3.2; Hood 2011) to estimate λ of deer based on estimated adult female age class-specific survival and fecundity rates. We evaluated 2 annual models for years 2010–2011. We assigned prior-year annual age class-specific survival rates to prime- and late-aged deer and used late-aged rates for yearlings because we believed our original estimates (0.97–1.00) did not reflect mortality patterns of yearling deer in northern ranges (DelGiudice et al. 2002), likely due to limited sample size (n = 9) across years. We assigned same-year fecundity rates to prime- and late-aged deer, but we reduced yearling fecundity to 0.70 of annual estimates to account for reduced fecundity of this cohort (Duquette et al. 2012); we did not observe evidence of reproductive senescence (DelGiudice et al. 2007). We assumed fawns did not contribute to population growth because they did not reproduce during the study (Duquette et al. 2012) and a 1:1 sex ratio at birth (Verme 1983). We developed the age-structured projection matrix,
composed of elements for fecundity (F y = yearling, F p = prime-aged, F l = 7.5 years, F th i = subsequent late-aged values [8.5–15.5 years]) in the first row and age class-specific survival (S y = yearling, S p = prime-aged, S l = late-aged [7.5 years], S th i = subsequent late-aged values) on subsequent off-diagonal rows, for i th age. Using the projection matrix, population size of each age-class (n th i ) and age structure (N) can be calculated between times t and t + 1 from the equation:
We constructed the base model using only the female portion of the population using a density independent model with a year time step. The left eigenvector of A gives the expected relative contribution of a female in a given age group to future population growth. We used equation 7.94 in Skalski et al. (2005) to estimate standard errors for λ.
Results
Deer capture and monitoring
We captured and radiomarked 87 individual adult female deer (30, 26, and 31 in 2009–2011, respectively) in Clover traps (n = 81) or air-powered cannon nets (n = 6) from 7 January–21 April 2009–2011; 95 % of deer were captured before 20 March. Three deer were recaptured in subsequent years following their initial capture in 2009 (n = 1) or 2010 (n = 2) and are included in analyses, resulting in N = 90. Deer age was similar among years (Kruskal–Wallis test, H 2 = 1.3, P = 0.52) and mean age was 6.8 years (SD = 4.2). Nine deer were yearlings, 37 were prime-aged, and 44 were late-aged.
We captured and radiomarked 127 fawns and estimated their birth dates from 14 May to 23 June 2009–2011. Of the 127 fawns, we captured 93 fawns opportunistically and 34 during vaginal implant transmitter searches.
Abundance modeling
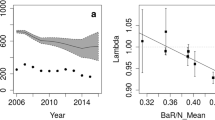
We recorded 9,812 images of deer from 54 cameras in 2009, 8,159 images from 54 cameras in 2010, and 6,749 images from 43 cameras in 2011. Eleven cameras malfunctioned during deployment in 2011. Camera density in 2009 and 2010 was 1/4.6 km2 and in 2011 was 1/5.8 km2. Radiomarked adult females were observed at 35 of 75 functioning cameras placed in known core use areas. No vegetation covariates of detection were correlated (Spearman-rank test, ρ = −0.07 to −0.46). Abundance models had no evidence of lack of fit (Chi-square goodness of fit test, χ 2 = 5.8–6.3, P = 0.17–0.98; Tables 1, 2). All covariates of deer detection competed (≤2 ΔAICc) with null models for adult females and fawns, but forest types were all negatively related to deer detection. Confidence intervals of detection coefficients for adult females and fawns overlapped indicating detection rates were similar between adult females and fawns. Mean adult female relative abundance was similar among years (Table 3) with densities of 4.8 ± 1.2, 4.9 ± 1.2, and 3.9 ± 0.7/km2 in 2009–2011, respectively. In contrast, fawn relative abundance was similar between 2009 and 2011, but greater in 2010 compared to other years (Table 3) with densities of 1.0 ± 0.2, 2.1 ± 0.4, and 1.3 ± 0.3/km2 in 2009–2011, respectively. Mean combined adult female and fawn population relative abundance was similar among years (Table 3) with densities of 5.8 ± 1.4, 6.8 ± 1.6, and 5.2 ± 0.9/km2 in 2009–2011, respectively.
Reproduction and recruitment
Overall deer fecundity was 94 % and 95 deer (87 of 88 adults, 8 of 10 yearlings, and 0 of 3 fawns) were confirmed pregnant; 6 females were not pregnant. Mean fawn recruitment increased by 97.0 % from 2009 to 2010, but standard errors showed estimates were similar. From 2010 to 2011 mean recruitment decreased 75.3 % and estimates did not overlap (Table 4). Similarly, mean fawn:adult female ratio increased 115.0 % from 2009 to 2010, but decreased 23.3 % from 2010 to 2011, though standard errors showed all ratios overlapped (Table 3). Mean fawn abundance supported trends in recruitment and ratios, increasing about 110 % from 2009 to 2010, but decreasing about 37 % from 2010 to 2011, with estimates overlapping only in 2009 and 2011 (Table 3).
Survival
Adult female survival was 0.78 in 2009 and 2010, but decreased 23 % from 2010 to 2011 (Table 4). Similarly, mean adult female abundance was nearly identical between 2009 and 2010, but decreased about 19 % between 2010 and 2011, though standard errors showed all estimates overlapped (Table 3). Estimated yearling survival was relatively constant and nearly 100 % across years, likely due to limited sample size. Prime- and late-aged deer survival was greatest in 2010, but late-aged deer exhibited a 33 % decrease in survival in from 2010 to 2011. Group (i.e., capture week) did not influence survival among years (Wald test, z = −0.26 to −1.34, P = 0.18–0.80). We censored 5 deer in 2011 due to presumed radiocollar failure, but all deer in other years were monitored for the complete annual period.
Population growth rate
Estimated λ based on radiomarked adult female survival and fawn recruitment showed the population increased about 10 % from 2009 to 2010, but decreased about 23 % from 2010 to 2011 (Table 4). Comparatively, mean pooled population growth estimated from camera trap data showed the population increased about 17 % from 2009 to 2010, but decreased about 24 % from 2010 to 2011, though standard errors showed all estimates overlapped (Table 3).
Discussion
Radiotelemetry data and Royle–Nichols occupancy models showed a similar trend in deer population growth, supporting our prediction. Moreover, these methods showed yearly population growth estimates of about the same magnitude, with generally similar increases between 2009 and 2010 and decreases between 2010 and 2011. Trends in abundance and survival estimates of adult females or abundance and recruitment of fawns also generally corroborated each other across years. We could not determine how well radiotelemetry or occupancy models estimate actual abundance because we did not have independent census estimates. Nonetheless, deer densities extrapolated from abundance estimates appeared to be reasonable based on our observations of deer prevalence across the study area. These results support the suggestion of Tempel and Gutiérrez (2013) that unmarked occupancy models using detection/non-detection data can provide reliable inferences on population trends. Although Royle–Nichols occupancy models appeared able to reflect trends in population growth, estimates had relatively wide variation, likely from using detection/non-detection data to estimate site density. Specifically, deer used some sites immediately and throughout the survey whereas others were not used at all, which likely produced greater heterogeneity in site density estimates. Nonetheless, monitoring trends in unmarked deer population growth may be of greater management interest than actual abundance estimates (DeCesare et al. 2012). Compared to occupancy modeling, radiotelemetry appeared to provide more precise estimates of deer population growth. Radiotelemetry likely provided greater precision because the fate of each animal was known and age-specific variation in survival and recruitment was directly incorporated into matrix models (Skalski et al. 2005).
Fawn:adult female ratios generally reflected radiomarked fawn recruitment, supporting our prediction and previous recommendations (Bender 2006; Harris et al. 2008) that age ratios are useful for understanding ungulate population dynamics. Also, remote cameras were useful for efficiently collecting large samples to estimate patterns in deer age ratios and supported results of previous camera-based surveys (Koerth et al. 1997; Ikeda et al. 2013). Although 2009 and 2010 age ratios closely reflected variation in fawn recruitment, the 2011 ratio did not proportionally decrease as much as 2011 recruitment. This discrepancy was likely due to the limited ability of occupancy models to estimate the decreased survival of adult females during 2011, resulting in a more even age ratio than expected based on recruitment. For this reason we recommend estimating consecutive years of age ratios (DeCesare et al. 2012) and concomitantly monitoring adult female survival via telemetry to better interpret age ratios (Caughley 1974; McCullough 1994). Additionally, we estimated age ratios when fawns were 4–5 months of age, which possibly biased abundance estimates and comparability among years because additional mortalities could have occurred after camera surveys. Despite this concern, mortality rates of radiomarked fawns after camera survey completion to 52 weeks of age were similar across years (10–15 %) and therefore mortality bias across years was likely minimal. Trends in fawn recruitment and age ratios were also supported by firearm deer hunter observations during November in the west-central Upper Peninsula, where hunters observed 44, 58, and 54 fawns per 100 adult females from 2009 to 2011, respectively (Michigan Department of Natural Resources 2009–2011). Finally, we did not separately estimate male and female fawn abundance due to our inability to differentiate sexes during camera surveys. In spite of this limitation we suggest trends in fawn abundance across years reflected variation in female fawn abundance because female fawns often have equal or greater recruitment than male fawns due to greater mortality rates of males (e.g., Jackson et al. 1972).
Occupancy modeling of unmarked deer using baited camera sites also has several biases which should be considered. First, we could not directly control for bait-induced heterogeneity in animal detection (Watts et al. 2008; McCoy et al. 2011) during camera surveys. Deer are known to form social hierarchies (Hawkins and Klimstra 1970), which could have potentially violated the assumptions of independent animal detections and constant detection probability of a single animal across time (McCoy et al. 2011). However, maternal defense of fawns should have been mainly dissolved by the time surveys took place (Ozoga et al. 1982) and continuous bait availability should have allowed all deer equal access to bait over time. Also, continuous bait potentially increased animal detection and decreased the width of abundance confidence intervals and may be preferred over unbaited cameras (Dougherty and Bowman 2012). Several images were often needed to identify the sex or age class of individual deer and bait likely increased duration of deer visits and facilitated identification. Finally, detection rates of radiomarked adult females were poor, with 35 radiomarked adult females detected at 75 cameras placed in known core use areas of radiomarked adult females. Poor detection rates likely resulted from sparse camera density, particularly because radiomarked deer often took several days to locate bait or did not find or use bait throughout the survey. We suggest researchers use a greater number of marked animals and/or increase camera density (e.g., 1 camera/65 ha; Jacobson et al. 1997) than our surveys when establishing a camera sampling area. We also suggest researchers consider exploring other unmarked occupancy models using count data (e.g., PCount model; Fiske and Chandler 2011) to estimate deer abundance and potentially reduce variation of site abundance associated with detection/non-detection data.
Radiotelemetry also has biases which can violate model assumptions when using these data to estimate population growth. First, animals are typically not equally catchable, which can bias vital rate estimates from radiotelemetry data (Millspaugh and Marzluff 2001). We suggest our sample of radiomarked deer was relatively representative of the population because adult females had relatively wide variation in body condition and age (Duquette et al. 2013). Also, nearly an equal number of male and female fawns were captured opportunistically and with organized searches throughout the study area. Second, researchers commonly do not monitor marked ungulate survival or recruitment throughout the entire year (e.g., Carstensen et al. 2009), which could suffice in landscapes where minimal mortality occurs after camera survey completion, as we observed. However, deer can experience substantial winter mortality (Fuller 1990; DelGiudice et al. 2002); suggesting vital rates should be monitored throughout the year to provide more accurate estimates for populations likely to experience greater winter mortality.
Radiotelemetry and unmarked occupancy each have advantages and disadvantages for estimating population growth. First, radiotelemetry provides estimates of age-specific survival (DelGiudice et al. 2006) and reproductive rates (Grund and Woolf 2004; Duquette et al. 2012), which cannot be directly estimated with the Royle–Nichols occupancy model. However, Roth and Amrhein (2010) showed unmarked occupancy models of a territorial avian species provided unbiased estimates of survival which closely reflected those from mark-recapture models. Second, radiotelemetry data and Royle–Nichols occupancy models can provide estimates of population growth, but occupancy models can also estimate species abundance. Nonetheless, occupancy model estimates can be difficult to interpret because abundance typically varies over sites and detection depends on abundance, which can introduce greater estimate bias (e.g., attenuation in detection; Welsh et al. 2013). This bias likely increased variation in our abundance estimates. Third, radiotelemetry is often labor intensive and expensive due to capture and marking of animals (Millspaugh and Marzluff 2001), which can limit these studies to small geographic areas (Tempel and Gutiérrez 2013). In contrast, occupancy studies allow geographically extensive areas to be surveyed because only detection/non-detection or count data of unmarked individuals is needed and can be collected with less overall labor (i.e., marking), compared to other remote camera-based estimators (e.g., Jacobson et al. 1997) that require identifying individuals. Remote cameras are particularly useful for simultaneously collecting ungulate abundance (Watts et al. 2008; Dougherty and Bowman 2012) and age ratios (Ikeda et al. 2013), which can provide information on maximum sustainable mortality for adult females (Bender 2006). Further, remote cameras can standardize sampling that can be biased with other methods (e.g., thermal imaging; Haroldson et al. 2003) because of variation in equipment operators and environmental conditions. Finally, many abundance estimators, including the Royle–Nichols occupancy model, can incorporate covariates of detection, such as habitat metrics (Anderson et al. 2013) or proportion of marked animals observed during surveys (DeYoung et al. 1989) to estimate variance of abundance. Radiomarked animals can be useful to estimate detection rates (Fuller 1990), but locating these animals may be tedious and require marked animals to have working transmitters and remain in the study area. In comparison, occupancy models use the detection history of unmarked animals to assess heterogeneity in site abundance (Fiske and Chandler 2011), which could be make this method preferable over other methods (e.g., aerial surveys; Haroldson et al. 2003; Storm et al. 2011) in forested landscapes with variable weather conditions.
We suggest the Royle–Nichols occupancy model and radiotelemetry data can provide useful methods of estimating deer population growth across a relatively large and forested area. We also suggest the Royle–Nichols occupancy model and radiotelemetry data are more advantageous than indices (e.g., pellet counts; Urbanek et al. 2012) because abundance can be directly estimated or incorporate corrections for detectability and confidence intervals can be estimated. If population trends and demography are being sought, we suggest the Royle–Nichols occupancy model using detection/non‐detection data collected from remote cameras may be preferable because marking deer is not required (e.g., Watts et al. 2008), reducing labor and costs (McCoy et al. 2011). Conversely, capturing and radiomonitoring deer provides more precise estimates of population growth, as well as estimates of vital rates which most influence population growth. Choice of population growth estimation method should depend on study objectives, logistics, and breadth and precision of data desired.
References
Anderson CW, Nielsen CK, Hubbard RD, Stroud JK, Schauber EM (2013) Comparison of indirect and direct methods of distance sampling for estimating density of white-tailed deer. Wildl Soc Bull 37:146–154
Bender LC (2006) Uses of herd composition and age ratios in ungulate management. Wildl Soc Bull 34:1125–1230
Beringer J, Hansen LP, Wilding W, Fischer J, Sheriff SL (1996) Factors affecting capture myopathy in white-tailed deer. J Wildl Manag 60:373–380
Burnham KP, Anderson DR (1998) Model selection and multimodel inference: a practical information-theoretic approach, 2nd edn. Springer, New York
Carstensen M, DelGiudice GD, Sampson BA (2003) Using doe behavior and vaginal-implant transmitters to capture neonate white-tailed deer in north-central Minnesota. Wildl Soc Bull 31:634–641
Carstensen M, DelGiudice GD, Sampson BA, Kuehn DW (2009) Survival, birth characteristics, and cause-specific mortality of white-tailed deer neonates. J Wildl Manag 73:175–183
Caughley G (1974) Interpretation of age ratios. J Wildl Manag 38:557–562
Clover MR (1956) Single-gate deer trap. Calif Fish Game 42:199–210
Collier BA, Ditchkoff SS, Raglin JB, Smith JM (2007) Detection probability and sources of variation in white-tailed deer spotlight surveys. J Wildl Manag 71:277–281
DeCesare NJ, Hebblewhite M, Bradley M, Smith KG, Hervieux D, Neufeld L (2012) Estimating ungulate recruitment and growth rates using age ratios. J Wildl Manag 76:144–153
DelGiudice GD, Riggs MR, Joly P, Pan W (2002) Winter severity, survival, and cause-specific mortality of female white-tailed deer in north-central Minnesota. J Wildl Manag 66:698–717
DelGiudice GD, Fieberg J, Riggs MR, Carstensen-Powell M, Pan W (2006) A long-term age-specific survival analysis of female white-tailed deer. J Wildl Manag 70:1556–1568
DelGiudice GD, Lenarz MS, Carstensen-Powell M (2007) Age-specific fertility and fecundity in northern free-ranging white-tailed deer: evidence for reproductive senescence? J Mamm 88:427–435
DeYoung CA, Guthery FS, Beasom SL, Coughlin SP, Heffelfinger JR (1989) Improving estimates of white-tailed deer abundance from helicopter surveys. Wildl Soc Bull 17:275–279
Dougherty SQ, Bowman JL (2012) Estimating sika deer abundance using camera surveys. Popul Ecol 54:357–365
Duong T (2007) ks: Kernel density estimation and kernel discriminant analysis for multivariate data in R. J Stat Softw 21:1–16 (package in R)
Duquette JF, Belant JL, Beyer DE Jr, Svoboda NJ (2012) Comparison of pregnancy detection in live white-tailed deer. Wildl Soc Bull 36:115–118
Duquette JF, Belant JL, Svoboda NJ, Beyer DE Jr (2013) Body condition and dosage effects on ketamine-xylazine immobilization of female white-tailed deer. Wildl Soc Bull 37:162–167
Ebert C, Sandrini J, Spielberger B, Thiele B, Hohmann U (2012) Non-invasive genetic approaches for estimation of ungulate population size: a study on roe deer (Capreolus capreolus) based on faeces. Anim Biodiv Conserv 35:267–275
Environmental Systems Research Institute, Inc (2010) ArcGIS 10.0. Redlands, California
Fiske I, Chandler R (2011) Unmarked: an R package for fitting hierarchical models of wildlife occurrence and abundance. J Stat Softw 43:1–23
Fuller TK (1990) Dynamics of a declining white-tailed deer population in north-central Minnesota. Wildl Monogr 110:3–37
Fuller TK (1991) Do pellet counts index white-tailed deer numbers and population change? J Wildl Manag 55:393–396
Gaillard JM, Festa-Bianchet M, Yoccoz NG, Loison A, Toïgo C (2000) Temporal variation in fitness components and population dynamics of large herbivores. Annu Rev Ecol Syst 31:367–393
Grund MD, Woolf A (2004) Development and evaluation of an accounting model for estimating deer population sizes. Ecol Model 180:345–357
Harris NC, Kauffman MJ, Mills LS (2008) Inferences about ungulate population dynamics derived from age ratios. J Wildl Manag 72:1143–1151
Haroldson BS, Wiggers EP, Beringer J, Hansen LP, McAninch JB (2003) Evaluation of aerial thermal imaging for detecting white-tailed deer in a deciduous forest environment. Wildl Soc Bull 31:1188–1197
Hatter IW, Bergerud WA (1991) Moose recruitment, adult mortality, and rate of change. Alces 27:65–73
Hawkins RE, Klimstra WD (1970) A preliminary study of the social organization of white-tailed deer. J Wildl Manag 34:407–419
Hood GM (2011) PopTools version 3.2.5. http://www.poptools.org
Ikeda T, Takahashi H, Yoshida T, Igota H, Kaji K (2013) Evaluation of camera trap surveys for estimation of sika deer herd composition. Mamm Study 38:29–33
Jackson RM, White M, Knowlton FF (1972) Activity patterns of young white-tailed deer fawns in south Texas. Ecology 53:262–270
Jacobson HA, Kroll JC, Browning RW, Koerth BH, Conway MH (1997) Infrared-triggered cameras for censusing white-tailed deer. Wildl Soc Bull 25:547–556
Karanth KU, Gopalaswamy AM, Kumar NS, Vaidyanathan S, Nichols JD, MacKenzie DI (2011) Monitoring carnivore populations at the landscape scale: occupancy modelling of tigers from sign surveys. J Appl Ecol 48:1048–1056
Koerth BH, Kroll JC (2000) Bait type and timing for deer counts using cameras triggered by infrared monitors. Wildl Soc Bull 28:630–635
Koerth BH, McKown CD, Kroll JC (1997) Infrared-triggered camera versus helicopter counts of white-tailed deer. Wildl Soc Bull 25:557–562
Kreeger TJ, Arnemo JM (2007) Handbook of wildlife chemical immobilization, 3rd edn. Laramie, Wyoming
Leslie PH (1945) On the use of matrices in certain population mathematics. Biometrika 3:183–212
MacKenzie DI, Nichols JD, Royle JA, Pollock KH, Bailey LL, Hines JE (2006) Occupancy estimation and modeling. Academic Press, Burlington
McCaffery KR (1976) Deer trail counts as an index to populations and habitat use. J Wildl Manag 40:308–316
McCoy JC, Ditchkoff SS, Steury TD (2011) Bias associated with baited camera sites for assessing population characteristics of deer. J Wildl Manag 75:472–477
McCullough DR (1994) In my experience: what do herd composition counts tell us? Wildl Soc Bull 22:295–300
McGarigal K, Marks BJ (1995) FRAGSTATS: spatial pattern analysis program for quantifying landscape structure. United States Forest Service General Technical Report PNW-GTR-351, Portland, Oregon
Michigan Department of Natural Resources (2009–2011) West U.P. deer camp survey. Michigan Department of Natural Resources, Wildlife Division, Gladstone, Michigan
Millspaugh JJ, Marzluff JM (2001) Radio tracking and animal populations. Academic Press, Burlington
Millspaugh JJ, Washburn BE, Milanick MA, Beringer J, Hansen LP, Meyer TM (2002) Non-invasive techniques for stress assessment in white-tailed deer. Wildl Soc Bull 30:899–907
Morellet N, Champely S, Gaillard JM, Ballon P, Boscardin Y (2001) The browsing index: new tool uses browsing pressure to monitor deer populations. Wildl Soc Bull 29:1243–1252
Naugle DE, Jenks JA, Kernohan BJ (1996) Use of thermal infrared sensing to estimate density of white-tailed deer. Wildl Soc Bull 24:37–43
Nelson ME (2001) Tooth extractions from live-captured white-tailed deer. Wildl Soc Bull 29:245–247
Ozoga JJ, Verme LJ, Bienz CS (1982) Parturition behavior and territoriality in white-tailed: impact on neonatal mortality. J Wildl Manag 46:1–11
Pradel R (1996) Utilization of capture-mark-recapture for the study of recruitment and population growth rate. Biometrics 52:703–709
R Core Team (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org
Roberts CW, Pierce BL, Braden AW, Lopez RR, Silvy NJ, Frank PA, Ransom D Jr (2006) Comparison of camera and road survey estimates for white-tailed deer. J Wildl Manag 70:263–267
Roth T, Amrhein V (2010) Estimating individual survival using territory occupancy data on unmarked animals. J Appl Ecol 47:386–392
Rowcliffe JM, Field J, Turvey ST, Carbone C (2009) Estimating animal density using camera traps without the need for individual recognition. J Appl Ecol 45:1228–1236
Royle JA, Nichols JD (2003) Estimating abundance from repeated presence-absence data or point counts. Ecology 84:777–790
Skalski JR, Ryding KE, Millspaugh JJ (2005) Wildlife demography: analysis of sex, age, and count data, 1st edn. Academic Press, Burlington
Stevens DL Jr, Olsen AR (2004) Spatially-balanced sampling of natural resources. J Am Stat Assoc 99:262–278
Storm DJ, Samuel MD, Van Deelen TR, Malcolm KD, Rolley RE, Frost NA, Bates DP, Richards BJ (2011) Comparison of visual-based helicopter and fixed-wing forward looking infrared surveys for counting white-tailed deer Odocoileus virginianus. Wildl Biol 17:431–440
Tempel DJ, Gutiérrez RJ (2013) Relation between occupancy and abundance for a territorial species, the California spotted owl. Conserv Biol 27:1087–1095
Therneau T (2012) Survival analysis, including penalized likelihood. Version 2.36-14. http://cran.r-project.org/web/packages/survival/survival.pdf
United States Geological Survey (2011) National landcover database 2006. http://www.mrlc.gov/nlcd2006.php
Urbanek RE, Nielsen CK, Preuss TS, Glowacki GA (2012) Comparison of aerial surveys and pellet-based distance sampling methods for estimating deer density. Wildl Soc Bull 36:100–106
Verme LJ (1969) Reproductive patterns of white-tailed deer related to nutritional plane. J Wildl Manag 33:881–887
Verme LJ (1983) Sex ratio variation in Odocoileus: a critical review. J Wildl Manag 47:573–582
Verme LJ (1989) Maternal investment in white-tailed deer. J Mamm 70:438–442
Watts DE, Parker ID, Lopez RR, Silvy NJ, Davis DS (2008) Distribution and abundance of endangered Florida Key deer on outer Islands. J Wildl Manag 72:360–366
Weckel M, Rockwell RF, Secret F (2011) A modification of Jacobson et al.’s (1997) individual branch-antlered male method for censusing white-tailed deer. Wildl Soc Bull 35:445–451
Welsh AH, Lindenmayer DB, Donnelly CF (2013) Fitting and interpreting occupancy models. PLos One 8:e52015
White GC, Burnham KP (1999) Program MARK: survival estimation from populations of marked animals. Bird Study 46(Suppl):120–138
White GC, Freddy DJ, Gill RB, Ellenberger JH (2001) Effect of adult sex ratio on mule deer and elk productivity in Colorado. J Wildl Manag 65:543–551
Wibisono HT, Linkie M, Guillera-Arroita G, Smith JA, Sunarto, Pusparini W, Asriadi, Baroto P, Brickle N, Dinata Y, Gemita E, Gunaryadi D, Haidir IA, Herwansyah, Karina I, Kiswayadi D, Kristiantono D, Kurniawan H, Lahoz-Monfort JJ, Leader-Williams N, Maddox T, Martyr DJ, Maryati, Nugroho A, Parakkasi K, Priatna D, Ramadiyanta E, Ramono WS, Reddy GV, Rood EJJ, Saputra DY, Sarimudi A, Salampessy A, Septayuda E, Suhartono T, Sumantri A, Susilo, Tanjung I, Tarmizi, Yulianto K, Yunus M, Zulfahmi (2011) Population status of a cryptic top predator: an island-wide assessment of tigers in Sumatran rainforests. PLos One 6:e25931
Zar JH (1999) Biostatistical analysis, 4th edn. Prentice Hall, Englewood Cliffs
Acknowledgments
This project was supported by the Federal Aid in Wildlife Restoration Act under Pittman-Robertson project W-147-R. We thank Michigan Department of Natural Resources and Mississippi State University Department of Wildlife, Fisheries and Aquaculture, the Mississippi State Carnivore Ecology Lab, and the Mississippi State Forest and Wildlife Research Center for logistical and financial support. We thank Safari Club International Foundation, and Safari Club International–Michigan Involvement Committee for additional financial support. Much gratitude to G. DelGiudice, G. Zuehlke, D. O’Brien, B. Roell, G. Sasser, J. Branen, T. Petroelje, R. Karsch, J. Edge, T. Swearingen, C. Corroy, L. Kreiensieck, K. Smith, H. Stricker, C. Ott-Conn, A. Nelson, M. Harrigan, N. Levikov, and D. Martell for field and technical support and participating landowners for land access. Finally, we thank B. D. Leopold and B. K. Strickland for useful comments on an earlier version of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Duquette, J.F., Belant, J.L., Svoboda, N.J. et al. Comparison of occupancy modeling and radiotelemetry to estimate ungulate population dynamics. Popul Ecol 56, 481–492 (2014). https://doi.org/10.1007/s10144-014-0432-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10144-014-0432-7