Abstract
This study explored a consumer-resource model including reproductive and nonreproductive subpopulations of the consumer to consider whether resource-dependent reproductive adjustment by the consumer would stabilize consumer-resource dynamics. The model assumed that decreasing (increasing) resource availability caused reproductive suppression (facilitation), and that the reproductive consumer had a higher mortality rate than the nonreproductive one (i.e., a trade-off between reproduction and survival). The model predicted that the variability would be reduced when the consumer had a strong tendency to suppress reproduction in response to low resource availability or when the cost of reproduction was high, although consumer extinction became more likely. Furthermore, when the consumer-resource dynamics converged to limit cycles, reproductive adjustment enhanced the long-term average of the consumer density. It was also predicted that if reproductive suppression enhanced resource consumption efficiency (i.e., a trade-off between reproduction and foraging), then it would destabilize the system by canceling the stabilizing effect of the reproductive adjustment itself. These results suggest that it is necessary not only to identify the costs of reproduction, but also to quantify the changes in individual-level performances due to reproduction in order to understand the ecological consequences of reproductive adjustment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Reproduction is a key life-history component that influences demography directly, as well as foraging and defense (Clutton-Brock 1988). Undoubtedly, reproduction has some costs such as reduced parental survival (reviewed by Reznick 1985, 1992; Stearns 1992; Harshman and Zera 2006), and the degree of the trade-off depends on environmental factors, such as predation risk (see below) or resource availability (Zera and Harshman 2001; note here that “resource” represents “food” in this study unless stated otherwise, because we will explore consumer-resource models below). Therefore, reproductive adjustment can evolve in terms of mating frequency, offspring number, or feeding effort in a temporally changing environment. Indeed, resource-dependent reproductive adjustment occurs in many taxa, including zooplankton (Pond et al. 1996; Gyllström and Hansson 2004), insects (Moehrlin and Juliano 1998; Kagata and Ohgushi 2001; Engqvist and Sauer 2003; Marden et al. 2003), fishes (Reznick and Yang 1993; Siems and Sikes 1998; Kolluru and Grether 2004), reptiles (Ford and Seigel 1989; Naulleau and Bonnet 1996; Shine and Madsen 1997; Doughty and Shine 1998; Madsen and Shine 1999; Du 2006), birds (Jacobsen et al. 1995; Erikstad et al. 1998; Meijer and Drent 1999; De Neve et al. 2004; Nagy and Holmes 2005), and mammals (Clutton-Brock et al. 1982; Saitoh 1989; Festa-Bianchet and Jorgenson 1998).
Recently, it has been argued that phenotypic plasticity could have a large impact on population or community dynamics (reviewed by Schmitz et al. 2003; Werner and Peacor 2003; Miner et al. 2005; Ohgushi 2005; Young et al. 2006; Ohgushi et al. 2007). This view has been widespread especially among theoretical researchers (reviewed by Abrams 2000; Bolker et al. 2003). However, little is known about the ecological consequences of resource-dependent reproductive adjustment despite the accumulating empirical evidence (see above), although only a few empirical studies indicated the stabilizing effect (see “Discussion”). This is because previous theoretical studies have considered mainly foraging or defense adaptations (Abrams 2000; Werner and Peacor 2003).
On the other hand, predator-induced reproductive suppression in voles is one of the well-studied examples linking reproductive adjustment and population dynamics (e.g., Ylönen 1994; Norrdahl and Korpimäki 1995; Kokko and Ranta 1996). Ruxton and Lima (1997) and Ruxton et al. (2002) explored prey–predator models including reproductive and nonreproductive subpopulations of the prey (i.e., voles). In the model, they assumed that reproductive prey suffered a higher predation rate than nonreproductive prey (i.e., a trade-off between reproduction and predator avoidance), and that increasing (decreasing) predator density enhanced behavioral switching from the reproductive (nonreproductive) into the nonreproductive (reproductive) stage. Gyllenberg et al. (1996) also worked on the same problem by exploring more specific models. In general, these models predict that predator-induced reproductive adjustment tends to be stabilizing, because reproductive suppression (facilitation) reduces (enhances) the population growth rate of the predator when the predator is abundant (scarce), which causes a negative feedback in the prey–predator dynamics (but see also Ylönen 1994).
Furthermore, Kokko and Ruxton (2000) explored a prey–predator model that included reproductive adjustment by both the prey and predator. However, because of the complexity of their model structure, we only know that the influences of reproductive adjustment on population stability would be complicated (see “Discussion” for further explanations). Therefore, more generalized and simple mathematical models are required to understand population dynamics in which reproductive behaviors change flexibly in response to resource availability.
In this study, we model the situation that increasing (decreasing) resource density enhances behavioral switching of the consumer from the reproductive (nonreproductive) into the nonreproductive (reproductive) stage, to examine whether resource-dependent reproductive adjustment would stabilize the consumer-resource dynamics. Although reproductive adjustment observed in nature is not necessarily a switching behavior, but rather a quantitative trait in many cases, we follow Ruxton and Lima (1997) and Ruxton et al. (2002) who assumed reproductive and nonreproductive subpopulations of prey (i.e., resource) but not of predator (i.e., consumer).
Furthermore, as cost of reproduction, we assume that the reproductive consumer has a higher mortality rate than the nonreproductive one, for example, because of energetic constraints (see above) or increased vulnerability to predation (Gwynne 1989) or parasitism (Sheldon and Verhulst 1996). These assumptions imply a trade-off between reproduction and survival, which is the most conventional trade-off (Reznick 1985; Stearns 1992). Although organisms in nature suffer from higher mortality and die from starvation when the resource availability is extremely low, we do not consider the extreme case for the purpose of highlighting potential demographic effects of resource-dependent reproductive adjustment (but see also “Discussion” for expected results). In addition, a decrease in foraging effort has also been reported as a cost of reproduction in many cases (e.g., Shine 1980; Oksanen and Lundberg 1995; Kolluru and Grether 2004; Nagy and Holmes 2005), which will also affect demographic effects of resource-dependent reproductive adjustment. Thus, we also consider the situation that the reproductive consumer has lower resource consumption efficiency than the nonreproductive one. We also discuss the support that our model predictions would provide for empirical studies.
Model
The model simply assumes that decreasing (increasing) resource availability causes reproductive suppression (facilitation) of the consumer, and that the reproductive consumer has a higher mortality rate than the nonreproductive one (i.e., a trade-off between reproduction and survival). These assumptions do not rigorously maximize consumer fitness, but the behavior will be adaptive to some degree, as suggested in previous empirical (see “Introduction”) and theoretical studies (Gyllenberg et al. 1996; Ruxton and Lima 1997; Ruxton et al. 2002).
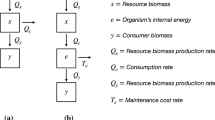
In the model, we consider one resource (R) and two subpopulations of consumer, reproductive (B) and nonreproductive (S), in a consumer-resource model.
where
r and K are the intrinsic growth rate and the carrying capacity of the resource (R), respectively. The functional response F is Holling’s type II (Eq. 1d), in which a and h represent the maximum resource consumption rate and the handling time, respectively. b is the conversion efficiency of reproductive energy by the consumer. d B and d S are the mortality rates of the reproductive and nonreproductive consumers, respectively. P and Q capture the magnitudes of reproductive suppression and facilitation, which depend on the per-individual foraging gain F by assumption. In this study, following Ruxton and Lima (1997) and Ruxton et al. (2002), we set P and Q as decreasing and increasing saturating functions of F, respectively:
These formulations assume that an increase in the foraging gain decreases (or increases) the per-individual switching rate from the reproductive into the nonreproductive stage (or vice versa). The maximum suppression and facilitation rates are governed by p 0 and q 0, respectively. p 1 and q 1 determine the saturation speed. In this model, we assume d B > d S , which implies a cost of reproduction. Substituting d B = c d S (c > 1), we denote the magnitude of the cost with c.
It is difficult to analyze the model, even to obtain the equilibrium density analytically. Accordingly, we analyze the properties of the system numerically by using computer simulations. Here, we first calculate the equilibrium densities numerically and then examine local stability from the Routh-Hurwitz criteria on the Jacobian matrix resulting from the linearization process. We examine parameter-dependences of local stability of the equilibria and conduct those procedures for different initial conditions to test whether bistability or chaotic dynamics exist. Furthermore, we also examine the long-term average of the total consumer density (i.e., B + S). This evaluation will be helpful to consider the adaptive significance of reproductive adjustment, although it does not rigorously indicate consumer fitness.
When the consumer changes the maximum resource consumption rate a due to reproductive suppression, the rate of resource increase (Eq. 1a) would be rewritten as follows:
where z represents the magnitude of the change in the resource consumption rate. In this case, we also conduct similar numerical simulations to examine demographic effects of reproductive adjustment.
Results
Figure 1 shows the dependence of local stability of the equilibria on several parameters (K, p 0, q 0, b, c). The model always predicted three types of dynamics: consumer extinction (black regions), coexistence equilibrium (white regions) and limit cycle coexistence (gray regions). It was unlikely that bistability existed, because the results did not change with the initial conditions. That is, when locally stable, the dynamics were always globally stable and when locally unstable, they approached limit cycles.
Parameter-dependence of the local stability of the equilibria for q 0 = a 0.01, b 0.1, c 0.5, and d 1.0. In each small panel, the horizontal and vertical axes represent the carrying capacity of the resource and the maximum reproductive suppression rate (K and p 0), respectively. Panels are arranged in order of the reproductive conversion efficiency (b = 0.2, 0.3, and 0.4) and the magnitude of the mortality cost of reproduction (c = 1.25, 1.5, and 2). In the black area, the trivial equilibrium is locally stable (consumer extinction). The white and gray areas represent the regions in which the internal equilibrium converges to coexistence equilibrium and limit cycles, respectively. Other parameter values are set as r = 1, a = 1, h = 1, d S = 0.1, p 1 = 1, and q 1 = 1
We found that the dynamic property at the equilibrium state would change from limit cycle coexistence (gray region) to consumer extinction (black region) via stable coexistence (white regions) with increasing maximum reproductive suppression rate p 0 or mortality cost of reproduction c and decreasing carrying capacity of the resource K, conversion efficiency of the reproductive energy b or maximum reproductive facilitation rate q 0 (Fig. 1). Large values of p 0 and small values of q 0 mean that the consumer has a strong tendency to suppress reproduction in response to resource deficiency. Small values of b and large values of c mean that the relative mortality cost of reproduction is high. Therefore, the obtained patterns in Fig. 1 will be explained by a well-known fact in consumer-resource interactions: if the population growth rate of the consumer is reduced, then the equilibrium resource density increases and the dynamics become more stable (Murdoch et al. 2003).
From Fig. 1, it can be inferred that reproductive adjustment reduces the variability (i.e., the amplitude of the population oscillations) of the consumer-resource dynamics, if they coexist. Figure 2 shows examples of the dynamics of resource and consumer densities without and with reproductive adjustment, illustrating the stabilizing effect of reproductive adjustment on population dynamics (i.e., reducing the variability) when it is not strong enough to stabilize the coexistence equilibrium. In both numerical simulations, the initial conditions were R(0) = K and B(0) = S(0) = 0.01. It is also notable that the variability of the system will be always reduced when the consumer has the capability to suppress reproduction (p 0 > 0) compared to when the consumer does not (p 0 = 0; Fig. 1), although the consumer did not persist when the parameter change was sufficiently large (black regions; Fig. 1).
Dynamics of resource and consumer densities a without and b with reproductive adjustment (bold lines). p 0 = a 0 and b 0.11; K = 5, b = 0.3, c = 1.5, and q 0 = 0.1. Other parameter values are the same as in Fig. 1. The solid line denotes breeders; the broken line denotes suppressors
Qualitatively similar patterns were obtained for different parameter settings of (r, a, h, d S, p 1, q 1) (not shown). Additionally, we observed that decreasing intrinsic growth rates of the resource r or increasing mortality rates of the nonreproductive consumer d S enhanced the stabilizing effect of reproductive adjustment on the variability of the consumer-resource dynamics (not shown). These results agree with the predictions in general consumer-resource models (Murdoch et al. 2003).
Figure 3 shows how the reproductive suppression rate p 0 would affect the long-term average of the total consumer density (i.e., B + S) for various values of the reproductive facilitation rates q 0. We obtained qualitatively similar patterns, where the time-averaged consumer density increased (decreased) when the consumer-resource dynamics converged to limit cycles (coexistence equilibrium) as p 0 increased.
Long-term average of total consumer density B + S for various values of the reproductive facilitation rates (q 0 = 0.01, 0.1, 0.5, and 1.0). The x axis represents the reproductive suppression rate p 0. Other parameter values are the same as in Fig. 2. The solid and broken lines denote that the dynamics converge to coexistence equilibrium and limit cycles, respectively
Finally, we found that, if the reproductive consumer has a lower resource consumption efficiency (Eq. 2), the variability was slightly augmented (compare Figs. 2b and 4), although the stability condition was almost invariant (not shown).
Dynamics of resource and consumer densities when reproductive suppression enhances the resource consumption efficiency. z = 2 and p 0 = 0.11. Other parameter values are the same as in Fig. 2
Discussion
In this study, we examined how resource-dependent (i.e., food-dependent) reproductive adjustment by the consumer would affect the consumer-resource dynamics. Previous theoretical models have also described reproductive plasticity in community ecological contexts (e.g., size-structured models; de Roos 1997). In those models, dynamic properties of populations were modified by plastic changes in interaction strength between consumer and resource (e.g., due to size-dependent consumption efficiency). In our models, however, the population growth rate of the consumer changed internally due to plasticity in reproductive efforts, which influenced the whole system through the interspecific interaction with constant strength.
We showed that resource-dependent reproductive adjustment would basically stabilize (i.e., reduce the variability of) the consumer-resource dynamics under a trade-off between reproduction and survival. In standard consumer-resource models (i.e., the Rosenzweig–MacArthur model; Rosenzweig and MacArthur 1963), the consumer population grows by exploiting the resource and starts to decrease when the resource density declines to a certain level. Subsequently, the resource begins to recover as the consumer population decreases (Fig. 2a). With reproductive adjustment, however, the resource is not depleted to such a low level because the consumer reduces reproductive investment and the population decreases earlier when the resource is scarce (Fig. 2b). Moreover, when the resource is abundant, the consumer invests more in reproduction and the population increases more quickly, which enhances resource consumption. As a result, these effects reduced the variability. On the other hand, reproductive adjustment was predicted to be destabilizing (i.e., enhance the variability) under a trade-off between reproduction and foraging. This destabilization effect will be attributable to the fact that the resource is consumed (grows) excessively due to an increase in the nonreproductive (reproductive) consumer with high (low) consumption efficiency when the resource is scarce (abundant).
A number of studies have shown that the costs of reproduction are resource-dependent and that parents adjust their reproductive investment based on resource availability (see “Introduction”). Although researchers have considered the ecological consequences of reproductive adjustment less often, there is some empirical evidence that agrees with our model predictions on the variability of population dynamics. For example, an herbivorous ladybird beetle Epilachna niponica shows egg resorption in response to increased herbivory, allowing it to overwinter and survive to the next reproductive season in the natural habitat (Ohgushi 1996). When the ladybird was introduced to a site with different host plant phenology, the introduced population shifted to early reproduction at the cost of reduced longevity, and as a result, egg resorption no longer occurred (Ohgushi 1991, 1998; Ohgushi and Sawada 1997a). Comparing the temporal dynamics of the source and introduced populations, it was found that the introduced population exhibited more variable dynamics (Ohgushi and Sawada 1997b, 1998; Ohgushi 1998), suggesting that egg resorption greatly contributed to stabilization of the ladybird population (Ohgushi and Sawada 1985, 1998; Ohgushi 1998). These results indicate a stabilizing effect of resource-dependent reproductive adjustment directly.
Another example is resting-egg production by zooplankton. Many zooplankton species produce resting (dormant) eggs in response to certain environmental changes, such as food deficiency (reviewed by Gyllström and Hansson 2004). Resting eggs stay at the bottom of a lake until the environment improves, and thus do not contribute to instantaneous population growth. Accordingly, nonresting individuals and resting eggs can be regarded as the reproductive and nonreproductive subpopulations, respectively, in our model. Resting eggs have extremely low levels of both mortality and hatching rates (Hairston et al. 1995), which would correspond to a large value of reproductive cost c and a small value of reproductive facilitation rate q 0. Moreover, resting eggs do not consume food, so z = 0. Therefore, our results predict that resting-egg production would have a stabilizing effect on the population dynamics. Indeed, McCauley et al. (1999) observed that resting-egg production stabilized the population dynamics in algae-Daphnia microcosm experimental systems, which is being tested using mathematical models (T. Nakazawa et al., in preparation).
Previous theoretical studies have predicted that predator-induced reproductive suppression of prey would stabilize the prey–predator dynamics (Gyllenberg et al. 1996; Ruxton and Lima 1997; see also Ylönen 1994; Ruxton et al. 2002). Therefore, together with our predictions, it is expected that if both prey and predator (i.e., resource and consumer) adjust their reproductive investment based on the density of the other, the system should be more stable. Conversely, Kokko and Ruxton (2000) argued that the ecological consequences of reproductive adjustment would be more complicated, explaining that reproductive adjustment either stabilized or destabilized the system and there seemed to be no simple general rule governing the effects.
To date, few arguments have been made about this inconsistency among the predictions of these previous models. One big difference between the assumptions of the models concerns the optimization process of reproductive investment. Ruxton and Lima (1997) and Ruxton et al. (2002) assumed a priori that the switching rates between the reproductive and nonreproductive subpopulations were determined by predator and prey densities, while Kokko and Ruxton (2000) optimized the reproductive investment to maximize fitness, which was defined as the sum of parental survival and offspring recruitment. However, we consider that a more important factor is the difference in stage-specific performances. For example, suppose that the juvenile generally has lower resource consumption efficiency than the adult, as Kokko and Ruxton (2000) assumed. When adults increase their reproductive investment in response to increasing resource availability, the adults suffer a higher mortality rate due to the cost of reproduction, which potentially has a destabilizing effect because a reduction in the consumer population facilitates overgrowth of the resource. Meanwhile, the increased reproductive investment may not enhance resource consumption, even with juvenile recruitment, if the consumption efficiency of the juvenile is very low. Consequently, reproductive adjustment can counterintuitively destabilize the system. Therefore, whether reproductive adjustment stabilizes or destabilizes the system will depend on the reproductive output, a cost of reproduction, and the relative performances (e.g., resource consumption efficiency or mortality rate) of the adult and juvenile. This hypothesis is further extended by our prediction that if the consumer enhances the resource consumption efficiency due to reproductive suppression, it would destabilize the system by canceling the stabilizing effect of reproductive adjustment itself (Fig. 4). Therefore, to understand the ecological consequences of reproductive adjustment, it is necessary not only to identify the costs of reproduction, but also to quantify the changes in age- or behavior-dependent individual-level performances due to reproduction.
In this study, we showed that reproductive adjustment would be not only stabilizing but also advantageous (disadvantageous) to the consumer when the consumer-resource dynamics converge to limit cycles (coexistence equilibrium) (Fig. 3). These patterns are compatible with the prediction that when the functional response of the consumer is nonlinear, the large amplitude cycles generally reduce the mean growth rate of the consumer (Abrams et al. 1997, 1998, 2003). We modeled reproductive adjustment by the consumer phenomenologically in the density-dependent way, without considering consumer fitness explicitly. Although there is still debate over the extent to which phenotypic plasticity is adaptive in behavioral ecology (Via et al. 2001; Pigliucci 2005; Ghalambor et al. 2007), it would also be interesting to see how model predictions would change by explicitly introducing optimal reproductive strategy, because it may be predicted to contribute to population stability.
We simply assumed an increasing mortality rate as a cost of reproduction. However, the cost may affect future performance (i.e., a time-delayed trade-off; Gustafsson and Sutherland 1988; Nilsson and Svensson 1996; Shine and Madsen 1997; Doughty and Shine 1998). In this case, it is necessary to model life-history optimization on a time scale shorter than the individual lifespan. A classic approach is to maximize the eigenvalue of the age-structured matrix model including life-history trade-offs (Caswell 1989). More recently, a state-dependent life-history model has been proposed in a dynamic optimization approach (e.g., Perrin and Sibly 1993; McNamara and Houston 1996). However, it is still difficult to apply these methods to community models that include the dynamics of other species (i.e., resource or predator). Therefore, we attempted to eliminate the influences of the time-delayed trade-off by assuming that life-history optimization would be achieved immediately on a long time scale in which the population dynamics reached the equilibrium state. More refined mathematical models are necessary for a rigorous investigation of how the population dynamics would be affected by life-history optimization under a time-delayed trade-off.
Furthermore, we also considered that the resource availability influenced only reproductive efforts of the consumer whereas other demographic parameters such as mortality rate were invariant. Therefore, our model is minimal in its structure, allowing us to highlight and investigate potential demographic effects of resource-dependent reproductive adjustment. However, the mortality rate, for example, of the consumer may increase with decreasing resource availability due to starvation. This assumption may reduce the stabilizing effect of reproductive adjustment because, when the resource availability is extremely low, a slight increase in the resource availability would enhance the population growth rate of the consumer considerably, which could rather exhaust the resource.
Finally, we would like to emphasize that phenotypic plasticity occurs in diverse reproductive traits: for example, in fish species, it has been reported that egg size (Reznick and Yang 1993), hatching date (Wedekind 2002), sex ratio (Römer and Beisenherz 1996), age at maturity (Stearns and Koella 1986), mating strategy (Klemetsen et al. 2003), and sexual role (Munday et al. 2005) change plastically in response to environmental changes. Researchers have rarely tested whether or how phenotypic plasticity in these traits affects population or community dynamics. More consideration should be given to phenotypic plasticity in reproductive traits in community ecological studies in addition to foraging or defense plasticity, considering the increasing concern over a linkage between individual levels of trait plasticity and community dynamics (Schmitz et al. 2003; Werner and Peacor 2003; Miner et al. 2005; Ohgushi 2005; Young et al. 2006; Ohgushi et al. 2007). This will provide profound insights into our understanding of community dynamics in nature, as will emerging new study fields linking community ecology and evolutionary biology (Johnson and Stinchcombe 2007).
References
Abrams PA (2000) The evolution of predator–prey interactions: theory and evidence. Annu Rev Ecol Syst 31:79–105. doi:10.1146/annurev.ecolsys.31.1.79
Abrams PA, Namba T, Mimura M, Roth JD (1997) The relationship between productivity and population densities in cycling predator–prey systems. Evol Ecol 11:371–373. doi:10.1023/A:1018424605347
Abrams PA, Holt RD, Roth JD (1998) Apparent competition or apparent mutualism? Shared predation when populations cycle. Ecology 79:201–212. doi:10.2307/176875
Abrams PA, Brassil CE, Holt RD (2003) Dynamics and responses to mortality rates of competing predators undergoing predator–prey cycles. Theor Popul Biol 64:163–176. doi:10.1016/S0040-5809(03)00067-4
Bolker B, Holyoak M, Kŕivan V, Rowe L, Schmitz S (2003) Connecting theoretical and empirical studies of trait-mediated interactions. Ecology 84:1101–1114. doi:10.1890/0012-9658(2003)084[1101:CTAESO]2.0.CO;2
Caswell H (1989) Matrix population models: construction, analysis, and interpretation. Sinauer, Sunderland
Clutton-Brock TH (1988) Reproductive success. University of Chicago Press, Chicago
Clutton-Brock TH, Guiness FE, Albon SD (1982) Red deer: behavior and ecology of two sexes. University of Chicago Press, Chicago
De Neve L, Soler JJ, Soler M, Pérez-Contreras T, Martín-Vivaldi M, Martínez JG (2004) Effects of a food supplementation experiment on reproductive investment and a post-mating sexually selected trait in magpies Pica pica. J Avian Biol 35:246–251. doi:10.1111/j.0908-8857.2004.03162.x
Doughty P, Shine R (1998) Reproductive energy allocation and long-term energy stores in a viviparous lizard (Eulamprus tympanum). Ecology 79:1073–1083. doi:10.1890/0012-9658(1998)079[1073:REAALT]2.0.CO;2
Du WG (2006) Phenotypic plasticity in reproductive traits induced by food availability in a lacertid lizard, Takydromus septentrionalis. Oikos 112:363–369. doi:10.1111/j.0030-1299.2006.13552.x
Engqvist L, Sauer KP (2003) Influence of nutrition on courtship and mating in the scorpionfly Panorpa cognata (Mecoptera, Insecta). Ethology 109:911–928. doi:10.1046/j.1439-0310.2003.00937.x
Erikstad KE, Fauchald P, Tveraa T, Steen H (1998) On the cost of reproduction in long-lived birds: the influence of environmental variability. Ecology 79:1781–1788. doi:10.1890/0012-9658(1998)079[1781:OTCORI]2.0.CO;2
Festa-Bianchet M, Jorgenson JT (1998) Selfish mothers: reproductive expenditure and resource availability in bighorn ewes. Behav Ecol 9:144–150. doi:10.1093/beheco/9.2.136, 10.1093/beheco/9.2.144
Ford NB, Seigel RA (1989) Phenotypic plasticity in reproductive traits: evidence from a viviparous snake. Ecology 70:1768–1774. doi:10.2307/1938110
Ghalambor CK, McKay JK, Carroll SP, Reznick DN (2007) Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Funct Ecol 21:394–407. doi:10.1111/j.1365-2435.2007.01283.x
Gustafsson L, Sutherland WJ (1988) The costs of reproduction in the collared flycatcher Ficedula albicollis. Nature 335:813–815. doi:10.1038/335813a0
Gwynne DT (1989) Does copulation increase the risk of predation? Trends Ecol Evol 4:54–56. doi:10.1016/0169-5347(89)90144-4
Gyllenberg M, Hanski I, Lindström T (1996) A predator–prey model with optimal suppression of reproduction in the prey. Math Biosci 134:119–152. doi:10.1016/0025-5564(95)00082-8
Gyllström M, Hansson LA (2004) Dormancy in freshwater zooplankton: induction, termination and the importance of benthic-pelagic coupling. Aquat Sci 66:274–295. doi:10.1007/s00027-004-0712-y
Hairston NG Jr, Van Brunt RA, Kearns CM (1995) Age and survivorship of diapausing eggs in a sediment egg bank. Ecology 76:1706–1711. doi:10.2307/1940704
Harshman LG, Zera AJ (2006) The cost of reproduction: the devil in the details. Trends Ecol Evol 22:80–86. doi:10.1016/j.tree.2006.10.008
Jacobsen KO, Erikstad KE, Sæther BE (1995) An experimental study of the costs of reproduction in the Kittiwake Rissa tridactyla. Ecology 76:1636–1642
Johnson MTJ, Stinchcombe JR (2007) An emerging synthesis between community ecology and evolutionary biology. Trends Ecol Evol 22:250–257. doi:10.1016/j.tree.2007.01.014
Kagata H, Ohgushi T (2001) Clutch size adjustment of a leaf-mining moth (Lyonetiidae: Lepidoptera) in response to resource availability. Ann Entomol Soc Am 95:213–217. doi:10.1603/0013-8746(2002)095[0213:CSAOAL]2.0.CO;2
Klemetsen A, Amundsen PA, Dempson JB, Jonsson B, Jonsson N, O’Connell MF, Mortensen E (2003) Atlantic salmon Salmo salar L., brown trout Salmo trutta L. and Arctic charr Salvelinus alpinus (L.): a review of aspects of their life histories. Ecol Freshw Fish 12:1–59. doi:10.1034/j.1600-0633.2003.00010.x
Kokko H, Ranta E (1996) Evolutionary optimality of delayed breeding in voles. Oikos 77:173–175. doi:10.2307/3545599
Kokko H, Ruxton GD (2000) Breeding suppression and predator–prey dynamics. Ecology 81:252–260. doi:10.2307/177148
Kolluru GR, Grether GF (2004) The effects of resource availability on alternative mating tactics in guppies (Poecilia reticulata). Behav Ecol 16:294–300. doi:10.1093/beheco/arh161
Madsen T, Shine R (1999) The adjustment of reproductive threshold to prey abundance in a capital breeder. J Anim Ecol 68:571–580. doi:10.1046/j.1365-2656.1999.00306.x
Marden JH, Rogina B, Montooth KL, Helfand SL (2003) Conditional tradeoffs between aging and organismal performance of Indy long-lived mutant flies. Proc Natl Acad Sci USA 100:3369–3373. doi:10.1073/pnas.0634985100
McCauley E, Nisbet RM, Murdoch WW, de Roos AM, Gurney WSC (1999) Large-amplitude cycles of Daphnia and its algal prey in enriched environments. Nature 402:653–656. doi:10.1038/45223
McNamara JM, Houston AI (1996) State-dependent life histories. Nature 380:215–221. doi:10.1038/380215a0
Meijer T, Drent R (1999) Re-examination of the capital and income dichotomy in breeding birds. Ibis 141:399–414
Miner BG, Sultan SE, Morgan SG, Padilla DK, Relyea RA (2005) Ecological consequences of phenotypic plasticity. Trends Ecol Evol 20:685–692. doi:10.1016/j.tree.2005.08.002
Moehrlin GS, Juliano SA (1998) Plasticity of insect reproduction: testing models of flexible and fixed development in response to different growth rates. Oecologia 115:492–500. doi:10.1007/s004420050546
Munday PL, Buston PM, Warner RR (2005) Diversity and flexibility of sex-change strategies in animals. Trends Ecol Evol 21:89–95. doi:10.1016/j.tree.2005.10.020
Murdoch WW, Briggs CJ, Nisbet RM (2003) Consumer-resource dynamics. Princeton University Press, Princeton
Nagy L, Holmes RT (2005) Food limits annual fecundity of a migratory songbird: an experimental study. Ecology 86:675–681. doi:10.1890/04-0155
Naulleau G, Bonnet X (1996) Body condition threshold for breeding in a viviparous snake. Oecologia 107:301–306. doi:10.1007/BF00328446
Nilsson JA, Svensson E (1996) The cost of reproduction: a new link between current reproductive effort and future reproductive success. Proc R Soc B 263:711–714. doi:10.1098/rspb.1996.0106
Norrdahl K, Korpimäki E (1995) Does predation risk constrain maturation in cyclic vole populations? Oikos 72:263–272. doi:10.2307/3546228
Ohgushi T (1991) Lifetime fitness and evolution of reproductive pattern in the herbivorous lady beetle. Ecology 72:2110–2122. doi:10.2307/1941563
Ohgushi T (1996) A reproductive trade-off in an herbivorous lady beetle: egg resorption and female survival. Oecologia 106:345–351. doi:10.1007/BF00334562
Ohgushi T (1998) Bottom-up population regulation of a herbivorous lady beetle: an evolutionary perspective. In: Dempster JP, McLean IFG (eds) Insect populations in theory and in practice. Kluwer, Dordrecht, pp 367–390
Ohgushi T (2005) Indirect interaction webs: herbivore-induced effects through trait change in plants. Annu Rev Ecol Evol Syst 36:81–105. doi:10.1146/annurev.ecolsys.36.091704.175523
Ohgushi T, Sawada H (1985) Population equilibrium with respect to available food resource and its behavioural basis in an herbivorous lady beetle, Henosepilachna niponica. J Anim Ecol 54:781–796. doi:10.2307/4378
Ohgushi T, Sawada H (1997a) A shift toward early reproduction in an introduced herbivorous ladybird. Ecol Entomol 22:90–96. doi:10.1046/j.1365-2311.1997.00024.x
Ohgushi T, Sawada H (1997b) Population stability in relation to resource availability in an introduced population of an herbivorous lady beetle. Res Popul Ecol 39:37–45
Ohgushi T, Sawada H (1998) What changed the demography of an introduced population of an herbivorous lady beetle? J Anim Ecol 67:679–688. doi:10.1046/j.1365-2656.1998.00225.x
Ohgushi T, Craig TP, Price PW (2007) Ecological communities: plant mediation in indirect interaction webs. Cambridge University Press, Cambridge
Oksanen L, Lundberg P (1995) Optimization of reproductive effort and foraging in mammals: the influence of resource level and predation risk. Evol Ecol 9:45–56. doi:10.1007/BF01237696
Perrin N, Sibly RM (1993) Dynamic models of energy allocation and investment. Annu Rev Ecol Syst 24:379–410. doi:10.1146/annurev.es.24.110193.002115
Pigliucci M (2005) Evolution of phenotypic plasticity: where are we going now? Trends Ecol Evol 20:481–486. doi:10.1016/j.tree.2005.06.001
Pond D, Harris R, Head R, Harbour D (1996) Environmental and nutritional factors determining seasonal variability in the fecundity and egg viability of Calanus helgolandicus in coastal waters off Plymouth, UK. Mar Ecol Prog Ser 143:45–63. doi:10.3354/meps143045
Reznick DN (1985) Costs of reproduction: an evaluation of the empirical evidence. Oikos 44:257–267. doi:10.2307/3544698
Reznick DN (1992) Measuring the costs of reproduction. Trends Ecol Evol 7:42–45. doi:10.1016/0169-5347(92)90104-J
Reznick DN, Yang A (1993) The influence of fluctuating resources on life history: patterns of allocation and plasticity in female guppies. Ecology 74:2011–2019. doi:10.2307/1940844
Römer U, Beisenherz W (1996) Environmental determination of sex in Apistogrammai (Cichlidae) and two other freshwater fishes (Teleostei). J Fish Biol 48:714–725. doi:10.1111/j.1095-8649.1996.tb01467.x
de Roos AM (1997) A gentle introduction to physiologically structured population models. In: Tuljapurkar S, Caswell H (eds) Structured-population models in marine, terrestrial, and freshwater systems. Chapman & Hall, New York, pp 119–204
Rosenzweig ML, MacArthur RH (1963) Graphical representation and stability conditions of predator–prey interactions. Am Nat 97:209–223. doi:10.1086/282272
Ruxton GD, Lima SL (1997) Predator-induced breeding suppression and its consequences for predator–prey population dynamics. Proc R Soc B 264:409–415. doi:10.1098/rspb.1997.0058
Ruxton GD, Khan QJA, Al-Lawatia M (2002) The stability of internal equilibria in predator–prey models with breeding suppression. IMA J Math Appl Med Biol 19:207–219. doi:10.1093/imammb/19.3.207
Saitoh T (1989) Effects of added food on some attributes of an enclosed vole population. J Mammal 70:772–782. doi:10.2307/1381711
Schmitz OJ, Adler FR, Agrawal AA (2003) Linking individual-scale trait plasticity to community dynamics. Ecology 84:1081–1082. doi:10.1890/1051-0761(2003)084[1081:LITPTC]2.0.CO;2
Sheldon BC, Verhulst S (1996) Ecological immunology: costly parasite defences and trade-offs in evolutionary ecology. Trends Ecol Evol 11:317–321. doi:10.1016/0169-5347(96)10039-2
Shine R (1980) “Costs” of reproduction in reptiles. Oecologia 46:92–100. doi:10.1007/BF00346972
Shine R, Madsen T (1997) Prey abundance and predator reproduction: rats and pythons on a tropical Australian floodplain. Ecology 78:1078–1086. doi:10.2307/2265859
Siems DP, Sikes RS (1998) Tradeoffs between growth and reproduction in response to temporal variation in food supply. Environ Biol Fish 53:319–329. doi:10.1023/A:1007407925835
Stearns SC (1992) The evolution of life histories. University of Oxford Press, Oxford
Stearns SC, Koella JC (1986) The evolution of phenotypic plasticity in life-history traits: predictions of reaction norms for age and size at maturity. Evolution 40:893–913. doi:10.2307/2408752
Via S, Gomulkiewicz R, De Jong G, Scheiner SM, Schlichting CD, Van Tienderen PH (2001) Adaptive phenotypic plasticity: consensus and controversy. Trends Ecol Evol 10:212–217. doi:10.1016/S0169-5347(00)89061-8
Wedekind C (2002) Induced hatching to avoid infectious egg disease in whitefish. Curr Biol 12:69–71. doi:10.1016/S0960-9822(01)00627-3
Werner EE, Peacor SD (2003) A review of trait-mediated indirect interactions in ecological communities. Ecology 84:1083–1100. doi:10.1890/0012-9658(2003)084[1083:AROTII]2.0.CO;2
Ylönen H (1994) Vole cycles and antipredatory behaviour. Trends Ecol Evol 9:426–430. doi:10.1016/0169-5347(94)90125-2
Young JL, Bornik ZB, Marcotte ML, Charlie KN, Wagner GN, Hinch SG, Cooke SJ (2006) Integrating physiology and life history to improve fisheries management and conservation. Fish Fish 7:262–283. doi:10.1111/j.1467-2979.2006.00225.x
Zera AJ, Harshman LG (2001) The physiology of life history trade-offs in animals. Annu Rev Ecol Syst 32:95–126. doi:10.1146/annurev.ecolsys.32.081501.114006
Acknowledgments
We thank three anonymous referees for their valuable comments. This research was financially supported in part by the Global COE Program A06 to Kyoto University. TN was also supported by a Japan Society for the Promotion of Science Research Fellowship for Young Scientists (1702360).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nakazawa, T., Ohgushi, T. & Yamamura, N. Resource-dependent reproductive adjustment and the stability of consumer-resource dynamics. Popul Ecol 51, 105–113 (2009). https://doi.org/10.1007/s10144-008-0101-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10144-008-0101-9