Abstract
We studied the indirect effects of an aphid Uroleucon nigrotuberculatum on density and performance of herbivorous insects through tending ants and modification of plant traits on a tall goldenrod Solidago altissima in Japan. To examine ant-mediated indirect effects of the aphid on the leafhopper and geometrid moth caterpillars, we conducted an experiment in which we manipulated aphid densities. The aphid decreased the density of these herbivorous insects through ant-mediated indirect effects, because honeydew scattered by the aphid-attracted ants that then removed them. To examine plant-mediated indirect effects of the aphid on two temporally separated insects, a scale insect and a grasshopper, we compared the density and performance of these herbivorous insects on aphid-inoculated plants and aphid-free plants. Aphid-induced plant modifications had different effects on the scale insect and grasshopper. The aphid indirectly decreased the density and survivorship of the scale insect. On the other hand, the number of grasshoppers increased as a result of the increased number of leaves and the increased nitrogen content induced by prior aphid feeding. However, aphid infestation did not affect the survival of the grasshopper. Thus, the aphid has large indirect effects on co-occurring herbivorous insects through the removal behavior of tending ants and on temporally separated herbivorous insects through changes in quality and quantity of the tall goldenrod.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Associations between aphids and ants are common on a wide variety of plants and are generally accepted as being mutualistic interactions (Way 1963; Kaplan and Eubanks 2005; Stadler and Dixon 2005). Aphids provide sugar-rich honeydew to ants, and, in return, tending ants provide protection to aphids from their natural enemies and/or competitors (Buckley 1987). Although previous studies have focused on tight aphid-ant mutualisms, Del-Claro and Oliveira (1996) reported that aphids that scattered honeydew to the leaves or ground also attracted ants. Aggressive ants attracted by aphid honeydew can decrease the density of other herbivores and their natural enemies (Del-Claro and Oliveira 1999, 2000; Stadler and Dixon 1999). Hence, even if there is only a loose association between aphids and ants, aphids may indirectly reduce the performance and abundance of other herbivorous insects and predators through the actions of their ant mutualists in removing or preying upon them.
Aphid feeding can alter the traits of host plants, such as plant growth, soluble nitrogen content, amino acid and secondary compound concentrations, and resource allocation to roots, shoots, and seeds (Moran and Whitham 1990; Waltz and Whitham 1997; Petersen and Sandström 2001; Wimp and Whitham 2007). Such alterations induced by aphids can reduce the fitness of their subsequent aphid generations (Dixon 1970; Tedders 1978; Tedders and Thompson 1981). These alternations can also affect the performance and preference of other temporally separated sap suckers, such as scale insects and leafhoppers, in either a negative (Sluss 1967; Bumroongsook and Harris 1992) or positive (Way and Banks 1967; Way and Cammell 1970; Dorschner et al. 1987) way. Few studies, however, have explored how such plant-mediated indirect effects of aphids impact other feeding guilds, such as leaf chewers and leaf miners (but see Waltz and Whitham 1997). This lack of interest in inter-guild interactions is probably the result of the traditional view that competition occurs more frequently among closely related taxa (Miller 1967; Denno et al. 1995, for a review). However, recent reviews have argued the prevalence of interspecific competition among herbivorous insects mediated by herbivore-induced changes in plant traits (Ohgushi 2005; Denno and Kaplan 2007; Kaplan and Denno 2007).
The aphid Uroleucon nigrotuberculatum Olive is a common herbivore on tall goldenrod, Solidago altissima Linn, in Japan from mid-May to early August. The aphid attracts an aggressive ant Formica japonica Motschulsky that consumes scattered aphid honeydew on adjacent leaves. Thus, the aphid could indirectly affect other co-occurring insects through ant-mediated interactions. We found a positive correlation between the aphid and ant densities, and negative correlations between the densities of the ant and a leafhopper Nephotettix cincticeps Uhler, and the ant and a geometrid moth caterpillar Ascotis selenaria Butler (Y. Ando and T. Ohgushi, unpublished data). Hence, it is important to examine whether the aphid indirectly decreases the abundance of N. cincticeps and the geometrid moth caterpillar through the removal behavior of tending ants. Aphid colonization also initiates the production of new leaves starting in late August when the aphids are no longer present. This morphological change suggests that the aphid could indirectly affect herbivorous insects that appear in autumn. In fact, there was a negative correlation between densities of the aphid and a scale insect Parasaissetia nigra Nietner and a positive correlation between the densities of the aphid and a grasshopper Atractomorpha lata Motschulsky (Y. Ando and T. Ohgushi, unpublished data). Therefore, it is critical to determine whether aphid-induced plant modification affects abundance or survival of these late-emerging insects.
In this study, we experimentally investigated the mechanisms underlying the aphid impact on these herbivorous insects on tall goldenrod: (1) the indirect effects of the aphid on abundance of the leafhopper and the geometrid moth caterpillar through the removal behavior of the ant in spring and early summer and (2) the indirect effects of the aphid on abundance and survival of the scale insect and the grasshopper in autumn.
Materials and methods
Plant
Tall goldenrod, S. altissima Linn (Compositae), is a rhizomatous perennial herb that was introduced to Japan from North America approximately 100 years ago (Shimizu 2003). It has spread all over Japan and become one of the most abundant weeds. It grows in open and disturbed areas and frequently invades abandoned agricultural fields. Ramets emerge from overwintering rhizomes as the ground warms in April, and shoots grow continuously until September. Flowering occurs from late October to November.
Insects
U. nigrotuberculatum Olive (Homoptera: Aphidinae) is a stem-feeding aphid. It was also introduced from North America in the early 1990s (Ôtake 1999), and it has become a common insect on S. altissima in Japan. The aphid feeds exclusively on terminal shoots of S. altissima. The aphid emerges from mid-May and disappears by early August. Formica japonica Motschulsky (Hymenoptera: Formicidae) is the most abundant ant species in a common garden at the Center for Ecological Research, Kyoto University in Japan. On the tall goldenrod, the ant appears to feed the aphid honeydew scattered in an early season from mid-May to early August and to feed on the honeydew of a scale insect P. nigra Nietner (Homoptera: Coccidae) in a late season from September to late October.
In the early season, not only U. nigrotuberculatum, but also a phloem-feeding leafhopper N. cincticeps Uhler (Homoptera: Deltocephalidae) and a leaf-chewing geometrid moth A. selenaria Butler (Lepidoptera: Geometridae) caterpillars are commonly observed on leaves of S. altissima. Although there are few insects on the tall goldenrod from mid-August to September, a scale insect P. nigra and a grasshopper A. lata Motschulsky (Orthoptera: Pyrgomorphidae) frequently appear in early October when the aphid had already disappeared. P. nigra, a phloem-sucking polyphagous insect, settles below the middle parts of the stem of the tall goldenrod. At the bottom of shoots of tall goldenrods in the common garden, P. nigra females oviposit > 800 eggs in September. When they hatch first instar nymphs move to other parts of the plant, where they settle down and start feeding. Once established, scales no longer move. The life history of the scale insect has not been previously described. It occurs from early October to early November on the tall goldenrod and the subsequent generation is not found on it in this study area (Y. Ando, personal observation). A. lata, a leaf-chewing grasshopper that feeds on several agricultural crops, feeds on mature leaves (Okuno et al. 1995). Most mature leaf damage in this season is caused by A. lata in this study area.
Experimental design
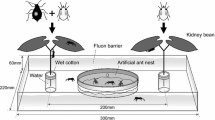
One hundred and eighty seedlings were randomly sampled from one patch of S. altissima at the Experimental Forest of Field Science Education and Research Center Kyoto University in Kyoto, and they were individually transplanted into pots containing peat-based soil in a greenhouse on 20 April 2002. On 1 May, we selected 30 plants for an aphid-manipulation experiment and randomly transplanted them into an experimental plot (6 m × 32 m) in a common garden at the Center for Ecological Research, Kyoto University, in Otsu, Shiga Prefecture, central Japan. They were set separately 1.5 m apart.
We randomly selected 52 potted plants for the aphid-inoculated treatment and 52 potted plants for the aphid-free treatment for experiments that were conducted in a laboratory and a greenhouse. For aphid inoculation, 1,040 adult aphids were collected from 40 aphid colonies at the common garden, and then 20 adult aphids were placed at a top of the apical stem of each aphid-inoculated plant on 25 May when the aphids appeared in the field. Each plant was fertilized with Hyponex liquid fertilizer (N–P–K, 6:10:15) and supplied with ample amounts of water. To avoid dispersal of aphids, all potted plants were individually covered with a nylon organdy that allowed normal plant growth. Plants were then placed in the greenhouse until the start of each experiment.
Aphid-manipulation experiment
To investigate whether the ant decreases the leafhopper and geometrid moth caterpillar in the aphid-present early season, an aphid manipulation experiment was conducted in the common garden from 25 May to 19 June in 2002. This experimental period was chosen to coincide with the timing of aphid appearance because the ant is present only on plants with aphids. We set up two treatments that alternated aphid-present and aphid-absent periods. Treatment A consisted of 20 aphids on the plants for the first 12 days (aphid-present period), no aphids for the next 12 days (aphid-absent period), and 20 aphids for the last 12 days (aphid-present period). In treatment B there were no aphids present for the first 12 days, 20 aphids for the next 12 days, and no aphids for the last 12 days. We also set up a control plant treatment where plants were exposed to aphid feeding continuously for 36 days. Each treatment had ten replicates. Once inoculated, no aphids were added during the experimental period. We counted the number of aphids, ants, leafhoppers, and geometrid moth caterpillars on each plant. Data were log (n + 1)-transformed and analyzed using a repeated-measure ANOVA to compare numbers of these herbivorous insects and the ant among three treatments. We also used Kendall’s rank correlation to determine the relationships between the leafhopper and the ant, and between the geometrid moth caterpillar and the ant in all treatments.
Effects of aphids on density and performance of the scale insect
To examine whether aphid feeding in the early season affects the density and performance of the late-emerging scale insect P. nigra, we conducted a greenhouse experiment using aphid-inoculated plants and aphid-free plants. We used 12 replicates each of aphid-inoculated and aphid-free plants. On 25 September, 24 mature females of the scale insect were collected from the common garden and were placed individually on each plant in the greenhouse. After most of the first instar nymphs had emerged and settled, the females were removed. On 1 October, the number of the established scale insects was counted to determine colonization success. The density of the scale insect was compared between treatments using a Mann–Whitney U-test. Then, the temporal change in numbers of the scale insects on each plant was examined from 1 to 30 October, by which time all of the scale insects had died and fallen off of the host plants. A Kaplan–Meier plot was constructed, and the remaining numbers of the scale insects was compared between treatments, using a Log-rank test.
Effects of aphids on density and performance of the grasshopper
To examine whether aphid feeding in the early season affects the performance of the late-emerging grasshopper A. lata, we conducted a laboratory experiment. We compared the survival period of grasshoppers provided with leaves of aphid-inoculated and aphid-free plants. On 7 October, 16 mature grasshopper females were collected from the common garden and reared individually in plastic cases (10 cm in diameter, 5.5 cm in depth) in an environmental chamber at 25°C, LD 14:10 h. After starvation for 24 h, eight grasshoppers were fed leaves from the aphid-inoculated plants, and the other eight grasshoppers were fed leaves from the aphid-free plants. As the grasshopper prefers mature leaves (Okuno et al. 1995), mature leaves were used in this experiment. Since S. altissima continuously flushes leaves throughout the season, new leaves were defined as leaves within 10 cm below a shoot tip, and mature leaves were defined as leaves that were more than 10 cm below the new leaves. We randomly collected mature leaves from the aphid-inoculated plants (n = 16) and aphid-free plants (n = 16), and provided each grasshopper with five leaves per day. This is more than a sufficient food supply as individual grasshoppers consume only two to three leaves a day. The number of days that individuals survived in the two treatments was compared using a Mann–Whitney U-test.
To examine the effects of leaf number and aphid treatment on grasshopper density, we conducted a greenhouse experiment. On 9 October, when the aphid was already absent, three grasshoppers were placed on each potted plant (n = 8) after the numbers of mature leaves were counted. The potted plants were placed close together so that the grasshopper could access both the aphid-inoculated and aphid-free plants. One hour later, we counted the number of grasshoppers on each plant. We compared the number of grasshoppers and leaves between aphid-inoculated and aphid-free plants, using a Mann–Whitney U-test. The number of grasshoppers was analyzed using ANCOVA with aphid treatment as a factor and the number of leaves as a covariate. To determine the relationship between leaf number and the number of grasshoppers, we conducted a linear regression for aphid-inoculated and aphid-free treatments.
Effects of aphid colonization on leaf quality and quantity
To examine whether aphid colonization affects host plant traits in the (aphid present) early season and the (aphid absent) late season, we recorded the number of leaves and measured the nitrogen and water content of leaves in a greenhouse. We selected eight each of the aphid-inoculated and aphid-free plants in early August 2002. After the numbers of new and mature leaves were counted, ten leaves were randomly taken from each plant for measurement of nitrogen and water content. In early September, before late-emerging herbivores were present, the late season measurement was conducted. The numbers of new and mature leaves on another eight aphid-exposed and eight aphid-free plants were counted. We then randomly collected ten leaves from each plant and measured leaf nitrogen and water content. Since S. altissima flushed leaves throughout the experiment, new leaves were defined as leaves within 10 cm of the top of shoot, and mature leaves were defined as any that were more than 10 cm below the new leaves. Individual leaves were weighted in the laboratory and oven-dried at 60°C for 48 h to calculate water content. After the dried leaves were powdered, nitrogen content was measured using an elemental analyzer (Macro Corder JM1000CN, J-Science, Kyoto, Japan). A Mann–Whitney U-test was used to compare these traits of aphid-inoculated and aphid-free plants.
Results
Effect of the aphid on the leafhopper and the geometrid moth
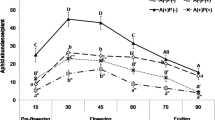
The aphid presence/absence experiments clearly showed that there was a large difference in the number of herbivorous insects, the leafhoppers and the geometrid moth caterpillars, and the ants, due to the presence or absence of aphids (Fig. 1). The numbers of aphids, leafhoppers, moth caterpillars, and ants remained relatively constant on control plants throughout the experiment. The numbers of leafhoppers and moth caterpillars on both treatment A and treatment B plants in the aphid-present periods were significantly lower than in aphid-absent periods. Conversely, the number of ants on treatments A and B plants in the aphid-present periods were significantly greater than in aphid-absent periods. The densities of leafhoppers, geometrid moth caterpillars, and ants significantly differed among treatment A, treatment B, and control plants (leafhoppers: F 2,27 = 32.50, P < 0.001; moth caterpillars: F 2,27 = 31.07, P < 0.001; ants: F 2,27 = 572.31, P < 0.00). There were significant interaction effects of treatment × day on the number of leafhoppers (F 70,945 = 30.73, P < 0.001), geometrid moth caterpillars (F 70,945 = 30.65, P < 0.001), and ants (F 70,945 = 636.95, P < 0.001). The numbers of leafhoppers and geometrid moths were negatively correlated with the number of ants (Kendall’s rank correlation: τ = −0.76, P < 0.001 for leaf hoppers and ants; τ = −0.65, P < 0.001 for moth caterpillars and ants).
Effects of aphids on density and survival of the scale insect and the grasshopper
Previous feeding by aphids negatively affected the density and survival of the scale insect P. nigra. Scale density was significantly lower on the aphid-inoculated plants than on the aphid-free plants (Mann–Whitney U-test: U = 0, P < 0.001, Fig. 2). The scale insects on the aphid-inoculated plants survived significantly differently, compared to those on the aphid-free plants (log-rank test, P < 0.001, Fig. 3).
On the other hand, previous aphid infestation did not have a significant effect on the survival period of the grasshopper A. lata [13.12 ± 1.31 days (mean ± SE) vs. 12.08 ± 2.48 days for aphid-inoculated and aphid-free plants, respectively; Mann–Whitney U-test: U = 14, P = 0.07]. In the greenhouse experiment, aphid infestation significantly enhanced leaf production (Mann–Whitney U-test: U = 2, P = 0.001) and increased grasshopper density (Mann–Whitney U-test: U = 0, P < 0.001, Fig. 4). The number of grasshoppers on the aphid-inoculated plants was greater than the initial number of grasshoppers released on each plant, but on the aphid-free plants there were fewer grasshoppers than the initial number released (Fig. 4). A regression analysis for each treatment detected a significantly positive relationship between the number of leaves and grasshopper density both in aphid-inoculated and aphid-free plants (Fig. 5). Also, analysis of covariance indicates that a significant effect of aphid infestation on grasshopper density was detected when leaf number was given as a covariate (ANCOVA: treatment: F 1,13 = 9.84, P = 0.008; Fig. 5), although treatment × leaf number interaction was not significant (ANCOVA: F 1,12 = 1.91, P = 0.193). These results indicate that the aphid infestation increased grasshopper density not only by increasing the number of leaves, but also by altering other plant characteristics.
Effects of aphids on leaf quality and quantity
In early August, no significant differences in leaf nitrogen, water content, and the numbers of new and mature leaves were found between the aphid-inoculated and aphid-free plants (Mann–Whitney U-test: nitrogen content: U = 20, P = 0.19 for new leaves, U = 24, P = 0.37 for mature leaves; water content: U = 31, P = 0.88 for new leaves, U = 29.5, P = 0.80 for mature leaves; number of leaves: U = 16, P = 0.08 for new leaves, U = 25, P = 0.44 for mature leaves, Table 1). On the other hand, in early October nitrogen content and leaf production were significantly greater in the aphid-inoculated plants than in the aphid-free plants (nitrogen content: U = 0, P < 0.001 for new leaves; U = 0, P < 0.001 for mature leaves; number of leaves: U = 0, P < 0.001 for new leaves; U = 0, P < 0.001 for mature leaves, Table 1), although water content did not differ between the treatments (U = 21, P = 0.25 for new leaves; U = 31, P = 0.88 for mature leaves).
Discussion
Ant-mediated indirect effects of aphids on herbivorous insects
Although the ant F. japonica did not contact the aphid directly, it climbed onto plants to consume honeydew scattered by the aphid colonies. As a result, the ant has a strong negative impact on the densities of the leafhopper and on the geometrid moth caterpillar. We frequently observed that ants removed these insects in the field. In the early season when aphids were present, the tending ants reduced the density of the leafhoppers by 87–100% and the density of the geometrid moth caterpillars by 73–100%. This implies that the aphid indirectly affected these co-occurring herbivores. It has been well documented that strong aphid-ant associations can reduce the density of other herbivorous insects and predators through the removal activities of tending ants (Messina 1981; Wimp and Whitham 2001, 2007). Although U. nigrotuberculatum is a common aphid on S. altissima in North America (Edson 1985; Pilson 1992), such strong indirect effects through tending ants have received little attention. This is probably because the aphid does not have a close interaction with ants in North America (Cappuccino 1987; Meyer 1993). Douglas and Sudd (1980) argued that scattered honeydew droplets were unlikely to be attractive to ants, because attending ants ignore them. However, our study clearly showed that even the honeydew scattered by the aphid could attract the ant, which led to a decrease in the number of other herbivorous insects. To our knowledge, there are no other studies establishing that aphids scattering honeydew on nearby leaves attract ants. However, Del-Claro and Oliveira (1996) reported that a treehopper Guayaquila xiphias scattered honeydew, which provided cues to ground-dwelling ants, and ants climbed onto the host plants and tended the treehopper. They found that 21 ant species, in spite of a lack of close association with the treehopper, protected the treehoppers from predators such as salticid spiders, syrphid flies, and parasitoid wasps.
The aphid may affect herbivorous insects in the early season through exploitative competition. Co-occurring phloem feeders often compete for assimilates in phloem vessels of host plants (Inbar et al. 1995; Denno and Kaplan 2007). Also, aphids can change leaf nitrogen and plant growth (Moran and Whitham 1990; Salt et al. 1996; Petersen and Sandström 2001), which may decrease food availability for the leafhoppers and geometrid moth caterpillars. These effects are, however, unlikely to have a great impact on these herbivores in our study because aphid infestation did not alter leaf traits in the early season. Also, significant direct effects of the aphid on these herbivores were not detected (Y. Ando and T. Ohgushi, unpublished data).
In our study, densities of these herbivorous insects changed, corresponding with changes in ant densities, and the density of each herbivorous insect was negatively correlated with ant density. Hence, the aphid is more likely to decrease co-occurring herbivore densities through ant-mediated indirect effects rather than through interspecific competition for plant resources.
Plant-mediated indirect effects of aphids on temporally separated herbivorous insects
Our study clearly illustrated that aphids negatively affected the colonization of the scale insect, P. nigra, which occurred in autumn when aphids were no longer present. The aphid decreased density of the nymphal scale insects and altered survival pattern of the established scale insects. Early season sap feeders often indirectly reduce performance and population growth of late-emerging sap feeders by altering the sap quality of shared host plants (Faeth 1986; Denno et al. 1995; Ohgushi 2005; Denno and Kaplan 2007). In particular, several studies have revealed that aphid infestation decreased the abundance of subsequent sap feeders by altering amino acid composition or by increasing allelochemicals (Salt et al. 1996; Petersen and Sandström 2001; Voelckel et al. 2004). Therefore, the early aphid infestation could have decreased phloem sap quality during the late season, resulting in a decrease in the scale insect density.
In contrast to effects on scale insects, the grasshopper density increased on the plants that were previously attacked by aphids in the greenhouse experiment. Compared to the initial number of grasshoppers released on each plant, the number of grasshoppers increased on the aphid-inoculated plants, but decreased on the aphid-free plants. This indicates that the grasshoppers preferred the aphid-inoculated plants to the aphid-free plants. Host plant regrowth following herbivory often has positive effects on herbivorous insects because of increased resource availability (Damman 1989; Mopper et al. 1991; Masters et al. 2001). Shortly, after aphid colonization, leaf flush on the aphid-inoculated plants continuously occurred, although this leaf flushing rarely occurred in the aphid-free plants. Because grasshoppers generally depend on poor-quality leaves, they need a large number of leaves for development (Joern and Behmer 1997). Hence, grasshoppers may prefer host plants with increased leaf biomass, and our study indicated that grasshopper density was correlated with the number of leaves on the plant.
The increase in grasshopper density could be due not only to increased leaf biomass, but also to increased leaf nitrogen. Heidorn and Joern (1987) examined the feeding preference in 16 grasshopper species at different leaf nitrogen levels of Calamovilfa longifolia and found that grasshoppers preferred leaves with higher nitrogen levels. It is likely that the aphid indirectly increased grasshopper density by increasing leaf nitrogen in the aphid-inoculated plants. On the other hand, aphid infestation did not affect the survival of the grasshopper in the laboratory experiment. Several studies have demonstrated that nitrogen concentration in leaves does not affect survival of grasshoppers (Joern and Behmer 1997; Berner et. al. 2005).
Our study clearly demonstrated that aphid-induced plant responses could affect temporally separated herbivorous insects in different ways, with the scale insect decreasing and the grasshopper increasing. Smith and Boyko (2007) argued that aphid feeding could trigger multiple signaling pathways in plants and induce various responses in a suit of traits, including an increase in photosynthesis, photorespiration, and production of allelochemicals. Thus, it is likely that herbivorous insects with different feeding modes respond differentially to herbivore-induced changes in plant traits.
Importance of indirect effects of aphids on the structure of insect communities
We clearly illustrated that the aphid colonization in the spring affected both co-occurring herbivores through ant-mediated indirect effects and temporally separated herbivores through plant-mediated indirect effects in the autumn. Our study also showed that the effects of aphid-induced plant modifications differed between the scale insect and the grasshopper. In contrast, the ant-mediated indirect effects of the aphid were consistently negative for herbivorous insects. This suggests that herbivore responses to plant trait modifications induced by the aphid are highly variable, depending on feeding mode. Since aphids are very common insects on a wide variety of woody and herbaceous plants, they are likely to play an important role in determining the structure of arthropod communities on many terrestrial plants (Wimp and Whitham 2007). To understand aphid effects on arthropod communities, we need to explore plant-mediated indirect effects of aphids on herbivorous insects, as well as ant-mediated indirect effects of aphids.
References
Berner D, Blanckenhorn WU, Körner C (2005) Grasshoppers cope with low host plant quality by compensatory feeding and food selection: N limitation challenged. Oikos 111:525–533
Buckley RC (1987) Ant-plant-homopteran interactions. Adv Ecol Res 16:53–85
Bumroongsook S, Harris MK (1992) Distribution, conditioning, and interspecific effects of blackmargined aphids and yellow pecan aphids (Homoptera: Aphididae) on pecan. J Econ Entomol 85:187–191
Cappuccino N (1987) Comparative population dynamics of two goldenrod aphids: spatial patterns and temporal constancy. Ecology 68:1634–1646
Damman H (1989) Facilitative interactions between two lepidopteran herbivores of Asimina. Oecologia 78:214–219
Del-Claro K, Oliveira PS (1996) Honeydew flicking by treehoppers provides cues to potential tending ants. Anim Behav 51:1071–1075
Del-Claro K, Oliveira PS (1999) Ant-Homoptera interactions in a neotropical savanna: the honeydew-producing treehopper, Guayaquila xiphias (Membracidae), and its associated ant fauna on Didymopanax vinosum (Araliaceae). Biotropica 31:135–144
Del-Claro K, Oliveira PS (2000) Conditional outcomes in a neotropical treehopper-ant association: temporal and species-specific variation in ant protection and homopteran fecundity. Oecologia 124:156–165
Denno RF, Kaplan I (2007) Plant-mediated interactions in herbivorous insects: mechanisms, symmetry, and challenging the paradigms of competition past. In: Ohgushi T, Craig TP, Price PW (eds) Ecological communities: plant mediation in indirect interaction webs. Cambridge University Press, Cambridge, pp 19–50
Denno RF, McClure MS, Ott JR (1995) Interspecific interactions in phytophagous insects: competition reexamined and resurrected. Annu Rev Entomol 40:297–331
Dixon AFG (1970) Stabilization of aphid populations by an aphid induced plant factor. Nature 227:1368–1369
Dorschner KW, Ryan JD, Johnson RC, Eikenbary RD (1987) Modification of host nitrogen levels by the greenbug (Homoptera: Aphididae)—its role in resistance of winter wheat to aphids. Environ Entomol 16:1007–1011
Douglas JM, Sudd JH (1980) Behavioural coordination between an aphis (Symydobius oblongus von Heyden; Hemiptera: Callaphidae) and the ant that attends it (Formica lugubris Zetterstedt; Hymenoptera: Formicidae): an ethological analysis. Anim Behav 28:1127–1139
Edson JL (1985) The influences of predation and resource subdivision on the coexistence of goldenrod aphids. Ecology 66:1736–1743
Faeth SH (1986) Indirect interactions between temporally separated herbivores mediated by the host plant. Ecology 67:479–494
Heidorn TJ, Joern A (1987) Feeding preference and spatial distribution of grasshoppers (Acrididae) in response to nitrogen fertilization of Calamovilfa longifolia. Funct Ecol 1:369–375
Inbar M, Eshel A, Wool D (1995) Interspecific competition among phloem-feeding insects mediated by induced host-plant sinks. Ecology 76:1506–1515
Joern A, Behmer ST (1997) Importance of dietary nitrogen and carbohydrates to survival, growth, and reproduction in adults of the grasshopper Ageneotettix deorum (Orthoptera: Acrididae). Oecologia 112:201–208
Kaplan I, Denno RF (2007) Interspecific interactions in phytophagous insects revisited: a quantitative assessment of competition theory. Ecol Lett 10:977–994
Kaplan I, Eubanks MD (2005) Aphids alter the community-wide impact of fire ants. Ecology 86:1640–1649
Masters GJ, Jones TH, Rogers M (2001) Host-plant mediated effects of root herbivory on insect seed predators and their parasitoids. Oecologia 127:246–250
Messina FJ (1981) Plant protection as a consequence of an ant-membracid mutualism: interactions on goldenrod (Solidago sp.). Ecology 62:1433–1440
Meyer GA (1993) A comparison of the impacts of leaf- and sap-feeding insects on growth and allocation of goldenrod. Ecology 74:1101–1116
Miller RS (1967) Pattern and process in competition. Adv Ecol Res 4:1–74
Mopper S, Maschinski J, Cobb N, Whitham TG (1991) A new look at habitat structure: consequences of herbivore-modified plant architecture. In: Bell SS, McCoy ED, Mushinsky HR (eds) Habitat structure: the physical arrangement of objects in space. Chapman and Hall, London, pp 260–280
Moran NA, Whitham TG (1990) Interspecific competition between root-feeding and leaf-galling aphids mediated by host-plant resistance. Ecology 71:1050–1058
Ohgushi T (2005) Indirect interaction webs: herbivore-induced effects through trait change in plants. Annu Rev Ecol Evol Syst 36:81–105
Okuno T, Tanaka Y, Kimura Y, Yoneyama S (1995) Diseases and pests of flowers and vegetables in color. Hoikusha, Osaka, in Japanese
Ôtake A (1999) Analytical study of fundatrix populations of Uroleucon nigrotuberculatum (Olive) (Hemiptera: Aphididae: Aphidinae) on an observation plot of the goldenrod Solidago altissima L. Appl Entomol Zool 34:435–442
Petersen MK, Sandström JP (2001) Outcome of indirect competition between two aphid species mediated by responses in their common host plant. Funct Ecol 15:525–534
Pilson D (1992) Relative resistance of goldenrod to aphid attack: changes through the growing season. Evolution 46:1230–1236
Salt DT, Fenwick P, Whittaker JB (1996) Interspecific herbivore interactions in a high CO2 environment: root and shoot aphids feeding on Cardamine. Oikos 77:326–330
Shimizu T (2003) Naturalized plants of Japan. Heibonsha, Tokyo, in Japanese
Sluss RR (1967) Population dynamics of the walnut aphid, Chromaphis juglandicola (Kalt.) in Northern California. Ecology 48:41–58
Smith CM, Boyko EV (2007) The molecular bases of plant resistance and defense responses to aphid feeding: current status. Entomol Exp Appl 122:1–16
Stadler B, Dixon AFG (1999) Ant attendance in aphids: why different degrees of myrmecophily? Ecol Entomol 24:363–369
Stadler B, Dixon AFG (2005) Ecology and evolution of aphid-ant interactions. Annu Rev Ecol Evol Syst 36:345–372
Tedders WL (1978) Important biological and morphological characteristics of the foliar-feeding aphids of pecan. US Dept Agric Tech Bull 1579:1–29
Tedders WL, Thompson JM (1981) Histological investigation of stylet penetration and feeding damage to pecan foliage by three aphids (Hemiptera (Homoptera): Aphididae). Misc Publ Entomol Soc Am 12:69–83
Voelckel C, Weisser WW, Baldwin IT (2004) An analysis of plant–aphid interactions by different microarray hybridization strategies. Mol Ecol 13:3187–3195
Waltz AM, Whitham TG (1997) Plant development affects arthropod communities: opposing impacts of species removal. Ecology 78:2133–2144
Way MJ (1963) Mutualism between ants and honeydew-producing Homoptera. Annu Rev Entomol 8:307–344
Way MJ, Banks CJ (1967) Intra-specific mechanisms in relation to the natural regulation of numbers of Aphis fabae Scop. Ann Appl Biol 59:189–205
Way MJ, Cammell M (1970) Aggregation behavior in relation to food utilization by aphids. In: Watson A (ed) Animal populations in relation to their food resources. Blackwell Scientific Publications, Oxford, pp 229–247
Wimp GM, Whitham TG (2001) Biodiversity consequences of predation and host plant hybridization on an aphid-ant mutualism. Ecology 82:440–452
Wimp GM, Whitham TG (2007) Host plants mediate ant-aphid mutualisms and their effects on community structure and diversity. In: Ohgushi T, Craig TP, Price PW (eds) Ecological communities: plant mediation in indirect interaction webs. Cambridge University Press, Cambridge, pp 275–305
Acknowledgments
We thank T. P. Craig and S. Utsumi for valuable comments on the manuscript. We also benefited from discussions with members of the Center for Ecological Research, Kyoto University. This study was supported by the Ministry of Education, Culture, Sports, Science and Technology Grant-in-Aid for Scientific Research (A-15207003) to T. Ohgushi, the 21st Century COE Program (A14), and the Global COE Program (A06).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ando, Y., Ohgushi, T. Ant- and plant-mediated indirect effects induced by aphid colonization on herbivorous insects on tall goldenrod. Popul Ecol 50, 181–189 (2008). https://doi.org/10.1007/s10144-007-0072-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10144-007-0072-2