Abstract
Competitive interactions between parasitoid species are traditionally evaluated when they compete for a single host species. Yet, the presence of additional host species can alter competitive interactions, even if the host is unsuitable for parasitoid development. In alfalfa of the mid-western USA, a native parasitoid species, Praon pequodorum, was once a dominant natural enemy, but it has become rare since the introduction of another parasitoid, Aphidius ervi. Despite A. ervi’s competitive superiority for their most common host, the pea aphid Acyrthosiphum pisum, P. pequodorum still persists at low densities. We performed a suite of laboratory and field studies to determine if the presence of an alternative host, the spotted alfalfa aphid Therioaphis maculata, may mitigate A. ervi’s competitive superiority and facilitate P. pequodorum’s persistence. We show that spotted alfalfa aphids reduce the foraging efficiency of both parasitoid species for pea aphids, despite spotted alfalfa aphids being an unsuitable host. This decrease in efficiency, however, was not symmetrical; the presence of spotted alfalfa aphids had a greater detrimental effect on A. ervi foraging for pea aphids. This might facilitate the persistence of the competitively inferior P. pequodorum. Our study suggests that indirect effects generated by the presence of alternative hosts are important for understanding parasitoid–host dynamics and overall insect community structure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Indirect community-level interactions that arise from three or more species interacting are often thought to be crucial in shaping community structure and altering competitive interactions (Holt and Lawton 1994; Wootton 1994; Müller and Godfray 1997; Hudson and Greenman 1998). For example, the presence of a shared parasitoid has been shown either to enhance competition between two host species (Chaneton and Bonsall 2000; van Veen et al. 2006) or to mitigate competition between hosts (van Veen et al. 2005). There has been much less work investigating the reciprocal effect: how multiple host species may alter competition between parasitoid species. Given that quantitative host–parasitoid food webs have demonstrated that parasitoid species frequently share multiple host species (Müller et al. 1999; Morris et al. 2004), competitive interactions between parasitoids may often be embedded within complex networks of potential hosts.
Since insect parasitoids often have numerous possible host species, they are frequently confronted with the decision of which species to attack and parasitize. This decision may have large fitness consequences if host species vary in suitability for parasitoid development; spending foraging time or eggs on less-suitable hosts will decrease parasitoid foraging success and ultimately decrease parasitoid population size (Hoogendoorn and Heimpel 2002; Heimpel et al. 2003). Alternatively, being able to choose among multiple suitable host species may also expand a parasitoid’s host range, increasing the parasitoid population (Landis et al. 2000). If there are several species of parasitoids that make different foraging decisions, they may differentially suffer the consequences of foraging for and attacking different host species. This in turn could potentially affect the relative competitive strengths of the parasitoid species. Those parasitoid species that can effectively use suitable hosts while wasting less time and fewer eggs on unsuitable hosts will gain in relative competitive ability when in an environment containing multiple host species.
We investigated the foraging behaviors of two parasitoid species (Hymenoptera: Braconidae), Aphidius ervi Haliday and Praon pequodorum Viereck, on two aphid host species (Homoptera: Aphididae): pea aphids Acyrthosiphon pisum Harris and spotted alfalfa aphids Therioaphis maculata Buckton. For both parasitoid species, pea aphids are a suitable host (Schellhorn et al. 2002), whereas we could find no published information on the suitability of spotted alfalfa aphids for parasitoid reproduction. We wanted to know whether spotted alfalfa aphids could be used as an alternative host, and whether the presence of spotted alfalfa aphids affected the attack rate of either parasitoid species on pea aphids.
Since A. ervi was introduced into North America in the 1950s for biological control the parasite guild attacking pea aphids has changed (Mackauer and Kambhampati 1986; Danyk 1993); populations of P. pequodorum have declined in alfalfa fields, and P. pequodorum is now rare (Danyk 1993; Schellhorn et al. 2002). Previous studies have investigated whether the competitive interactions between A. ervi and P. pequodorum could help explain this pattern. In direct larval competition, P. pequodorum is the better competitor (Danyk 1993; Danyk and Mackauer 1996). However, A. ervi has been shown to have higher attack rates than P. pequodorum on pea aphids (Schellhorn et al. 2002). Furthermore, A. ervi shows little inhibition from ovipositing in previously parasitized hosts, whereas P. pequodorum avoids superparasitism (Schellhorn et al. 2002). Because alfalfa fields are harvested roughly every 4–6 weeks over the summer growing season, population densities of pea aphids fluctuate widely, placing a premium on high parasitoid population growth rates and hence high foraging efficiency. Consequently, a simple population model suggests the rapid attack and lack of inhibition can give A. ervi a competitive edge over P. pequodorum in alfalfa fields in southern Wisconsin, USA (Schellhorn et al. 2002). Nonetheless, P. pequodorum persists throughout the state, often in areas with relatively little alfalfa acreage (K. J. Tilmon, personal communication).
Here, we ask how the presence of spotted alfalfa aphids affects A. ervi and P. pequodorum foraging, and whether the presence of spotted alfalfa aphids could in part mitigate the competitive superiority of A. ervi over P. pequodorum by providing an alternative host or by decreasing A. ervi’s foraging efficiency relative to P. pequodorum. First, we performed a choice test with single parasitoid females designed to determine the relative preferences of both parasitoid species for pea aphids versus spotted alfalfa aphids. Our second experiment investigated the foraging efficiencies of A. ervi and P. pequodorum females placed together in microcosms containing either pea aphids alone or both pea and spotted alfalfa aphids. This experiment tested whether the presence of spotted alfalfa aphids affected the relative competitive abilities of A. ervi and P. pequodorum and whether spotted alfalfa aphids were suitable for parasitoid development. Our third experiment was designed to give more information about how the presence of spotted alfalfa aphids affected the foraging rate of A. ervi for pea aphids; it consisted of detailed behavioral observations of A. ervi foraging for pea aphids in the presence or absence of spotted alfalfa aphids. Finally, we conducted a mesocosm experiment in large cages in an alfalfa field to investigate the effect of spotted alfalfa aphid density on the relative competitive abilities of A. ervi and P. pequodorum at the population scale.
Materials and methods
Study system
Aphidius ervi is a solitary endoparasitoid of aphids. It was introduced to North America from Europe to control pea aphids and has since spread throughout the United States and Canada to become the dominant pea aphid parasitoid in North America (Danyk 1993). Praon pequodorum is a native North American solitary endoparasitoid of aphids and a known generalist of a number of hosts, including the pea aphid in alfalfa (Schellhorn et al. 2002). Praon pequodorum larvae spin a cocoon within and below the mummy, firmly securing it to the leaf with a visible pedestal of silk. Aphidius ervi larvae spin their cocoons entirely inside the mummy, instead of below it. This difference makes mummies of the two species easily distinguishable.
The pea aphid is an Old World species that was introduced to North America sometime in the nineteenth century (Hagen et al. 1976; Mackauer and Kambhampati 1986). It is considered a pest of some species but rarely causes economic damage in alfalfa (Rauwald and Ives 2001). The spotted alfalfa aphid was first found in the United States in the 1950s (Smith 1959) and it is also an occasional pest of alfalfa in Wisconsin. Pea aphids, however, are by far the dominant species of aphid in alfalfa. Nonetheless, spotted alfalfa aphids occur regularly, particularly under hot, dry conditions (Forbes et al. 2005).
In all experiments, P. pequodorum and A. ervi were used from field-gathered mummies or mummies from recently established greenhouse colonies reared on pea aphids. The pea aphid colony was maintained on fava beans and the spotted alfalfa aphid colony on alfalfa.
Host preferences
We conducted experiments on both parasitoid species in petri dish arenas to determine if either parasitoid would attack spotted alfalfa aphids and, if so, each species’ preference for the aphid species. One alfalfa trifoliate infested with spotted alfalfa aphids was placed in a petri dish, and aphids were removed using forceps until five remained. Five pea aphids were then added to the petri dish. A single mated female parasitoid that had not yet experienced aphids was introduced into the petri dish and observed for 10 min. If the parasitoid came into contact with an aphid and examined the aphid with its antennae, this event was scored as an encounter. If, upon encountering an aphid, the parasitoid proceeded to insert her ovipositor into the aphid, this event was scored as an attack. Insertion of the ovipositor does not necessarily lead to oviposition (Ives et al. 1999), so attacks are not necessarily ovipositions. If no aphids were attacked in the first 5 min of observation, the trial was terminated. We conducted a total of 63 trials, using new aphids and parasitoids in each trial. Of the 63 trials, 16 were with A. ervi and 47 trials used P. pequodorum. We conducted more trials with P. pequodorum because of their much lower overall rates of aphid encounters and attacks.
We used data from all completed trials to estimate the probability of attacking an aphid once it was encountered, Prob(attack|encounter), as the proportion of encounters leading to attacks. To compare parasitoid species, we calculated the ratio Prob(A. ervi attack|encounter)/Prob(P. pequodorum attack|encounter) for each aphid species; the greater this ratio, the more likely that A. ervi was willing to attack an encountered aphid compared to P. pequodorum. Statistical tests of host acceptance were complicated by the structure of the data; some parasitoids encountered many more aphids than others, generating a strong effect of individual variation. Therefore, we performed randomization tests by creating simulated data sets in which the association of aphid species with the Prob(attack|encounter) was randomized. The data were organized into three columns giving the parasitoid individual, the aphid species, and whether or not an encounter was followed by an attack. To simulate a data set, we randomly assigned each record of a parasitoid individual to one of the two aphid species. This makes the experimental result (whether the encounter was followed by an attack) independent of the identity of the aphid species. For statistical tests, 10,000 data sets were simulated, and statistical confidence was determined by comparing the true data to the distribution of Prob(A. ervi attack|encounter)/Prob(P. pequodorum attack|encounter) from the simulated data. Thus, the null hypothesis is that individual parasitoids attacked aphid individuals randomly with respect to aphid species.
Laboratory microcosms
Experiments on single plants were conducted to determine if spotted alfalfa aphids are suitable for larval development for either parasitoid and to see if the presence of spotted alfalfa aphids influences the relative reproductive success of A. ervi versus P. pequodorum on pea aphids. Single alfalfa stems were enclosed in a cage consisting of a tube of transparent mylar with screen windows. Eighteen randomly selected plants were inoculated with 10 pea aphids, and 15 additional plants were inoculated with 10 pea aphids and 10 spotted alfalfa aphids in an additive design. Aphids were allowed to acclimate for approximately 2 h, and then one mated female parasitoid from each species was added to each plant. After 20 h, parasitoids were removed. Plants were checked daily for mummies of each parasitoid species, and any mummies found were removed and the species of parasitoid noted by closely checking for external cocoons which indicated that it was P. pequodorum and not A. ervi.
For this experiment, we chose an additive rather than substitutive design to test the null hypothesis that the presence/absence of spotted alfalfa aphids has no effect of the relative foraging efficiencies of both parasitoid species for pea aphids. Although the additive design means that the treatment with spotted aphids has a greater total number of aphids, we found that spotted aphids were unsuitable hosts for the development of both parasitoid species (see Results). Therefore, the additive design maintains the same number of suitable hosts (and hence resources for reproduction) in the two treatments.
To test for differences in the number of mummies formed by each parasitoid species, we performed a Poisson regression in which the number of pea aphid mummies found on a plant was the dependent variable, and the species of parasitoid mummy (A. ervi or P. pequodorum) and treatment (presence/absence of spotted alfalfa aphids) were the independent variables; plants were treated as random effects, and we blocked the data into two groups that were performed at different times of year (summer and fall). We used a Poisson regression that assumes the number of mummies on the plant are Poisson distributed because the number of mummies was often small or zero. Analyses were performed using geepack (Yan and Fine 2004) in the R statistical package.
Effect of spotted alfalfa aphids on A. ervi behavior
Behavioral observation experiments were conducted to quantify the effect of spotted alfalfa aphids on the attack rate of A. ervi on pea aphids. We observed the foraging behavior of A. ervi on single alfalfa stems with either pea aphids or a combination of pea aphids and spotted alfalfa aphids. Plants and cages were established as in the previous experiment except that aphid density was increased to either 20 pea aphids or 20 pea aphids and 20 spotted alfalfa aphids. Again, an additive design was used to test the null hypothesis that the presence/absence of the (unsuitable) spotted aphids affected foraging efficiencies on pea aphids. Aphids were allowed to acclimate for 30 min before a single, naïve (having no prior oviposition opportunities), mated female parasitoid was added to the plant. Each parasitoid was observed continuously and its behavior noted to the nearest second. Behavior was classified as moving, sitting, or cleaning, and we counted the number of attacks on pea and spotted alfalfa aphids. Ten A. ervi were observed on plants with only pea aphids and ten A. ervi were observed on plants with pea aphids and spotted alfalfa aphids. Parasitoids were observed until the parasitoid failed to attack an aphid in 10 min, or after a maximum of 1 h. Preliminary observations indicated that parasitoids that failed to attack an aphid in 10 min were extremely unlikely to attack another aphid for an extended period. The average length of an observation was 51.2 ± 2.7 min.
We used t tests to analyze how the presence of spotted alfalfa aphids affected the number of attacks on pea aphids and the total number of attacks on both aphid species. We also compared the foraging behavior of wasps in the presence or absence of spotted alfalfa aphids. To account for the different length of observation periods, time budget data were converted to the proportion of time each parasitoid spent moving, sitting, and cleaning. Because the proportion of time spent in each of the three categories must sum to one, there were only two independent responses (Cisneros and Rosenheim 1998). We therefore analyzed data using a multivariate linear regression model with the proportions of time spent moving and sitting (square root arcsine transformed) as dependent variables, and the presence/absence of spotted alfalfa aphids as the independent variable (SAS Institute 2000).
Field mesocosms
We conducted field-cage experiments to determine the population-level effect of spotted alfalfa aphids on A. ervi, P. pequodorum, and their competition for a shared host. Experiments were conducted in an alfalfa field at the University of Wisconsin, Arlington Agricultural Research Station using 20 2 × 2 × 2 m field cages. Eight of the cages were uprooted during a high-speed wind storm midway through the experiment, so we report data from only the remaining 12 cages. On 30 August 2005, cages were placed in alfalfa that had recently been cut and then vacuumed using a D-vac insect sampler. This method removed almost all natural enemies and greatly reduced densities of parasitized and unparasitized aphids. Cages were mounded with soil around the base to stop organisms entering the cages and to keep the desired organisms inside cages. After the alfalfa reached 15–20 cm high, cages were inoculated with aphids from greenhouse colonies. Half of the cages were randomly selected and inoculated with 100 pea aphids, and the other half were inoculated with 100 pea aphids and 100 spotted alfalfa aphids. Of the 12 surviving cages, each treatment was represented by 6 cages.
Over the course of the first 2 weeks of the experiment, five P. pequodorum and three A. ervi females were added to every cage. The number of parasitoids added was based on the assumption that A. ervi would be in cages at low densities given their natural abundance in the field and their imperfect removal prior to the experiment. We sampled 100 alfalfa stems in each cage every 2–4 days to determine aphid densities, at which time we also performed extensive visual surveys within each cage to count the number of mummies of each parasitoid species. The experiment was terminated after 20 days following a second high-speed wind storm.
Results
Host preferences
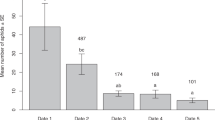
Parasitoids encountered aphids in all 63 trials, but only attacked aphids in 22 trials. Both P. pequodorum and A. ervi were observed to attack both of the two aphid species. Praon pequodorum differentiated between the two aphids, being almost twice as likely to attack pea aphids following an encounter than it was to attack spotted alfalfa aphids after an encounter (Fig. 1); however, the randomization test showed this difference to be marginally non-significant (P < 0.075). Aphidius ervi were more likely to attack aphids following an encounter than was P. pequodorum and did not differentiate between the two aphids (randomization test, P > 0.1).
To compare the relative attack rates of each parasitoid species on pea aphids versus spotted alfalfa aphids, we calculated the ratio Prob(A. ervi attack|encounter)/Prob(P. pequodorum attack|encounter) for each aphid species; for pea aphids, it was 3.2, and for spotted alfalfa aphids, it was 17.4, which was statistically significantly higher (randomization test, P < 0.04). Therefore, although P. pequodorum was less likely to attack either aphid species given an encounter than was A. ervi, this likelihood was much less for spotted alfalfa aphids than for pea aphids, implying that P. pequodorum is much less likely to accept spotted alfalfa aphids than A. ervi.
Laboratory microcosms
In the single plant experiment, no mummies were produced on spotted alfalfa aphids on any of the 15 plants with both spotted alfalfa and pea aphids. In contrast, 107 mummies were produced on the 18 plants with just pea aphids, and 24 mummies were produced on pea aphids on the 15 plants with both aphids.
Although no mummies were found on spotted alfalfa aphids, the presence of spotted alfalfa aphids reduced parasitism of pea aphids by each of the two parasitoids. More P. pequodorum and A. ervi mummies were found on plants with only pea aphids than on plants with both aphid species (P. pequodorum: 3.94 ± 1.42 vs 1.20 ± 0.71; A. ervi: 2.00 ± 0.76 vs 0.40 ± 0.19). The result that A. ervi showed a lower parasitism rate than P. pequodorum was somewhat unexpected, since A. ervi had a higher parasitism rate at the smaller scale (host preferences experiment; Fig. 1) and in previous experiments using individual plants (Schellhorn et al. 2002). However, these previous experiments showed that parasitism rates by these species can be highly variable (Schellhorn et al. 2002), perhaps due to experimental conditions.
In these laboratory microcosms, we also wanted to observe how spotted alfalfa aphids affected competition between the parasitoid species on the scale of a single plant. Extremely variable parasitism rates by both parasitoid species made this difficult to ascertain; we found mummies of both parasitoid species on only 6 of 33 plants (5 on plants without spotted alfalfa aphids and 1 on plants with spotted alfalfa aphids). Therefore, we could not make a valid comparison of the effect of spotted alfalfa aphids on competition within individual plants. We can draw some tentative conclusions by looking at parasitism rates across all plants. Praon pequodorum held a competitive advantage over A. ervi both on plants with and without spotted alfalfa aphids, accounting for a higher proportion of pea aphid parasitism than A. ervi (Table 1). Furthermore, summing over all plants, P. pequodorum accounted for a higher proportion of pea aphid parasitism on plants where spotted alfalfa aphids were present compared to plants with just pea aphids (0.75 vs 0.66). While this suggests that the presence of spotted alfalfa aphids increased the competitive superiority of P. pequodorum over A. ervi, it was not statistically significant (the interaction between treatment and parasitoid species, Table 1).
Effect of spotted alfalfa aphids on A. ervi behavior
In observations of A. ervi foraging, the presence of spotted alfalfa aphids reduced the frequency of A. ervi attacks on pea aphids (Fig. 2) (21.3 ± 2.8 attacks/trial without spotted alfalfa aphids vs 9.2 ± 1.1 attacks/trial with spotted alfalfa aphids; t 19 = 4.0, P < 0.001). As in the first experiment, we observed A. ervi attacking spotted alfalfa aphids when they were available (10.0 ± 1.6 attacks/trial). Combining attacks on both aphid species, the presence of spotted alfalfa aphids did not influence the parasitoid’s overall attack rate (21.3 ± 2.8 attacks/trial on pea aphids in absence of spotted alfalfa aphids vs 19.2 ± 2.4 attacks/trial on either pea or spotted alfalfa aphids when both present; t 19 = 0.6, P = 0.58). The time budget of A. ervi in both treatments was similar, although parasitoids spent a greater proportion of their time sitting and less time moving when on plants with spotted alfalfa aphids compared to plants without spotted alfalfa aphids (proportion of time sitting: 0.14 vs 0.07, time moving: 0.69 vs 0.78; F 1,18 = 4.86, P = 0.041).
Field mesocosms
Our manipulation of spotted alfalfa aphid densities was not successful. Spotted alfalfa aphids were found in all cages, and there was no statistically significant effect of treatment on the density of spotted alfalfa aphids at the end of the experiment (t 10 = 1.00, P = 0.338). Nonetheless, there was a wide range of spotted alfalfa aphid densities relative to pea aphids, with spotted alfalfa aphids making up 0–43% of the aphid population, with an average of 7 ± 2%.
Given the high unmanipulated variability in spotted alfalfa aphid densities among cages, we analyzed the experiment with regression. Mummy densities increased steadily through time, so we focused our analyses on the last day of the experiment (day 20). There was no statistically significant effect of the proportion of spotted alfalfa aphids in the aphid community on percent parasitism by either A. ervi or P. pequodorum (Fig. 3a, b). A partial correlation accounting for pea aphid density indicates that there is a non-significant negative correlation between the proportion of spotted alfalfa aphids and A. ervi parasitism (r = −0.354, P < 0.20) and a non-significant positive correlation between the proportion of spotted alfalfa aphids and P. pequodorum parasitism (r = 0.258, P < 0.076). Nonetheless, there was a positive correlation between the proportion of spotted alfalfa aphids in a cage and the proportion of mummies that were P. pequodorum (t 11 = 2.43, P < 0.035; Fig. 3c). This indicates that P. pequodorum did relatively better than A. ervi in cages where spotted alfalfa aphids made up a greater proportion of the aphid community. Observing an affect of spotted alfalfa aphids on the competitive abilities of P. pequodorum versus A. ervi in the absence of statistically significant effects of spotted alfalfa aphids on P. pequodorum and A. ervi parasitism rates individually is not surprising given uncontrolled variation among cages in the absolute performance of parasitoids. While the absolute performance of parasitoids is variable, the relative performances of P. pequodorum versus A. ervi within cages is less sensitive to cage-by-cage variation in performance. As in the laboratory microcosms, we found no evidence that either parasitoid species can develop mummies in spotted alfalfa aphids. Of 650 observed mummies from both parasitoid species, none were parasitized spotted alfalfa aphids.
Proportion of aphids parasitized by a Aphidis ervi. b Praon pequodorum on day 20 of a field cage experiment as a function of the proportion of spotted alfalfa aphids in the cage, measured as [spotted alfalfa aphids/(spotted alfalfa aphids + pea aphids)]. c Proportion of mummies in cages that were Praon pequodorum, measured as [P. pequodorum/(P. pequodorum + A. ervi)] as a function of the proportion of spotted alfalfa aphids in the cage
Discussion
We performed a suite of experiments to determine the effect of spotted alfalfa aphids on the foraging behavior of two parasitoid species, asking whether the presence of spotted alfalfa aphids might affect their attack rates on pea aphids and ultimately their relative competitive abilities. Our results consistently demonstrated that both parasitoid species were willing to attack spotted alfalfa aphids, but that neither could complete larval development on this species. This suggests that spotted alfalfa aphids are equally unsuitable for both parasitoid species, but that spotted alfalfa aphids could still affect parasitoids by influencing their behaviors. Our results showed that the presence of spotted alfalfa aphids decreased parasitism of pea aphids by both parasitoid species in laboratory petri dishes and trials on single plants. A similar reduction in A. ervi parasitism of pea aphids has been demonstrated in the presence of other aphid species (van Veen et al. 2005; Harvey 2006). Likewise, generalist predators can be less effective at controlling an aphid species when a second species is added (e.g., Bergeson and Messina 1997, 1998). In our system, the reduction in parasitism is apparently caused by parasitoids spending time evaluating and attacking spotted alfalfa aphids—a mechanism we demonstrated in detail for A. ervi.
In addition to decreasing the attack rate on pea aphids, the presence of spotted alfalfa aphids might also increase the competitive ability of P. pequodorum relative to A. ervi if spotted alfalfa aphids have a greater negative effect on A. ervi efficiency. Changes in the relative competitive abilities of parasitoid species were suggested in petri dish choice experiments, in which P. pequodorum was less likely to attack spotted alfalfa aphids than pea aphids following encounters, whereas A. ervi was equally likely to attack both aphid species. We had a non-significant trend in the same direction with the overall results of the single-plant experiment. In the field experiment, spotted alfalfa aphid did not have a significant effect on P. pequodorum parasitism of pea aphids, but they had a negative effect on A. ervi parasitism. Consequently, mummy production by P. pequodorum relative to A. ervi increased as the relative density of spotted alfalfa aphids increased, suggesting a possible effect of spotted alfalfa aphids on competitive abilities at the population level.
Our experiments used an additive experimental design that kept pea aphid density constant, rather than a substitutive design that would have kept total aphid density constant but changed the density of pea aphids. Therefore, the design tests the null hypothesis that the presence/absence of spotted alfalfa aphids has no effect on parasitism of pea aphids. If spotted alfalfa aphids had been suitable hosts, it would have been best to use both additive and substitutive designs to disentangle the effects of varying composition and density of hosts. In our case, however, the additive design maintained the number of suitable hosts. Even though we did not perform experiments varying pea aphid numbers, previous work on A. ervi (Ives et al. 1999) suggests that over the range of pea aphid numbers we used, there is little variation in A. ervi foraging efficiency. This weak effect of pea aphid density is corroborated by the total attack rates in A. ervi behavioral observations which were similar despite a change in total aphid density (Fig. 2).
Evaluating and attacking an unsuitable host can waste a parasitoid’s time and eggs, which can be detrimental to the parasitoid under certain circumstances (Hoogendoorn and Heimpel 2002; Heimpel et al. 2003). While neither P. pequodorum nor A. ervi are known to be egg-limited over the course of their lifetimes (as defined in Heimpel et al. 1996; De Vis et al. 2003), in laboratory experiments the presence of spotted alfalfa aphids decreased their parasitism rate. The field mesocosms at least partially corroborated the laboratory evidence; A. ervi parasitism was negatively affected by spotted alfalfa aphids while there was no significant effect of spotted alfalfa aphids on P. pequodorum. In a similar study, A. ervi parasitism was also negatively affected by the presence of a second aphid species (van Veen et al. 2005). These results suggest that the effect of spotted alfalfa aphids on parasitoid foraging behavior and ultimately population density was likely due to a time limitation that at least A. ervi experiences in the natural setting. In addition, recent work has emphasized the potential role of plants in mediating community interactions (Ohgushi et al. 2007). It is possible in our system that spotted alfalfa aphids can modify plant quality or some other factor that ultimately influences the ability of each parasitoid species to attack pea aphids.
Aphidius ervi’s competitive superiority for the ubiquitous pea aphid could have played a role in the decrease of P. pequodorum populations coincident with the arrival and growth of A. ervi populations (Schellhorn et al. 2002). However, P. pequodorum has not completely vanished, and our findings suggest that, when the sporadically available spotted alfalfa aphids are present, P. pequodorum may have a local advantage that helps it persist in the face of a normally superior competitor. This relative advantage could be even more widespread than our findings suggest; A. ervi parasitism of pea aphids seems negatively affected by other aphid species residing within the same habitat (pea aphids and Megoura viciae in fava beans; van Veen et al. 2005) and even from aphids in other habitats (Aphis glycines from soybeans; Harvey 2006).
Our results suggest that attention should be given to more than just the density of a target host species when studying the searching efficiency of parasitoids; alternative hosts, even if unsuitable, may nonetheless affect parasitoid searching efficiencies. Similarly, the relative competitive abilities of parasitoid species may depend on the presence of other species. Although we have focused on a case in which an additional species has a negative effect on parasitoid foraging, there are numerous other cases in which additional species can have positive effects on parasitoids (Tylianakis et al. 2004). For example, plant-based resources such as nectar dramatically influence parasitoids in a number of systems (Wäckers et al. 2005). They do so by enhancing parasitoid fecundity or longevity (Andow 1991; Jervis et al. 1993; Landis 1996), or by attracting parasitoids into specific areas (Liang and Huang 1994; Schellhorn et al. 2000). Additional resources gained from hosts by host-feeding (Heimpel and Collier 1996) or feeding on by-products like honeydew (Wäckers and Steppuhn 2003) can also benefit parasitoids through similar mechanisms. If any of these resources differentially affect competing parasitoid species, as was the case for the distraction effect observed in this study, they may affect the outcome of parasitoid competition. Future work is needed to determine how much of parasitoid competition is dependent upon the availability of multiple hosts or other resources, and when alternative resources are helping inferior competitors to persist in the environment. More work of this type is also necessary to better understand how indirect effects can modify ecological interactions with biological control agents (Pearson and Callaway 2003), or more generally those between native and introduced species (White et al. 2006).
References
Andow DA (1991) Vegetational diversity and arthropod population response. Annu Rev Entomol 36:561–586
Bergeson E, Messina FJ (1997) Resource versus enemy-mediated interactions between cereal aphids (Homoptera: Aphididae) on a common host plant. Ann Entomol Soc Am 90:425–432
Bergeson E, Messina FJ (1998) Effect of a co-occurring aphid on the susceptibility of the Russian wheat aphid to lacewing predators. Entomol Exp Appl 87:103–108
Chaneton EJ, Bonsall MB (2000) Enemy-mediated apparent competition: empirical patterns and the evidence. Oikos 88:380–394
Cisneros JJ, Rosenheim JA (1998) Changes in the foraging behavior, within-plant vertical distribution, and microhabitat selection of a generalist insect predator: an age analysis. Environ Entomol 27:949–957
Danyk TP (1993) Competitive interactions between the pea aphid parasitoids, Aphidius ervi and Praon pequodorum (Hymenoptera: Aphididae): influence of guild composition in southern British Columbia. Thesis, Simon Fraser University, Burnaby
Danyk TP, Mackauer M (1996) An extraserosal envelope in eggs of Praon pequodorum (Hymenoptera: Aphidiidae), a parasitoid of pea aphid. Biol Control 7:67–70
De Vis RMJ, Mendez H, van Lenteren JC (2003) Comparison of foraging behavior, interspecific host discrimination, and competition of Encarsia formosa and Amitus fuscipennis. J Insect Behav 16:117–152
Forbes A, Harvey C, Tilmon K, Survey F (2005) Variation in the responses of spotted alfalfa aphids, Therioaphis maculata Buckton (Homoptera: Aphididae) and pea aphids, Acythosiphon pisum Harris (Homoptera: Aphididae) to drought conditions in alfalfa (Medicago sativa L. Fabaceae). J Kansas Entomol Soc 78:387–389
Hagen KS, Viktrorov GA, Yasumatsu K, Shuster MF (1976) Biological control of pests of range, forage, and grain crops. In: Huffaker CB, Messenger PS (eds) Theory and practice of biological control. Academic, New York, pp 397–442
Harvey CT (2006) Indirect effects and an invasive species: complexity in a simple food web. PhD Dissertation, University of Wisconsin
Heimpel GE, Collier TR (1996) The evolution of host-feeding behaviour in insect parasitoids. Biol Rev Camb Philos Soc 71:373–400
Heimpel GE, Rosenheim JA, Mangel M (1996) Egg limitation, host quality, and dynamic behavior by a parasitoid in the field. Ecology 77:2410–2420
Heimpel GE, Neuhauser C, Hoogendoorn M (2003) Effects of parasitoid fecundity and host resistance on indirect interactions among hosts sharing a parasitoid. Ecol Lett 6:556–566
Holt RD, Lawton JH (1994) The ecological consequences of shared natural enemies. Annu Rev Ecol Syst 25:495–520
Hoogendoorn M, Heimpel GE (2002) Indirect interactions between an introduced and a native ladybird beetle species mediated by a shared parasitoid. Biol Control 25:224–230
Hudson P, Greenman J (1998) Competition mediated by parasites: biological and theoretical progress. Trends Ecol Evol 13:387–390
Ives AR, Schooler SS, Jagar VJ, Knuteson SE, Grbic M, Settle WH (1999) Variability and parasitoid foraging efficiency: a case study of pea aphids and Aphidius ervi. Am Nat 154:652–673
Jervis MA, Kidd NAC, Fitton MG, Huddleston T, Dawah HA (1993) Flower-visiting by hymenopteran parasitoids. J Nat Hist 27:67–105
Landis DA (1996) Habitat management for insect biological control. In: Reports of the NC-205 committee, p 219
Landis DA, Wratten SD, Gurr GM (2000) Habitat management to conserve natural enemies of arthropod pests in agriculture. Annu Rev Entomol 45:175–201
Liang W, Huang M (1994) Influence of citrus orchard ground cover plants on arthropod communities in China: a review. Agr Ecosys Environ 50:29–37
Mackauer M, Kambhampati S (1986) Structural changes in the parasite guild attacking the pea aphid in North America. In: Hodek I (ed) Ecology of Aphidophaga. Academia, Prague, pp 347–356
Morris RJ, Lewis OT, Godfray HCJ (2004) Experimental evidence for apparent competition in a tropical forest food web. Nature 428:310–313
Müller CB, Godfray HCJ (1997) Apparent competition between two aphid species. J Anim Ecol 66:57–64
Müller CB, Adriaanse ICT, Belshaw R, Godfray HCJ (1999) The structure of an aphid-parasitoid community. J Anim Ecol 68:346–370
Ohgushi T, Craig TP, Price PW (2007) Ecological communities: plant mediation in indirect interaction webs. Cambridge University Press, Cambridge
Pearson DE, Callaway RM (2003) Indirect effects of host-specific biological control agents. Trends Ecol Evol 18:456–461
Rauwald KS, Ives AR (2001) Biological control in disturbed agricultural systems and the rapid recovery of parasitoid populations. Ecol Appl 11:1224–1234
SAS Institute (2000) JMP statistics and graphics guide, version 4. SAS Institute, Cary
Schellhorn NA, Harmon JP, Andow DA (2000) Using cultural practices to enhance insect pest control by natural enemies. In: Rechcigl JE, Rechcigl NA (eds) Insect pest management: techniques for environmental protection. Lewis, Boca Raton, pp 147–170
Schellhorn NA, Kuhman TR, Olson AC, Ives AR (2002) Competition between native and introduced parasitoids of aphids: non-target effects and biological control. Ecology 83:2745–2757
Smith RF (1959) The spread of the spotted alfalfa aphid, Therioaphis maculata (Buckton), in California. Hilgardia 28:647–683
Tylianakis JM, Didham RK, Wratten SD (2004) Improved fitness of aphid parasitoids receiving resource subsidies. Ecology 85:658–666
van Veen FJF, van Holland PD, Godfray HCJ (2005) Stable coexistence in insect communities due to density and trait-mediated indirect effects. Ecology 86:3182–3189
van Veen FJF, Morris RJ, Godfray HCJ (2006) Apparent competition, quantitative food webs, and the structure of phytophagous insect communities. Annu Rev Entomol 51:187–208
Wäckers FL, Steppuhn A (2003) Characterizing nutritional state and food source use of parasitoids collected in fields with high and low nectar availability. IOBC WPRS Bull 26:203–208
Wäckers FL, van Rijn PCJ, Bruin J (2005) Plant-provided food for carnivorous insects: a protective mutualism and its applications. Cambridge University Press, Cambridge
White EM, Wilson JC, Clarke AR (2006) Biotic indirect effects: a neglected concept in invasion biology. Divers Distrib 12:443–455
Wootton JT (1994) The nature and consequences of indirect effects in ecological communities. Annu Rev Ecol Syst 25:443–466
Yan J, Fine JP (2004) Estimating equations for association structures. Stat Med 23:859–880
Acknowledgments
The authors wish to thank Chad Harvey for his suggestions in improving this manuscript and help in performing experiments. We also wish to thank the University of Wisconsin, Arlington Agricultural Research Station, for the care and maintenance of experimental alfalfa fields.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Meisner, M., Harmon, J.P. & Ives, A.R. Presence of an unsuitable host diminishes the competitive superiority of an insect parasitoid: a distraction effect. Popul Ecol 49, 347–355 (2007). https://doi.org/10.1007/s10144-007-0054-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10144-007-0054-4