Abstract
We investigated the combined effects of herbivore damage and soil fertility on shoot growth patterns of Quercus serrata and Q. crispula saplings at both the shoot and individual levels. Saplings were grown in herbivore-damaged or undamaged areas of the greenhouse with the two fertilization treatment levels, low or high. We measured the leaf area loss, number of flushes, length of extension units (EUs; the first vs the higher), number of leaves on each individual, and number of EUs. At the shoot level, the leaf area loss at high soil fertility was significantly greater than that at low soil fertility among the highest EUs of Q. serrata, while this difference was not significant in Q. crispula, suggesting that effect of soil fertility on leaf area loss is species-specific. Furthermore, herbivore damage was associated with a significant increase in the number of EUs and a reduction in the length of the higher EUs under both soil fertility treatments, although saplings had a tendency to produce significantly more flushes and longer individual EUs under the high soil fertility. At the individual level, herbivore-damaged saplings exhibited a significant increase in leaf numbers; however, the total length of the EUs in Q. serrata or Q. crispula was not significantly affected by herbivore damage, regardless of soil fertility. These results suggest that Q. serrata and Q. crispula saplings produce shorter EUs in response to herbivore damage in order to reduce the cost of mechanical support and spread the risk for any subsequent herbivore damage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Compensation, i.e., recovering from the loss of vegetative tissue, is one plant strategy for overcoming herbivory damage (e.g., Marquis 1996; Strauss and Agrawal 1999). For example, the compensatory responses in woody plants include increasing shoot regrowth (Wilson 1993; Gadd et al. 2001) and photosynthetic rates (Heichel and Turner 1983). Thus, compensating responses in woody plants might affect plant growth patterns, especially patterns of subsequent shoot growth.
Shoot growth patterns are also affected by environmental factors such as the availability of soil nutrients, light, and water (e.g., Harmer 1989; Charr et al. 1997a; Gardiner and Hodges 1998). In particular, high nutrient availability increases plant biomass production, the number of leaves, leaf area (e.g., Berger and Glatzel 2001), and the nitrogen concentration in leaves (e.g., Harmer 1989; Forkner and Hunter 2000; Berger and Glatzel 2001). Because increased nitrogen levels in plant tissues cause increased herbivory, soil nutrient availability indirectly determines the intensity of herbivory (Lower et al. 2003). Therefore, soil fertility might modify shoot growth patterns and determine the intensity of herbivory, suggesting the importance of soil nutrient availability to the interaction between herbivorous insects and plants.
This interaction has been studied mainly using herbaceous plants: individual-level responses to herbivore damage and soil fertility is assessed by seed production and relative growth rate (e.g., Meyer 2000), which are used indicators of the individual fitness (e.g., Strauss et al. 2002). However, in woody plants, it is difficult to assess individual-level responses, because (1) woody plants have complex branching structures that are formed through the repetitive production of shoots (White 1979), and (2) the branches within an individual woody plant are autonomous (Sprugel et al. 1991). These results suggest that branch-level responses to environmental factors are not always equal to individual-level responses (Küppers 1989). Most studies has assessed the effect of herbivore damage on woody plants only at branch level (e.g., Heichel and Turner 1984; Charr et al. 1997b). However, only branch-level responses could not fully describe plant behavior to environmental factors: a combination of branch- and individual-level responses is much needed to clarify the plant behavior especially in woody plants. Therefore, it is important to scale up branch- to individual-level responses to describe plant behavior for herbivory and soil fertility.
Quercus trees have a rhythmic growth pattern (e.g., Borchert 1975; Charr et al. 1997a,b). Quercus saplings can produce several growth flushes per growing season, and the number of flushes varies according to environmental factors (Hanson et al. 1986; Charr et al. 1997a,b). In highly fertile soil, Quercus saplings have longer shoots (Harmer 1989), and artificial defoliation (i.e., simulated herbivory damage) in spring reduces the shoot length and the number of leaves in the second flush (Charr et al. 1997b). These results suggest that the growth of shoots at a subsequent flush is determined by both soil nutrient conditions and the level of herbivory at the time of the previous flush.
Quercus trees have the richest herbivore fauna of any temperate plant genus (e.g., Southwood 1961; Teramoto 1993; Wagner et al. 1995). In Japan, 10% of all lepidopterous species use Quercus as a host plant; 346 and 260 lepidopterous species use Quercus serrata Thunb. ex Murray and Q. crispula Blume, respectively (Teramoto 1996). These data suggest that Q. serrata and Q. crispula have constantly been exposed to herbivory, implying that Q. serrata and Q. crispula might have developed adaptive responses to herbivory. Q. serrata and Q. crispula are deciduous trees of temperate forests in Japan; Q. serrata is a dominant species of secondary forests (e.g., Ozawa et al. 2000), while Q. crispula is a dominant climax species of cool temperate forests (e.g., Koike 1988). Responses of Q. crispula to herbivory have been studied mainly from the viewpoint of leaf quality (Kudo 1996; Nabeshima et al. 2001), and the results have clarified that leaf damage causes changes in the physical and chemical qualities of a leaf: leaf mass per area, concentration of nitrogen, and amount of condensed tannin. Compensative shoot growth might play an important role in the tolerance of Quercus saplings to herbivory damage, because Quercus saplings are capable of repeated flushes of shoot growth (Borchert 1975); herbivory damage modifies shoot growth patterns through compensatory shoot growth.
Weltzin et al. (1998) showed that seedlings depend upon a plant’s resistance to herbivory during its establishment. Furthermore, McGraw et al. (1990) stressed the role of resource availability in tolerance to artificial leaf damage. Therefore, the effects of both insect damage and soil nutrient levels should be simultaneously investigated under controlled conditions, because saplings growing in a range of soil nutrient availabilities might experience different herbivory attacks in nature.
In this study, we clarified the interaction between herbivory damage and the availability of soil nutrients on growth patterns of Q. serrata and Q. crispula saplings at both the shoot and individual levels.
Materials and methods
Plant material
We purchased saplings of two oak species, Q. serrata and Q. crispula, from Kutsuki Village Forest Association, Shiga, Japan. One-hundred-and-twenty saplings of Q. serrata and Q. crispula (240 in total) were transplanted into plastic pots (44 cm in diameter, 24 cm in depth) on 19 and 20 December 2001. At the bottom of each pot was placed 500 cc of kamuma soil (pumice), and then the pot was filled with sand. To ensure uniform soil conditions, the roots of the saplings were washed to remove previous soil before transplanting. The mean sapling heights were 43.4±0.4 (mean ± SE) cm for Q. serrata and 43.5±0.4 cm for Q. crispula. Sapling height did not differ significantly among the treatments (Scheffe’s range test, P>0.05). The saplings were grown in two greenhouses (H1, 10×7.5 m, 4 m in height; H2, 9.5×4.4 m, 3.5 m in height). The greenhouses were located at the Kitashirakawa Experimental Station of Kyoto University in Kyoto, Japan. Bud-break occurred between 2 and 15 April 2002 for Q. serrata saplings and between 8 and 24 April 2002 for Q. crispula . During the growing season, most of the saplings had more than one flush. The bud-break at the last flush occurred in early October 2002. This characteristic indicates that Quercus saplings can be used to assess treatment effects within one growing season. Q. serrata is found naturally distributed around the experimental station, whereas the experimental station is located about 15 km south of the natural southern limit of Q. crispula distribution.
Treatments
All experiments were conducted according to a two-way factorial design to assess the effects of herbivory damage and soil fertility in the two greenhouses. Nylon mesh (1×1 mm) divided each greenhouse into two separate blocks (herbivory-damaged and undamaged). The four sides of the herbivory-undamaged block were made of 1×1-mm mesh to exclude insect herbivores; this mesh size effectively reduced herbivory damage (the leaf area loss in herbivore-undamaged blocks were less than 3%), although a few insect invasions did occur. Therefore, we checked all saplings and removed invasive insects every 2 days. The three sides of the herbivory-damaged block were made of 20×20-mm nylon mesh to allow insect herbivores free access. Saplings in each category were assigned to different fertilization treatments. We applied 25:5:20 (N:P:K) fertilizer (Peters Professional, HYPONeX JAPAN) every 2 weeks from April to November 2002. Half of the saplings in each block were grown under low soil fertility (20 kg N/ha/year); the other half were grown under high soil fertility (200 kg N/ha/year). The pots were arranged in random order with respect to the fertilizer treatment. All saplings were watered to saturation for 10 min daily by an automatic sprinkler (Sprinkler Thinker DC-1, Irrigation Control Equipment, Galcon).
Measurements
Quercus saplings can exhibit several flushes during one growing season. The EU is defined as the portion of a shoot that elongates during one flush. In this study, EU was divided into two categories according to flush times. The EUs formed during the first flush in April were defined as ‘the first EUs’; the EUs from the following flush (up to the seventh flush) were defined as ‘the higher EUs’.
A two-dimensional diagram was drawn to describe the branching structure of each sapling during the growing season when new shoots elongated. Each EU was numbered on the diagram, and the date on which the EU appeared was recorded. For each EU, we measured the length and the number of leaves after elongation and leaf growth of the EU had finished from early May to early October 2002. At the same time, leaves were roughly categorized into one of seven leaf damage classes based on the visually estimated percentage of leaf area loss. Defoliation can cause mortality of saplings, as young seedlings and saplings of woody plants allocate a large fraction of their biomass to leaves (Poorter and Nagel 2000). Therefore, we assessed the level of leaf damage. The leaf damage at this experimental station was caused mainly by generalist herbivorous insects: larvae of Lepidoptera belonging to the families Oecophoridae, Timyridae, Noctuidae, Geometridae, Lymantriidae, and Arctiidae; larvae of Hymenoptera belonging to the family Tenthredinidae; and adults of Coleoptera belonging to the families Attelabidae and Scarabaeidae (Ishii and Osawa, unpublished data).
Data analysis
The percentage of leaf area loss for an individual was analyzed from Mann–Whitney’s U-test under low and high soil fertility. We estimated the leaf area loss for each leaf using the following percentages for each leaf damage class: 0, 0%; 1, 2.5%; 2, 15%; 3, 30%; 4, 62.5%; 5, 87.5%; and 6, 100%. The leaf area loss for the first and higher EUs was calculated using the average values of these percentages for each individual sapling. The preliminary analysis of EU length in Q. serrata and Q. crispula revealed a distribution pattern that was significantly different from normal, so we applied non-parametric tests to compare the frequency distributions and the means of these variables. The frequency distributions of each treatment were analyzed with the Kolmogorov–Smirnov test, and the means were analyzed with the Mann–Whitney’s U-test. We used Bonferroni correction to adjust significance levels in the multiple comparisons.
Variables other than leaf area loss and EU length were compared using a two-way ANOVA. The number of flushes was defined as the maximum flush number in a sapling. The total EU length was defined as the sum of the individual EU lengths in a sapling. The total number of leaves was defined as the sum of the leaf numbers in a sapling. All statistical analyses were performed with StatView-J ver. 5.0 (SAS 1998).
Results
Soil fertility and herbivory damage in Q. serrata and Q. crispula
In Q. serrata (Fig. 1a), soil fertility significantly affected the leaf area loss of the higher EUs (Mann–Whitney’s U-test: P=0.0115), but not that of the first EUs (Mann–Whitney’s U-test: P=0.8941). In Q. crispula (Fig. 1b), soil fertility did not significantly affect leaf area loss of either the first (Mann–Whitney’s U-test: P=0.6952) or the higher EUs (Mann–Whitney’s U-test: P=0.6579).
Shoot-level consequences in Q. serrata and Q. crispula
In Q. serrata, the effect of herbivory damage on the mean length of the first EUs was significant under high soil fertility but was not significant under low soil fertility (Fig. 2); the effect of soil fertility on the mean first EU length was not significant in either herbivory-damaged or undamaged saplings (Fig. 2). Herbivory damage significantly affected the distribution pattern of first EU length under high soil fertility but not under low soil fertility (Fig. 2); the effect of soil fertility on the length distribution pattern was significant in herbivory-damaged saplings but was not significant in undamaged saplings (Fig. 2).
Frequency distributions of EU length in Q. serrata under low and high soil fertilities with herbivory-damaged and undamaged saplings at the first and the higher EUs. Arrows indicate mean values of the EU length. Means with different letters are significantly different from Mann–Whitney’s U-test at each EU (Bonferroni corrected P<0.0083). The different letters at skewness indicate significantly different distributions according to Kolmogorov–Smirnov test at each EU (Bonferroni corrected P<0.0083)
In Q. serrata, among the higher EUs, the effect of herbivory damage on the mean length was significant under both low and high soil fertilities, while the effect of soil fertility was significant in herbivory-damaged saplings but not in undamaged saplings (Fig. 2). Parallel results were obtained for the distribution patterns of the mean length of the higher EUs (Fig. 2).
In Q. crispula, among the first EUs, the effect of herbivory damage on the mean length was significant under high soil fertility but not under low soil fertility (Fig. 3); the effect of soil fertility was not significant in either herbivory-damaged or undamaged saplings (Fig. 3). Parallel results were obtained for the distribution patterns of first EU lengths (Fig. 3).
Frequency distributions of EU length in Q. crispula under low and high soil fertilities with herbivory-damaged and undamaged saplings at the first and the higher EUs. Arrows indicate mean values of the EU length. Means with different letters are significantly different from Mann–Whitney’s U-test at each EU (Bonferroni corrected P<0.0083). The different letters at skewness indicate significantly different distributions according to Kolmogorov–Smirnov test at each EU (Bonferroni corrected P<0.0083)
In Q. crispula, the effect of herbivory damage on the mean length of the higher EUs was significant under both low and high soil fertilities (Fig. 3), while the effect of soil fertility was significant in herbivory-damaged saplings but not in undamaged saplings (Fig. 3). The same results were obtained for the distribution patterns of the mean length of the higher EUs (Fig. 3).
Individual-level consequences in Q. serrata and Q. crispula
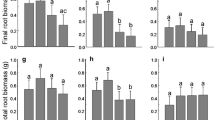
The effects of soil fertility and herbivory damage on the number of flushes in Q. serrata and Q. crispula were significant (two-way ANOVA: soil fertility F 1,116=41.575, P<0.0001; F 1,116=70.893, P<0.0001, herbivory damage F 1,116=39.199, P<0.0001; F 1,116=25.522, P<0.0001, respectively) (Fig. 4a,b). There was no significant interaction between soil fertility and herbivory damage in Q. serrata or Q. crispula (two-way ANOVA: F 1,116=0.009, P=0.9257; F 1,116=2.836, P=0.0949, respectively) (Fig. 4a,b)
Soil fertility, herbivory damage, or their interaction did not affect the number of first EUs in Q. serrata or Q. crispula (two-way ANOVA: soil fertility F 1,116=0.334, P=0.5644; F 1,116=0.395, P=0.5309, herbivory damage F 1,116=2.565, P=0.1120; F 1,116=1.043, P=0.3092, soil fertility × herbivory damage, F 1,116=1.014, P=0.3159; F 1,116=0.395, P=0.5309) (Fig. 5a,b). In contrast, both soil fertility and herbivory damage significantly affected the number of higher EUs in Q. serrata and Q. crispula (two-way ANOVA: soil fertility F 1,116=32.350, P<0.0001; F 1,116=11.114, P=0.0012, herbivory damage F 1,116=23.967, P<0.0001; F 1,116=25.420, P<0.0001) (Fig. 5a,b). However, there was no significant interaction between soil fertility and herbivory damage related to the number of higher EUs in Q. serrata or Q. crispula (two-way ANOVA: F 1,116=0.340, P=0.5610; F 1,116=2.136, P=0.1465) (Fig. 5a,b). Soil fertility and herbivory damage each had a significant effect on the total number of EUs in Q. serrata and Q. crispula (two-way ANOVA: soil fertility F 1,116=17.726, P<0.0001; F 1,116=5.057, P=0.0264, herbivory damage F 1,116=9.240, P=0.0029; F 1,116=18.181, P<0.0001) (Fig. 5a,b), but they did not have a significant interaction in Q. serrata and Q. crispula (two-way ANOVA: F 1,116=0.992, P=0.3214; F 1,116=2.671, P=0.1049) (Fig.5a,b).
Soil fertility had a significant effect on the total EU length in Q. serrata and Q. crispula (two-way ANOVA: F 1,116=54.143, P<0.0001; F 1,116=34.970, P<0.0001) (Fig. 6a,b), but herbivory damage did not (two-way ANOVA: F 1,116=0.054, P=0.8161; F 1,116=0.047, P=0.8279) (Fig. 6a,b). Furthermore, there was no significant interaction between soil fertility and herbivory damage in Q. serrata or Q. crispula (two-way ANOVA: F 1,116=0.382, P=0.5380; F 1,116=0.210, P=0.6474) (Fig. 6a,b).
Herbivory-damaged saplings had a greater number of leaves than did undamaged saplings of both Q. serrata and Q. crispula (two-way ANOVA: F 1,116=5.483, P=0.0209; F 1,116=11.058, P=0.0012) (Fig. 7a,b). Moreover, saplings of both Q. serrata and Q. crispula had greater numbers of leaves under high soil fertility conditions than under low soil fertility conditions (two-way ANOVA: F 1,116=32.175, P<0.0001; F 1,116=19.284, P<0.0001) (Fig. 7a,b). There was no significant interaction between soil fertility and herbivory damage in Q. serrata, but the interaction was significant in Q. crispula (two-way ANOVA: F 1,116=0.323, P=0.5707; F 1,116=4.584, P=0.0344) (Fig. 7a,b).
Discussion
Effect of soil fertility on herbivory damage
This study showed that the leaf area loss from the higher EUs of saplings grown under conditions of high soil fertility was significantly greater than that from saplings grown under conditions of low soil fertility, whereas the leaf area loss from the first EU in Q. serrata was not significantly different between the conditions of high and low soil fertility (Fig. 1a). Generally, soil fertility influences leaf qualities such as nitrogen concentration (e.g., Harmer 1989; Forkner and Hunter 2000; Berger and Glatzel 2001), and leaf quality is a major determinant of the distribution and abundance of herbivores (e.g., Kytö et al. 1996; Lill and Marquis 2001); this implies that the leaf area loss from a plant grown under conditions of high soil fertility would be greater than that from a plant grown under conditions of low soil fertility. Thus, the large leaf area loss from the higher EUs of Q. serrata under high soil fertility conditions was due mainly to intense herbivory damage produced by a concentration of herbivores. Because spring leaf expansion depends largely on the total amount of nitrogen supplied during the previous year (Millard and Proe 1991; Dyckmans and Flessa 2001), and because in this study we began fertilization in April 2002, the effect of soil fertility did not emerge in the first EUs in Q. serrata. Therefore, the absence of an effect of high or low soil fertility on leaf area loss from the first EUs in Q. serrata in this study might be due to similar insect densities, and the subsequent similar amount of herbivory under both low and high soil fertility. This study also showed that leaf area losses from the first and higher EUs in Q. crispula were not significantly different under either nutrient condition (Fig. 1b). The discrepancy between the effect of soil fertility on leaf area loss from the higher EUs in Q. serrata and Q. crispula suggests species-specific patterns of resource use and allocation.
Shoot-level responses and the consequences
Herbivory damage significantly reduced EU length, especially in the higher EUs in Q. serrata and Q. crispula under both low and high soil fertilities (Figs. 2,3). Similar results were obtained by Heichel and Turner (1984) and Chaar et al. (1997b), who showed that artificial defoliation simulating herbivorous insect damage reduced the lengths of subsequent shoots. With high soil fertility, the lengths of the higher EUs were significantly increased (Figs. 2,3). Therefore, the compensating ability of Q. serrata and Q. crispula might be higher under high soil fertility than under low soil fertility, although the leaf area loss in Q. serrata suggests that highly fertile soil may cause severe herbivory, especially in the higher EUs.
Individual-level responses and the consequences
Shorter shoots generally display a greater number of leaves per stem length than do longer shoots (e.g., Yagi and Kikuzawa 1999). In this study, herbivory-damaged saplings had a greater number of shorter EUs than did herbivory-undamaged saplings in Q. serrata and Q. crispula (Figs. 2,3,5a,b), indicating that a damaged sapling with a large number of shorter shoots would have more leaves than would an undamaged sapling, at an individual level (Fig. 7a,b). Increasing the number of leaves per shoot could increase mutual shading among leaves within a shoot (e.g., Takenaka 1994), implying that mutual shading would also increase at an individual level. However, Yagi and Kikuzawa (1999) showed that leaf size on shorter shoots is smaller than that on longer shoots in Q. crispula, suggesting that the display of smaller leaves on the shorter shoots of herbivory-damaged Q. serrata and Q. crispula saplings would avoid mutual shading.
The number of flushes is affected by environmental factors, such as frost, shading, and defoliation (Chaar et al. 1997a), as well as by soil nutrients (Harmer 1989). In this study, herbivory damage and soil fertility significantly affected the number of flushes (Fig. 4a,b). It is known that defoliation in Quercus seedlings increases the number of flushes (Hilton et al. 1987; Chaar et al. 1997b). One explanation for this adaptive behavior in Quercus is that increased flushes compensate for the lost foliage and provide the required annual production at an individual level. In this study, herbivory damage resulted in an increased number of flushes and number of EUs (Figs. 4a,b,5a,b), with the result that herbivory damage did not significantly affect the total length of shoot elongation in Q. serrata or Q. crispula (Fig. 6a,b). Additionally, the number of flushes tended to be greater in Q. serrata than in Q. crispula (Fig. 4a,b). Moreover, Q. serrata saplings had more branches with several flushes than did Q. crispula saplings. These results suggest that Q. serrata saplings have a greater ability to respond to the intensity of herbivory damage and the level of soil nutrients than do Q. crispula saplings.
Scaling up shoot- to individual-level responses
Herbivory damage resulted in shorter EUs and more EUs, especially higher EUs (Figs. 2,3). However, in individual-level responses, herbivory damage did not significantly affect the total length of shoot elongation (Fig. 6a,b), indicating that the damaged and undamaged saplings invest nearly equivalent resources in shoot production. There are two possible explanations for this adaptive shoot production pattern. First, the partitioning of resources into the many small shoots observed in the saplings of the two species may be an adaptive strategy for reducing herbivore damage and avoiding localized damage by spreading the risk of herbivory damage; concentrated herbivory and artificial damage at the leaf and/or shoot level tend to have a greater effect on plant growth at the individual level than does dispersed damage (Marquis 1996). Second, herbivory-damaged saplings more effectively reduce the costs of mechanical support because the proportion of photosynthetic shoot tissue is greater in shorter shoots than in longer shoots (Niinemets and Kull 1995), indicating that the mechanical requirement for support (i.e., the proportion of non-photosynthetic tissue) is less in shorter shoots (Mori and Takeda 2004). Generally, shorter shoots have greater leaf area per stem length than do longer shoots (e.g., Takenaka 1997). Therefore, herbivory damage can promote the production of short shoots at an individual level and, thereby, result in the low-cost production of photosynthetic tissue, the spreading of the risk of herbivory damage, and the reduction of costs for mechanical support at an individual level.
In this study, we scale up shoot-level responses to individual-level responses in order to reveal whether the compensatory responses are adaptive foraging behaviors in Q. serrata and Q. crispula. The partitioning of resources into the many small shoots represent adaptive strategies at shoot- and individual-level in Q. serrata and Q. crispula, allowing effective use of limited resources and avoidance of herbivore damage.
References
Berger TW, Glatzel G (2001) Response of Quercus petraea seedlings to nitrogen fertilization. For Ecol Manage 149:1–14
Borchert R (1975) Endogenous shoot growth rhythms and indeterminate shoot growth in oak. Physiol Plant 35:152–157
Chaar H, Colin F, Collet C (1997a) Effects of environmental factors on the shoot development of Quercus petraea seedlings: a methodological approach. For Ecol Manage 97:119–131
Chaar H, Colin F, Leborgne G (1997b) Artificial defoliation, decapitation of the terminal bud, and removal of the apical tip of the shoot in sessile oak seedlings and consequences on subsequent growth. Can J For Res 27:1614–1621
Dyckmans J, Flessa H (2001) Influence of tree internal N status on uptake and translocation of C and N in beech: a dual 13C and 15N labeling approach. Tree Physiol 21:395–401
Forkner RE, Hunter MD (2000) What goes up must come down? Nutrient addition and predation pressure on oak herbivores. Ecology 81:1588–1600
Gadd ME, Young TP, Palmer TM (2001) Effects of simulated shoot and leaf herbivory on vegetative growth and plant defense in Acacia drepanolobium. Oikos 92:515–521
Gardiner ES, Hodges JD (1998) Growth and biomass distribution of cherrybark oak (Quercus pagoda Raf.) seedlings as influenced by light availability. For Ecol Manage 108:127–134
Hanson PJ, Dickson RE, Isebrands JG, Crow TR, Dixon RK (1986) A morphological index of Quercus seedling ontogeny for use in studies of physiology and growth. Tree Physiol 2:273–281
Harmer R (1989) The effect of mineral nutrients on growth, flushing, apical dominance and branching in Quercus petraea (Matt.) Liebl. Forestry 62:383–395
Heichel GH, Turner NC (1983) CO2 assimilation of primary and regrowth foliage of red maple (Acer rubrum L.) and red oak (Quercus rubra L.): response to defoliation. Oecologia 57:14–19
Heichel GH, Turner NC (1984) Branch growth and leaf numbers of red maple (Acer rubrum L.) and red oak (Quercus rubra L.): response to defoliation. Oecologia 62:1–6
Hilton GM, Packham JR, Willis AJ (1987) Effects of experimental defoliation on a population of pedunculate oak (Quercus robur L.). New Phytol 107:603–612
Koike T (1988) Leaf structure and photosynthetic performance as related to the forest succession of deciduous broad-leaved trees. Plant Species Biol 3:77–87
Kudo G (1996) Herbivory pattern and induced responses to simulated herbivory in Quercus mongolica var. grosseserrata. Ecol Res 11:283–289
Küppers M (1989) Ecological significance of above-ground architectural patterns in woody plants: a question of cost-benefit relationships. Trends Ecol Evol 4:375–379
Kytö M, Niemelä P, Larsson S (1996) Insects on trees: population and individual response to fertilization. Oikos 75:148–159
Lill JT, Marquis RJ (2001) The effects of leaf quality on herbivore performance and attack from natural enemies. Oecologia 126:418–428
Lower SS, Kirshenbaum S, Orians CM (2003) Preference and performance of a willow-feeding leaf beetle: soil nutrient and flooding effects on host quality. Oecologia 136:402–411
Marquis RJ (1996) Plant architecture, sectoriality and plant tolerance to herbivores. Vegetatio 127:85–97
McGraw JB, Gottschalk KW, Vavrek MC, Chester AL (1990) Interactive effects of resource availabilities and defoliation on photosynthesis, growth, and mortality of red oak seedlings. Tree Physiol 7:247–254
Meyer GA (2000) Interactive effects of soil fertility and herbivory on Brassica nigra. Oikos 88:433–441
Millard P, Proe MF (1991) Leaf demography and the seasonal internal cycling of nitrogen in sycamore (Acer pseudoplatanus L.) seedlings in relation to nitrogen supply. New Phytol 117:587–596
Mori A, Takeda H (2004) Functional relationships between crown morphology and within-crown characteristics of understory saplings of three co-dominating conifers in a subalpine forest in central Japan. Tree Physiol 24:661–670
Nabeshima E, Murakami M, Hiura T (2001) Effects of herbivory and light conditions on induced defense in Quercus crispula. J Plant Res 114:403–409
Niinemets Ü, Kull O (1995) Effects of light availability and tree size on the architecture of assimilative surface in the canopy of Picea abies: variation in shoot structure. Tree Physiol 15:791–798
Ozawa H, Itoh K, Hori Y (2000) Shoot structure and dynamics of saplings and canopies of three deciduous broad-leaved trees of a coppice forest in central Japan. Trees 14:206–214
Poorter H, Nagel O (2000) The role of biomass allocation in the growth response of plants to different levels of light, CO2, nutrients and water: a quantitative review. Aust J Plant Physiol 27:595–607
SAS (1998) Statview (ver. 5.0 J). SAS Institute, Cary, NC
Southwood TRE (1961) The number of species of insect associated with various trees. J Anim Ecol 30:1–8
Sprugel DG, Hinckley TM, Schaap W (1991) The theory and practice of branch autonomy. Annu Rev Ecol Syst 22:309–334
Strauss SY, Agrawal AA (1999) The ecology and evolution of plant tolerance to herbivory. Trends Ecol Evol 14:179–185
Strauss SY, Rudgers JA, Lau JA, Irwin RE (2002) Direct and ecological costs of resistance to herbivory. Trends Ecol Evol 17:278–285
Takenaka A (1994) Effects of leaf blade narrowness and petiole length on the light capture efficiency of a shoot. Ecol Res 9:109–114
Takenaka A (1997) Structural variation in current-year shoots of broad-leaved evergreen tree saplings under forest canopies in warm temperate Japan. Tree Physiol 17:205–210
Teramoto N (1993) Catalogue of host plants of lepidopterous insects in Japan (Fagaceae) (in Japanese). Bull Shiga Agric Exp Stn [Extra issue] 1:1–161
Teramoto N (1996) Studies on lepidopterous insect fauna on Fagaceous plants, as the food plants of the wild silk moth, Antheraea yamamai (in Japanese). Special Bull Shiga Agric Exp Stn 19:1–216
Wagner DL, Peacock JW, Carter JL, Talley SE (1995) Spring caterpillar fauna of oak and blueberry in a Virginia deciduous forest. Ann Entomol Soc Am 88:416–426
Weltzin JF, Archer SR, Heitschmidt RK (1998) Defoliation and woody plant (Prosopis glandulosa) seedling regeneration: potential vs. realized herbivory tolerance. Plant Ecol 138:127–135
White J (1979) The plant as a metapopulation. Annu Rev Ecol Syst 10:109–145
Wilson BF (1993) Compensatory shoot growth in young black birch and red maple trees. Can J For Res 23:302–306
Yagi T, Kikuzawa K (1999) Patterns in size-related variations in current-year shoot structure in eight deciduous tree species. J Plant Res 112:343–352
Acknowledgements
We thank the members of the Kitashirakawa Experimental Station, Field Science Education and Research Center, Kyoto University, for their supports in this experiment. We also thank Prof. H. Takeda, Dr. A. Mori, Mr. H. Ishii, and Dr. K. Kawamura, Laboratory of Forest Ecology, Kyoto University, and Prof. K. Kikuzawa, Laboratory of Forest Biology, Kyoto University for their helpful advice, encouragement, and statistical assistance. Thanks are also due to all the members of the Laboratory of Forest Ecology, Kyoto University, for useful discussion. This study was supported in part by a Grant-in-Aid for Science Research (No. 13306012, to N.O.) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mizumachi, E., Osawa, N., Akiyama, R. et al. The effects of herbivory and soil fertility on the growth patterns of Quercus serrata and Q. crispula saplings at the shoot and individual levels. Popul Ecol 46, 203–211 (2004). https://doi.org/10.1007/s10144-004-0188-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10144-004-0188-6