Abstract
The purpose of this research was to demonstrate the effectiveness and clinical outcome of an external carotid artery-radial artery graft-posterior cerebral artery (ECA-RAG-PCA) bypass in the treatment of complex vertebrobasilar artery aneurysms (VBANs) in a single-center retrospective study. An ECA-RAG-PCA bypass may be a last and very important option in the treatment of complex VBANs when conventional surgical clipping or endovascular interventions fail to achieve the desired outcome. This study retrospectively analyzed the clinical presentation, case characteristics, aneurysm location, size and morphology, choice of surgical strategy, complications, clinical follow-up, and prognosis of the patients enrolled. The data involved were analyzed by the appropriate statistical methods. A total of 24 patients with complex VBANs who met the criteria were included in this study. Eighteen (75.0%) were male and the mean age was 54.1 ± 8.83 years. The aneurysms were located in the vertebral artery, the basilar artery, and in the vertebrobasilar artery with simultaneous involvement. All patients underwent ECA-RAG-PCA bypass surgery via an extended middle cranial fossa approach, with 8 (33.3%) undergoing ECA-RAG-PCA bypass only, 3 (12.5%) undergoing ECA-RAG-PCA bypass combined with aneurysm partial trapping, and 12 (50.0%) undergoing ECA-RAG-PCA bypass combined with proximal occlusion of the parent artery. The average clinical follow-up was 22.0 ± 13.35 months. The patency rate of the high-flow bypass was 100%. At the final follow-up, 15 (62.5%) patients had complete occlusion of the aneurysm, 7 (29.2%) patients had subtotal occlusion of the aneurysm, and 2 (8.3%) patients had stable aneurysms. The rate of complete and subtotal occlusion of the aneurysm at the final follow-up was 91.7%. The clinical prognosis was good in 21 (87.5%) patients and no procedure-related deaths occurred. Analysis of the good and poor prognosis groups revealed a statistically significant difference in aneurysm size (P = 0.034, t-test). Combining the results of this study and the clinical experience of our center, we propose a surgical algorithm and strategy for the treatment of complex VBANs.
The technical approach of ECA-RAG-PCA bypass for complex VBANs remains important, even in an era of rapid advances in endovascular intervention. When conventional surgical clipping or endovascular intervention has failed, an ECA-RAG-PCA bypass plays a role that cannot be abandoned and is a very important treatment option of last resort.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the treatment of intracranial aneurysms (IAs), surgical clipping and endovascular intervention are the two main modalities, but they often fail to achieve satisfactory clinical results for complex cases, particularly complex vertebrobasilar artery aneurysms (VBANs) of the posterior circulation. Flow-diverter device (FD) is a valuable tool in the treatment of aneurysms, but there are still shortcomings and the long-term results of FD in the treatment of complex IAs remain to be clinically proven [1, 2]. In recent years, intracranial-extracranial (EC-IC) bypass combined with aneurysm trapping or proximal/distal occlusion of the parent artery have shown promising results in the treatment of complex IAs [3]. A paper by Lawton et al. describes in detail the different bypass approaches for the treatment of complex IAs and the technical requirements of the vascular neurosurgeon [4]. In this study, we describe a single-center treatment experience through the clinical results of an external carotid artery-radial artery graft-posterior cerebral artery (ECA-RAG-PCA) bypass for complex VBANs. The purpose of this study was to demonstrate the choice of surgical algorithm and the effectiveness and clinical prognosis of an ECA-RAG-PCA bypass in the treatment of complex VBANs.
Materials and methods
Patients
The study protocol was approved by the Institutional Ethics Committee of Tianjin Huanhu Hospital. All patients gave their written consent. Inclusion criteria for this study were as follows: (1) patients were aged 18–80 years, (2) aneurysms were located in the vertebrobasilar artery system, (3) diagnostic criteria for complex IAs were met, and (4) treatments all included an ECA-RAG-PCA bypass. Exclusion criteria were as follows: (1) the lesion was not a complex aneurysm, (2) the aneurysm was located in the anterior circulation system, and (3) the patient had a combination of severe underlying disease (for example, severe cardiopulmonary disease, severe endocrine system disease or multiple organ dysfunction syndrome, and other conditions that make it impossible to tolerate surgery). Based on the inclusion and exclusion criteria, we retrospectively analyzed cases of complex VBANs treated by an ECA-RAG-PCA bypass at our center. Clinical data and follow-up information included medical history, clinical presentation, physical examination, surgical algorithm, complications, and radiological follow-up every 3 months for the first year after surgery and every 6 months thereafter. The primary surgeon involved in this study was the corresponding author, and the other authors were blinded to the case data collection and clinical follow-up. All data in this study were blinded to minimize the risk of bias. The modified Rankin Scale (mRS) score was used to assess the clinical prognosis of all patients at postoperative follow-up.
Based on previous research experience, although there is no clear definition of a complex aneurysm, generally accepted characteristics of a complex aneurysm include the following: large or giant aneurysm, morphological manifestations of dissecting or dolichoectatic morphology, wide neck involving a perforator artery, intra-aneurysmal thrombosis, atherosclerosis of the aneurysm wall, crucial branch arteries originating from the aneurysm wall, and recurrent aneurysm after previous therapy [5,6,7,8]. For the purposes of this study, aneurysms meeting one or more of these characteristics were considered complex VBANs.
Surgical procedure
All patients in this study underwent EC-IC high-flow bypass surgery. The choice of whether to combine ECA-RAG-PCA bypass with aneurysm partial trapping or proximal occlusion of the parent artery was individualized according to the patient’s primary characteristics. Not every patient has undergone a balloon occlusion test (BOT), as the reliability of BOT is poor. A published meta-analysis showed that even with the BOT passing, there was still an overall incidence of 3.7% of ischemic stroke events [9]. Therefore, the BOT was not routinely performed in every patient in this study, as it is still unreliable even when the leptomeningeal collateral circulation is adequate. Each patient underwent preoperative radial arteriography or ultrasonography. All patients passed the Allen test, which showed adequate collateral circulation through the ulnar artery and palmar arch of the hand. All patients were started on aspirin 100 mg/day 1 week before surgery and did not stop taking it on the day of surgery. Postoperatively, patients were asked to continue taking it regularly. No anticoagulation regimen was routinely performed in the perioperative period.
The ECA-RAG-PCA bypass is performed under general anesthesia through an extended middle cranial fossa approach. The patient is maintained in a supine position with the head tilted to the opposite side so that the cheekbone is at its highest point. The surgical corridor of the middle skull base and the anterior cervical triangle is fully visualized. The carotid sheath is incised in the neck to expose the ipsilateral common carotid artery (CCA), external carotid artery (ECA), and internal carotid artery (ICA). The curved head incision starts approximately 1 cm anterior to the tragus, crosses the zygomatic arch, and continues across the midline to extend into the contralateral hairline. The ends of the zygomatic arches are sectioned, the temporalis muscle is turned completely downwards through the interfascial space, which gives a broader corridor of the middle skull base for surgery. A frontotemporal bone flap is removed using a milling cutter. The sphenoid ridge to the anterior clinoid process and the bones of the middle skull base are sufficiently drilled. The dura mater was carefully incised, and the cerebrospinal fluid was released. The temporal lobe is gently retracted so that the P2 segment of the posterior cerebral artery (PCA) and the corresponding aneurysm can be clearly visualized under the operating microscope. For some complex IAs located in the middle and lower segments of the basilar artery (BA), further grinding and exploration can be performed in the posteromedial (Kawase’s) triangle. The ECA is dissected at the beginning, before issuing branches, with the proximal end set aside and the distal end ligated. A radial artery (RA) approximately 20 cm in length was extracted from the forearm and pressure distended with heparinized papaverine warm saline to prevent vasospasm. The proximal RA was anastomosed in an end-to-end fashion with the proximal ECA by interrupted 8–0 nylon sutures and then tunneled through the skin into the intracranial space. We chose the P2 segment of the PCA without the perforator arteries and occluded both sides with temporary aneurysm clips. The distal end of the RA was anastomosed in an end-to-side fashion with the P2 segment at the distal location of the aneurysm using interrupted 10–0 nylon sutures. The anastomosis cuffs between the recipient and donor vessels were opposed in an everted fashion to ensure that both endothelial layers of the vessels at both ends could be attached together and to promote reliable patency of the anastomosis. Intraoperative indocyanine green fluorescein angiography was performed to assess bypass patency in all patients. Digital subtraction angiography (DSA) was used to observe intraoperative bypass surgery-induced alterations in cerebral hemodynamics and to guide surgical strategies.
Surgical algorithm
Patients included in this study had complex VBANs according to the inclusion and exclusion criteria. All enrolled patients underwent intraoperative DSA in the immediate post-bypass period. As shown in Fig. 1, the surgical algorithm was determined primarily by whether the aneurysm has ruptured or not. An important reference for surgical strategy is the intraoperative DSA and the ability of the bypass graft flow to return to the BA. For patients with ruptured aneurysms, we will apply aneurysm clips to localize the rupture site while performing ECA-RAG-PCA bypass to minimize the risk of re-rupture bleeding from the aneurysm. With an adequate return of the bypass graft blood flow to the middle to upper segment of the BA, an ECA-RAG-PCA bypass combined with aneurysm partial trapping was used if the aneurysm and the surrounding anatomical structure could be fully exposed, and conversely, an ECA-RAG-PCA bypass combined with proximal occlusion of the parent artery was used. If the intraoperative DSA of a patient with a ruptured aneurysm shows that there is insufficient bypass graft flow to return to the BA, we recommend an ECA-RAG-PCA bypass and a partial clip of the ruptured aneurysm and then an evaluation of the lesion based on DSA 1–3 months after the procedure. Proximal occlusion of the parent artery is then performed according to the principles of individualized treatment. The aim of this surgical strategy for ruptured aneurysms is to minimize intra-aneurysmal flow pressure and reduce the risk of re-rupture of the aneurysm. In patients with unruptured aneurysms, the majority develop because of ischemic stroke symptoms or occupying effects. With adequate return of the bypass graft blood flow to the middle to upper segment of the BA according to intraoperative DSA, if a recurrent aneurysm has been previously treated (including interventional embolization or surgical clipping), an ECA-RAG-PCA bypass with aneurysm partial trapping, or at least an ECA-RAG-PCA bypass with proximal occlusion of the parent artery should be performed if possible. If the intraoperative DSA of a patient with a recurrent aneurysm shows that there is insufficient bypass graft flow to return to the BA, we recommend first performing an ECA-RAG-PCA bypass and then evaluating the lesion based on the DSA 1–3 months after the procedure, followed by an aneurysm partial trapping or a proximal occlusion of the parent artery according to the principles of individualized treatment. Because recurrent aneurysms are more likely to have a poor clinical prognosis, strategies are used to minimize antegrade blood flow within the aneurysm. For complex VBANs treated for the first time, if the aneurysm is a saccular aneurysm it is likely that the full aneurysm will be exposed and an ECA-RAG-PCA bypass combined with an aneurysm partial trapping strategy or a proximal occlusion of the parent artery will be performed. The prerequisite is that, the bypass graft is able to provide adequate reverse blood flow to the BA. Otherwise, we recommend performing an ECA-RAG-PCA bypass first and deciding on further proximal occlusion of the parent artery based on the DSA 1–3 months after surgery. Nevertheless, the following is a description of some aneurysms that cannot be trapped. If the aneurysm is not a saccular aneurysm, but a complex aneurysm such as a dissecting aneurysm or a dolichoectatic aneurysm with extensive involvement of the vertebrobasilar artery system, the surgical strategy will be determined by an intraoperative DSA in the immediate post-bypass period. If the bypass graft blood flow is able to return to the middle to upper segment of the BA, an ECA-RAG-PCA bypass is performed in combination with a proximal occlusion of the parent artery. This approach will ensure adequate blood supply to the crucial perforators of the basilar artery, while blocking antegrade flow to the complex aneurysm and facilitating its disappearance. If the bypass graft is not able to return blood flow to the middle to upper segment of the BA, the bypass graft is not flowing sufficiently, and there is a high risk of brainstem infarction from the occlusion of the proximal part of the parent artery, so only an ECA-RAG-PCA bypass strategy is performed. This approach can be used to treat the aneurysm by hedging the reverse flow of the bypass graft with the antegrade flow of the vertebrobasilar artery system, altering the intra-aneurysmal hemodynamics and promoting intra-aneurysmal thrombosis. For patients with intraoperative reversal of flow in the bypass graft but not fully adequate, considering the higher risk of cerebral infarction caused by the occlusion of the proximal part of the parent artery during the same period, interventional surgery or craniotomy to occlude the proximal part of the parent artery can be performed 1–3 months after bypass surgery. The blood flow in the bypass graft would be judged to be stable according to DSA, which may be a safer and more effective surgical approach. The surgical algorithm is not set in stone, and this is only a basic reference principle. The specific surgical plan should be based on the principle of individualized treatment.
Clinical follow-up
Rigorous postoperative follow-up is particularly necessary. During the follow-up phase of the study, inpatient review follow-up, outpatient follow-up, radiological follow-up, or telephone follow-up are recommended. In this study, the mRS score was used to obtain clinical outcomes of neurological function. As patients with complex VBANs have more severe symptoms of neurological deficit, we divided all patients for whom follow-up data were available into two groups according to functional changes at the last postoperative follow-up: patients with an mRS score of 0–3 were defined as having a “good prognosis” and patients with an mRS score of 4–6 were defined as having a “poor prognosis”. Obliteration of aneurysm was defined as “complete occlusion”, reduction in aneurysm volume from preoperative level was defined as “subtotal occlusion”, and no significant change in aneurysm volume from preoperative level was defined as “stabilization”. Aneurysms that are “complete occlusion” or “subtotal occlusion” are defined as “treatment effective”, while aneurysms that are larger than before surgery or “stable” are defined as “treatment ineffective”.
Statistical analysis
Quantitative data were presented as mean ± standard deviation (SD). Qualitative data are expressed as rates or composition ratios. Statistical methods of t-test, chi-square (χ2) test or Fisher’s exact test were used in the analysis of differences between quantitative and qualitative data, respectively. Two sides value of P < 0.05 was considered to be statistically significant. All statistical analyses were performed using IBM SPSS software, version 26 (IBM Software Group, Chicago, IL). All statistical diagrams were performed with GraphPad Prism (version 8.0 software package, GraphPad Software, CA, USA).
Results
Patient and aneurysm characteristics
Over a period of more than 4 years from January 2019 to March 2023, we treated a total of 24 patients with complex VBANs for a total of 24 aneurysms by means of ECA-RAG-PCA bypass surgery, with the exception of 2 patients who were lost to follow-up. Details of the 24 patients are shown in Table 1. A total of 18 (75.0%) male patients and 6 (25.0%) female patients were treated. The age of the patients ranged from 43 to 68 years, with a mean age of 54 ± 8.83 years. In this study, 3 (12.5%) patients had subarachnoid hemorrhage (SAH), 12 (50.0%) patients had cerebral ischemic syndrome, 4 (16.7%) were patients with recurrent aneurysms, 4 (16.7%) had a mass effect, and 1 (4.1%) was incidental finding. Twelve (50.0%) aneurysms were located in the BA, 1 (4.2%) was in the vertebral artery and 11 (45.8%) involved the vertebrobasilar artery. Of the 12 BA aneurysms, 4 were located in the basilar apex, 1 in the mid basilar trunk, 1 in the lower basilar trunk, and the remaining 6 involved the truly holobasilar artery. Aneurysm sizes ranged from 12.4 to 55.0 mm, with a mean size of 31.5 ± 10.47 mm. Except for two saccular aneurysms, most of the aneurysms were dissecting or dolichoectatic morphology with extensive involvement of the vertebrobasilar artery system, most of which were giant aneurysms combined with thrombosis. The preoperative mRS scores of the patients were 1 in 1 (4.2%) case, 2 in 4 (16.7%), 3 in 6 (25.0%), 4 in 7 (29.2%), and 5 in 6 (25.0%), respectively. Detailed information on patient and aneurysm characteristics is given in Table 2.
Treatment outcomes and follow-up
All patients were treated with ECA-RAG-PCA bypass surgery and satisfactory patency of the bypass was confirmed by DSA after surgery. In our study, bypass surgery was performed via an extended middle cranial fossa approach. All enrolled patients underwent intraoperative DSA in the immediate post-bypass period. Intraoperative DSA in 8 (33.3%) patients showed insufficient return of bypass graft blood flow to the BA, and intraoperative DSA in the remaining 16 (66.7%) patients showed that bypass graft blood flow could be able to return to the middle and upper segment of the BA. According to the surgical algorithm described in the previous section, 3 (12.5%) patients underwent ECA-RAG-PCA bypass combined with aneurysm partial trapping, 12 (50.0%) patients underwent ECA-RAG-PCA bypass combined with proximal occlusion of the parent artery, and 8 (33.3%) patients underwent ECA-RAG-PCA bypass only. Special mention needs to be made about the treatment of the 12 BA aneurysms. Three of the four cases located in the basilar apex underwent ECA-RAG-PCA bypass combined with aneurysm partial trapping, and one underwent ECA-RAG-PCA bypass combined with proximal occlusion of the parent artery. One case in the mid basilar trunk and one case in the lower basilar trunk underwent ECA-RAG-PCA bypass combined with proximal occlusion of the parent artery. Six cases with involvement of the holobasilar artery underwent ECA-RAG-PCA bypass only in three cases and ECA-RAG-PCA bypass combined with proximal occlusion of the parent artery in three other cases. The choice of surgical strategy was based on both intraoperative DSA and the patient’s clinical symptoms. In our study, patients with ruptured aneurysm were treated by ECA-RAG-PCA bypass combined with aneurysm partial trapping or proximal occlusion of the parent artery to reduce the risk of re-rupture of the aneurysm (illustration case, Fig. 2). These patients had a satisfactory prognostic outcome during follow-up. The duration of clinical follow-up of the patients ranged from 3 to 45 months, with a mean follow-up of 22.0 ± 13.35 months. During clinical follow-up, there were no procedure-related deaths. Four patients developed ischemic stroke complications and two recovered well clinically after rehabilitation therapy. One developed central respiratory failure and had a poor clinical prognosis. The mRS scores at the last postoperative follow-up were 0 in 4 (16.7%), 1 in 4 (16.7%), 2 in 8 (33.3%), 3 in 5 (20.8%), 4 in 2 (8.3%), and 5 in 1 (4.2%), respectively. There were 11 (45.8%) patients with 0–3 in preoperative mRS score but 21 (87.5%) patients with 0–3 in late mRS score. The clinical prognosis of the patients at the final follow-up was significantly improved compared to the preoperative period. A comparison of the patients’ preoperative and final postoperative follow-up mRS scores is shown in Fig. 3. In this study, the final follow-up showed good patency of the bypass grafts in all patients, with a 100% high-flow bypass patency rate. Based on the definitions described previously and the clinical data at the final follow-up, 15 (62.5%) patients had complete occlusion of the aneurysm, 7 (29.2%) patients had subtotal occlusion of the aneurysm and 2 (8.3%) patients had stable aneurysms. The effective rate of treatment of complex VBANs was 91.7%. A good clinical prognosis was achieved in 21 (87.5%) of the patients and a poor clinical prognosis in 3 (12.5%). A patient with a large BA dissecting aneurysm was treated with ECA-RAG-PCA bypass combined with proximal occlusion of the parent artery with good results. During clinical follow-up, this complex aneurysm almost completely disappeared (illustration case, Fig. 4). Please see Table 3 for details of surgical treatment and clinical outcome.
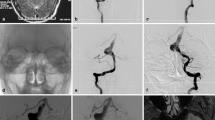
A 59-year-old woman with coma visiting our hospital was diagnosed as a massive SAH with CT scan (a), who was definitively recognized complex basilar aneurysms and treated by ECA-RAG-PCA bypass with aneurysms partial trapping. HR-MRI and DSA revealed the aneurysms and involvement of PCA or other perforating arteries clearly (b and c). We performed right ECA-RAG-PCA bypass with aneurysms partial trapping using silver clips. The anastomosis was located in the P2 segment of the PCA (d and e). Intraoperative indocyanine green fluorescein angiography was utilized to confirm graft artery patency (f). Immediately after the procedure, slow blood flow in the aneurysms and graft patency were observed (g). Re-examination at 6 months after surgery showed near disappearance of aneurysms and a good patency of graft (h). This patient recovered from mRS 5 to mRS 3 in the clinical follow up of 16 months. CT = computed tomography, HR-MRI = high-resolution magnetic resonance imaging.
This statistical figure reflects the changes in preoperative and late mRS scores at the late follow-up. The late mRS scores are significantly better than the preoperative mRS scores. The red bar graph represents the preoperative mRS score. The green bar graph represents the late mRS score. There were no cases with mRS score = 6.
A 45-year-old male patient was admitted to hospital with hydrocephalus. CT and HR-MRI images revealed hyper-vascular space-occupying lesions with diameters of 5.5 × 2.9 cm in the front of the brain stem (a and b). Pre-operative DSA and HR-MRI clearly showed a giant dissecting aneurysm involved in the left vertebral artery and basilar artery (c). Firstly, we performed a left ECA-RAG-PCA bypass (d and e). Intraoperative indocyanine green fluorescein angiography was utilized to confirm graft artery patency (f). Immediately after the bypass surgery, DSA was performed to verify the good patency of bypass and retrograde blood flow in the basilar artery (g and h). One month later after the first bypass procedure, the dissecting aneurysm was treated by endovascular embolization to occlude proximal parent artery. At 1-year re-examination during clinical follow-up, postoperative DSA demonstrated good patency of the bypass and retrograde blood flow filling into the upper segment of the basilar artery. The aneurysm almost disappeared and the branch arteries involved in the aneurysm had good patency (i and j).
Results of statistical analysis
Based on the definition and methodology of the prognostic grouping of the mRS score described previously, the prognosis of the patients at the last follow-up was divided into a “good prognosis” group and a “poor prognosis” group. Detailed information is shown in Table 4. Statistical analysis showed no statistically significant differences between the different types of surgery and the final prognosis for the three different procedures: ECA-RAG-PCA bypass alone, ECA-RAG-PCA bypass with aneurysm partial trapping, and ECA-RAG-PCA bypass with proximal occlusion of the parent artery (P = 0.217, Fisher exact test). No statistically significant difference was found between the aneurysm location and the final clinical prognosis for complex aneurysms located in the VA, the BA, or in different locations involving the vertebrobasilar artery (P = 0.125, Fisher exact test). In terms of aneurysm outcome, there was no statistically significant difference between complete occlusion of the aneurysm, incomplete occlusion of the aneurysm, and stabilization of the aneurysm (P = 0.256, Fisher exact test). In terms of patient age, no statistically significant differences were found between the two groups with good and poor prognosis (P = 0.906, t-test). However, a statistically significant difference was found between the “good prognosis” and “poor prognosis” groups in terms of aneurysm size (P = 0.034, t-test). This result suggested that the larger the aneurysm volume, the worse the prognosis.
Discussion
In our study, all cases underwent flow-replacement bypass surgery via ECA-RAG-PCA bypass to the posterior circulation. The results of this study, which showed 24 patients with complex VBANs treated with ECA-RAG-PCA bypass, confirmed that ECA-RAG-PCA bypass combined with an aneurysm partial trapping or proximal occlusion of the parent artery surgical strategy still plays a very important role in the treatment of this type of disease. The blood flow from the bypass graft not only fills the distal PCA in an antegrade fashion, but also fills the proximal PCA and BA retrogradely, as well as the rest of the vertebrobasilar circulatory system in a reverse direction. Although, the number of aneurysms treated by craniotomy is decreasing with the development of endovascular therapy, complex IAs continued to face treatment challenges. Revascularization techniques continue to play an important role in the treatment of complex IAs when endovascular interventions and traditional craniotomy procedures for clipping aneurysms encounter bottlenecks. The results of this study also confirm that ECA-RAG-PCA bypass techniques combined with aneurysm partial trapping or proximal occlusion of the parent artery can achieve satisfactory clinical outcomes for complex VBANs.
Characteristics and current status of treatment for complex VBANs
Complex IAs are currently defined acceptably as those with large or giant dimensions, dissecting or dolichoectatic morphology, wide necks, intra-aneurysmal thrombosis, atherosclerosis of the aneurysmal wall, critical branch arteries originating from the lateral wall of the aneurysm, or recurrent aneurysms that have been previously treated [5,6,7,8]. Direct surgical clipping or endovascular intervention is not entirely effective and safe for complex VBANs in the posterior circulation. In conventional procedures, when the perforators are inevitably blocked, collateral circulation can be inadequate and cerebral infarction may occur. In addition to the deep location of the vertebrobasilar aneurysm, the complex anatomical relationship with the surrounding vital neurovascular structures is a great challenge.
In the current era of endovascular treatment, interventional therapies are the main treatment strategy for cerebrovascular disease. However, endovascular therapy still leaves something to be desired in the treatment of complex IAs. Thus, microsurgical revascularization holds significant importance [10]. Although FD has shown strong clinical efficacy, serious complications such as intracranial hemorrhage and thromboembolism cannot be ignored [1, 11]. The dual antiplatelet therapy of aspirin and clopidogrel is necessary to prevent thrombosis in the parent artery after FD placement of a ruptured aneurysm, but this may increase the risk of re-rupture of the aneurysm. A study by Kulcsár Z et al. in 12 different centers documented 13 patients with delayed aneurysm rupture after FD treatment. According to the results, reverse remodeling and aggressive thrombus-related autolysis of the aneurysm wall after FD placement may lead to delayed rupture [12]. The high metal coverage rate by FD is accompanied by a high rate of thromboembolic events, increasing the risk of ischemic infarction, particularly in the posterior circulation [13]. One study showed an unfavorable outcome in 29% of patients [14]. In patients with BA aneurysms using FDs, the rate of infarction in the region of the perforator artery demonstrated in the Phillips TJ’s study was as high as 14% [15]. A retrospective study of 131 posterior circulation aneurysms treated with FDs showed complete and near-complete occlusion of the aneurysm in 78.1% of cases [2]. Siddiqui AH et al. applied FDs to treat 27 cases of dolichoectatic vertebrobasilar fusiform aneurysms. The rates of hemorrhage complications were 7.7% and 28.6% in the dual-antiplatelet therapy (DAPT) and DAPT plus anticoagulation groups, respectively, and the rates of aneurysm complete occlusion were 54.4% and 25% in the two groups, respectively [16]. In contrast, in the present study of complex VBANs treated with ECA-RAG-PCA bypass, complete and near-complete occlusion of the aneurysm was seen in 91.7% of cases, which is better than in previous studies. Therefore, radical treatment of complex VBANs should be reconsidered with caution. Microsurgical revascularization is therefore of great importance.
Microsurgical revascularization techniques
High-flow bypass combined with aneurysm trapping or proximal occlusion of the parent artery is an option for complex aneurysms that are not amenable to direct surgical clipping or endovascular treatment. Based on the advantage of fully exposing the neurovascular anatomy associated with the aneurysm, Lawton et al. suggested that microsurgery combined with revascularization techniques would be more advantageous in complex and recurrent aneurysms [17]. In this study, all patients with complex VBANs were treated by ECA-RAG-PCA bypass techniques. The clinical treatment rate was 91.7%, which was generally satisfactory. Superficial temporal artery (STA)-middle cerebral artery (MCA) bypass was first performed by Yasargil in the 1960s and is widely used to treat moyamoya disease, cerebral vascular stenosis, or occlusion [18]. Since then, various revascularization techniques have gradually been reported.
EC-IC vascular reconstruction surgery is an important tool in the treatment of complex IAs [19]. The treatment of aneurysms is based on the principle of individualized treatment. For the purpose of supplemental blood flow, low-flow bypass is sufficient. Otherwise, a high-flow bypass is essential for replacing blood flow in the region of the collateral circulation. The major low-flow cerebral revascularization procedures in the posterior circulation include STA-PCA, STA-superior cerebellar artery (SCA), occipital artery (OA)-anterior inferior cerebellar artery (AICA), occipital artery (OA)-posterior inferior cerebellar artery (PICA), and PICA-PICA anastomosis [20, 21]. However, when revascularizing complex VBANs or larger areas, high-flow bypasses such as ECA-RAG-PCA bypass combined with RAG or saphenous vein graft (SVG) are required to provide adequate blood flow and higher pressure [20]. In our study, all patients were diagnosed with complex VBANs, the vast majority involving the entire length of the parent artery or vital branches. All cases in this study were treated with ECA-RAG-PCA bypass surgery, with the option of occluding the proximal portion of the parent artery or partially trapping the aneurysm according to individual principles. The SVG is a common vessel for revascularization. Because of its very thin walls and the monodirectional flow in the longitudinal vessels with venous valves, the SVG is susceptible to injury and lacks sufficient elasticity. According to Tomizawa M et al., 19.9% of SVG and 8.1% of RAG showed occlusion on follow-up angiograms [22]. Matsukawa H et al. showed that in the case of complex aneurysms treated with EC-IC high-flow bypasses, only the SVG was associated with graft occlusion, and the RAG became progressively larger in diameter postoperatively [23]. In contrast, the intimal, medial, and adventitia layer of the RAG are intact, and the vessel wall is thicker compared to the SVG. More importantly, the diameter values of the RAG match well with the PCA and MCA. The disadvantage of the RAG is that it is prone to vasospasm. Interestingly, vasospasm in the RAG can be overcome by performing hydrostatic pressure distension prior to bypass [24]. The most common donor vessels for high-flow bypass are the ECA or the internal maxillary artery (IMA). The ECA was chosen as the donor vessel in this study. The reasons for this are as follows: on the one hand, it is difficult to find the IMA through an intracranial approach because of its complex anatomical relationships [25]. Vasospasm is another frequent intraoperative complication during the dissection of the IMA. In a study on the radiological management of epistaxis, the operator placed the guiding catheter in the proximal part of the ECA instead of the IMA, in order to avoid vasospasm of the IMA [26]. This also confirms that the IMA is more prone to vasospasm, which in turn affects subsequent bypass surgery operations. The IMA is a terminal branch of the ECA and does not provide adequate and stable blood flow compared to the main ECA [27]. In the management of complex aneurysms in the posterior circulation, a high and stable blood flow is required for revascularization with replacement flow options. In terms of being a donor vessel, the IMA is less advantageous than the ECA trunk. Therefore, all patients in this study underwent surgery with the ECA trunk as the donor artery for high-flow bypass. The rationale for a high-flow bypass for complex aneurysms is to reduce the antegrade blood flow to the aneurysm by redirecting the flow, either in combination with aneurysm trapping or proximal occlusion of the parent artery, in order to promote thrombosis within the aneurysm for therapeutic purposes [28]. On the other hand, a high-flow bypass can provide the corresponding perforator arteries with retrograde blood flow to ensure adequate blood supply to the perforator arteries and, to a certain extent, to counteract the antegrade flow from the parent artery [29, 30]. Thus, complex VBANs are potentially curable. A study on the hemodynamic effects of revascularization on intracranial aneurysms showed a 40–70% reduction in pressure and wall shear stress within the aneurysm compared to the pre-surgical period [31]. The hemodynamic changes following high-flow bypass surgery not only reduced pressure and wall shear stress in the aneurysm, but also largely facilitated the formation of intra-aneurysmal thrombus. High-flow bypass surgery provides adequate blood flow and stable pressure in the area of parent artery occlusion. In our cohort study, 33.3% of patients underwent ECA-RAG-PCA bypass surgery only, without disposing of the aneurysm or performing a proximal occlusion of the parent artery. This was due to the intraoperative DSA findings of inadequate reversal of blood flow from the bypass graft to the BA. Given the extremely high risk of ischemic stroke from direct management of complex aneurysms or performing proximal occlusion of the parent artery in this situation, only ECA-RAG-PCA bypass surgery was performed. By counteracting the retrograde flow of the bypass graft with the antegrade flow of the parent artery, the pressure in the aneurysm is reduced and intra-aneurysmal thrombosis is promoted for therapeutic purposes, while the blood supply to the corresponding perforator area is ensured to reduce the risk of cerebral infarction. This approach may not completely cure the aneurysm in the short term, but it may lead to a reduction or stabilization of the complex aneurysm. Long-term follow-up clinical outcomes are expected to be safe and effective. Thus, ECA-RAG-PCA bypass techniques offer a safe and alternative treatment option for complex VBANs.
Surgical approach for bypass
The extended middle cranial fossa approach provides adequate anterior frontal, temporal, and posterior surgical corridors. When additional operative space is required, sphenoid ridge to the anterior clinoid process and the bones of the middle skull base osteotomy provide additional space in the Glasscock’s triangle and Kawase’s triangle to create vascular anastomosis space for complex IAs. Common surgical approaches to treat complex VBANs in the posterior circulation include: (1) an expanded orbitozygomatic approach for intracranial aneurysms located in the upper two-fifths of the BA region, (2) a transpetrosal approach for IAs located in the middle fifth of the BA region, (3) an extended far lateral approach for IAs located in the lower two-fifths of the BA region and in the intradural segment of the VA, and (4) for complex IAs between regions or spanning multiple regions, a combined surgical approach is used [32]. For the treatment of IAs in the P2 segment of the PCA, the subtemporal approach is a classic surgical approach and has been used for many years. Professor Drake had made an outstanding contribution to the treatment of aneurysms of the vertebrobasilar system by the subtemporal approach [33, 34]. Strikingly, the obvious disadvantage of the subtemporal approach is the severe damage and potential damage to the temporal lobe due to brain retraction [35]. In the present study, the extended middle cranial fossa approach was used in all patients with complex VBANs. By severing the zygomatic arch and simultaneously grinding away the sphenoid ridge, anterior clinoid process and the middle skull base bone through the epidural space, we were able to better visualize the aneurysm and gain more surgical space to facilitate the procedure. By extended middle cranial fossa approach, a greater epidural space can be obtained, which can reduce the retraction on the temporal lobe, reduce surgical complications, and improve clinical prognosis.
Surgical algorithm and safety assessment
Based on our experience, we classify complex VBANs into two main categories: ruptured and unruptured. These are shown in Fig. 1. In patients with ruptured aneurysms combined with SAH, where preoperative DSA excludes significant vasospasm, our center’s treatment principle is to treat them as early as possible, unless Hunt-Hess 4–5 or severe cerebral vasospasm is present. Otherwise, surgery is delayed for about 14 days. Due to the high risk of re-rupture and bleeding of the ruptured aneurysm in the short term, we opt for ECA-RAG-PCA bypass combined with an aneurysm partial trapping to largely isolate the aneurysm from the circulation and completely cure the aneurysm if the anatomy around the aneurysm can be fully visualized. This is illustrated in Fig. 5a. If the aneurysm cannot be fully visualized, we choose an ECA-RAG-PCA bypass combined with proximal occlusion of the parent artery to minimize antegrade flow to the aneurysm and reduce the risk of re-rupture and bleeding. For unruptured aneurysms, if the lesion is a previously treated recurrent aneurysm, depending on whether the overall structure of the aneurysm can be fully exposed, we use ECA-RAG-PCA bypass combined with aneurysm isolation or proximal occlusion of the parent artery to minimize antegrade blood flow to the aneurysm. Because recurrent aneurysms are often clinically severe and complex, failure to treat the aneurysm effectively is likely to result in a poor prognosis [36,37,38]. We aim to achieve a clinical cure for recurrent aneurysms, while reducing the likelihood of repeat surgery or even multiple surgeries in patients with recurrent aneurysms and reducing the cost and financial strain on patients. For patients with unruptured complex aneurysms treated for the first time, we adopt different surgical strategies depending on the results of intraoperative DSA. If intraoperative DSA after bypass reveals that the bypass graft is able to reverse the flow to the middle and upper segment of the BA, this suggests that the bypass graft is able to provide sufficient flow to the original supply area of the parent artery, and that the risk of embolism of the perforator artery is low, in which case it is safe to occlude the proximal part of the parent artery. This is illustrated in Fig. 5b. If intraoperative DSA after bypass reveals that the bypass graft is unable to reverse the flow to the superior middle BA, this indicates that the bypass graft is unable to provide sufficient blood flow to the original supply area of the parent artery, in which case the risk of embolism of the perforator artery and cerebral infarction from the occlusion of the parent artery is extremely high and even life-threatening, so we adopt an ECA-RAG-PCA bypass technique alone. The retrograde flow from the bypass graft is counteracted by the antegrade flow from the parent artery, altering the intra-aneurysm hemodynamics and thereby treating the aneurysm, while at the same time ensuring blood supply to the perforator vessels in the affected area and reducing the risk of ischemic stroke events. This is illustrated in Fig. 5c.
Black arrows represent antegrade blood flow from the parent artery. Blue arrows represent retrograde blood flow from the bypass graft. a ECA-RAG-PCA bypass + aneurysm partial trapping. b ECA-RAG-PCA bypass + proximal occlusion of the parent artery. c ECA-RAG-PCA bypass alone.
The present center’s experience is to make full use of flow-counteraction strategies to treat complex aneurysms. For aneurysms that cannot be trapped directly, such as those with crucial perforator branches, giant aneurysms that block the exposure of the proximal parent artery, or bypass grafts that do not provide adequate flow in the opposite direction, it is possible to perform a high-flow bypass merely at the distal end of the aneurysm, making full use of the regulation of blood flow itself, so that the flow from the bypass graft through the anastomosis supplies blood in the distal direction on the one hand and in the opposite direction on the other, thus promoting thrombosis in the aneurysm. This allows the blood flow from the bypass graft to be supplied through the anastomosis in a cascade to the distal end and in a reverse direction to the aneurysm, thus counteracting the antegrade flow of the parent artery and promoting thrombosis of the aneurysm. This is a simple procedure with fewer potential risks. This surgical approach can suppress the further development of the aneurysm lesion. The treatment principle has been confirmed in our preliminary research [39]. As for the choice of anastomosis location, if the recipient artery has no perforator vessels, it is placed as close as possible to the distal end of the aneurysm to ensure adequate reverse flow pressure; if the recipient artery has a perforator branch, a suitable location distal to the aneurysm is chosen avoiding the perforator branch. The weak part of the aneurysm may be partially clipped to reinforce the aneurysm wall and allow it to withstand the shock of changing flow pressures. For aneurysms where the anatomy around the aneurysm can be directly exposed, as the proximal parent artery or main blood supply artery is a key factor in the formation and progression of the aneurysm, the main responsible vessel (proximal to the parent artery) can be blocked to promote thrombosis within the aneurysm. A high-flow bypass can be performed at the distal end of the aneurysm where there are no perforator branches. The blood flow from the bypass graft can be supplied to the distal end through the anastomosis on the one hand, while the blood supply to the perforator vessels involved in the aneurysm is ensured by retrograde flow. This technique in effect completes the partial trapping of the aneurysm, and long-term follow-up reveals that the aneurysm is stable, shrinking or has disappeared, thus achieving the goal of treatment.
In our study, only 3 (12.5%) of the 24 cases are presented with SAH on admission. These 3 patients with ruptured aneurysms were all younger than 60 years of age, and clinical follow-up revealed an essentially satisfactory prognosis, with no re-ruptured aneurysm bleeding. The remaining 21 patients in this group did not rupture during clinical follow-up. One patient with incomplete occlusion of aneurysm who was followed up for 45 months in this study developed a cerebellar infarction after surgery, with an mRS score of 4 at final follow-up. This patient had a poor prognosis, but his condition had been stable. The patient had no cerebral hemorrhage on regular follow-up brain CT at the local hospital. The other three patients who were followed up for more than 40 months had a satisfactory clinical prognosis and did not develop clinical symptoms of cerebral hemorrhage. Our analysis of the possible reasons for this phenomenon is due to the fact that the high-flow bypass reduces the load of the parent artery on the aneurysmal body from the impact of the antegrade blood flow, which greatly reduces the intra-aneurysmal pressure. The retrograde flow-counteraction effect of ECA-RAG-PCA bypass promotes thrombus formation within the aneurysm, thus strengthening the aneurysm wall to a certain extent. With hemodynamic changes at the lesion site after completion of the bypass procedure, the aneurysm would be in a state of stabilization or even cure. These may account for the greatly reduced risk of aneurysm rupture after a high-flow bypass.
A review by Sia SF et al. showed that patency rates after high-flow bypass surgery ranged from 73 to 100% [40]. Our experience has shown a 100% patency rate for ECA-RAG-PCA bypass at long-term follow up, with overall results better than previously reported in the literature. Posterior circulation complex VBANs themselves are extremely difficult to treat, with severe clinical symptoms and serious treatment complications, and have long been a serious challenge for the medical community. Raper DMS et al. showed that as techniques related to preoperative assessment and postoperative complication identification and management continue to develop, bypass graft patency rates are increasing and revascularization techniques are becoming safer and more effective for specific patients [41]. Our center has been able to effectively treat 91.7% of complex VBANs and achieve a good clinical prognosis in 87.5% of patients by combining ECA-RAG-PCA bypass with aneurysm partial trapping or proximal occlusion of the parent artery according to individualized treatment principles. The effectiveness and safety of surgical treatment at the present center is satisfactory.
The main limitation is the small number of cases in a single center, which limits the statistical analysis. Further studies with larger samples are warranted to validate the significance of the results of this study. The generalization of the experience of this study may be potentially limited.
Conclusion
The treatment of complex vertebrobasilar aneurysms has long been a challenge for neurosurgeons. When conventional surgical clipping of aneurysms and endovascular interventions lead to frustrating results in complex VBANs, ECA-RAG-PCA bypass combined with aneurysm partial trapping or proximal occlusion of the parent artery remains an alternative and preferable option. The results of our study show that ECA-RAG-PCA bypass for complex VBANs has yielded satisfactory clinical results. Even in an era of increasingly advanced interventional therapy, the extremely skilled micro-neurosurgical technique continues to play an important role in the treatment of complex VBANs.
Data Availability
Data available on request from the authors. The data that support the findings of this study are available from the corresponding author, [Xiaoguang Tong], upon reasonable request.
References
Awad AJ, Mascitelli JR, Haroun RR, De Leacy RA, Fifi JT, Mocco J (2017) Endovascular management of fusiform aneurysms in the posterior circulation: the era of flow diversion. Neurosurg Focus 42(6):E14. https://doi.org/10.3171/2017.3.Focus1748
Griessenauer CJ, Ogilvy CS, Adeeb N et al (2018) Pipeline embolization of posterior circulation aneurysms: a multicenter study of 131 aneurysms. J Neurosurg 130(3):923–935. https://doi.org/10.3171/2017.9.Jns171376
Hara T, Arai S, Goto Y, Takizawa T, Uchida T (2016) Bypass surgeries in the treatment of cerebral aneurysms. Acta Neurochir Suppl 123:57–64. https://doi.org/10.1007/978-3-319-29887-0_8
Lawton MT, Lang MJ (2019) The future of open vascular neurosurgery: perspectives on cavernous malformations, AVMs, and bypasses for complex aneurysms. J Neurosurg 130(5):1409–1425. https://doi.org/10.3171/2019.1.Jns182156
Tayebi Meybodi A, Huang W, Benet A, Kola O, Lawton MT (2017) Bypass surgery for complex middle cerebral artery aneurysms: an algorithmic approach to revascularization. J Neurosurg 127(3):463–479. https://doi.org/10.3171/2016.7.Jns16772
Abla AA, Lawton MT (2014) Anterior cerebral artery bypass for complex aneurysms: an experience with intracranial-intracranial reconstruction and review of bypass options. J Neurosurg 120(6):1364–1377. https://doi.org/10.3171/2014.3.Jns132219
Kaku Y, Takei H, Miyai M, Yamashita K, Kokuzawa J (2016) Surgical treatment of complex cerebral aneurysms using interposition short vein graft. Acta Neurochir Suppl 123:65–71. https://doi.org/10.1007/978-3-319-29887-0_9
Zhu W, Liu P, Tian Y et al (2013) Complex middle cerebral artery aneurysms: a new classification based on the angioarchitecture and surgical strategies. Acta Neurochir (Wien) 155(8):1481–1491. https://doi.org/10.1007/s00701-013-1751-8
Butterfield JT, Chen CC, Grande AW, Jagadeesan B, Tummala R, Venteicher AS (2021) The rate of symptomatic ischemic events after passing balloon test occlusion of the major intracranial arteries: meta-analysis. World Neurosurg 146:e1182–e1190. https://doi.org/10.1016/j.wneu.2020.11.134
Kalani MY, Ramey W, Albuquerque FC et al (2014) Revascularization and aneurysm surgery: techniques, indications, and outcomes in the endovascular era. Neurosurgery 74(5):482–497. https://doi.org/10.1227/neu.0000000000000312. (discussion 497-488)
Kulcsár Z, Wetzel SG, Augsburger L, Gruber A, Wanke I, Rüfenacht DA (2010) Effect of flow diversion treatment on very small ruptured aneurysms. Neurosurgery 67(3):789–793. https://doi.org/10.1227/01.Neu.0000372920.39101.55
Kulcsár Z, Houdart E, Bonafé A et al (2011) Intra-aneurysmal thrombosis as a possible cause of delayed aneurysm rupture after flow-diversion treatment. AJNR Am J Neuroradiol 32(1):20–25. https://doi.org/10.3174/ajnr.A2370
Alghamdi F, Morais R, Scillia P, Lubicz B (2015) The silk flow-diverter stent for endovascular treatment of intracranial aneurysms. Expert Rev Med Devices 12(6):753–762. https://doi.org/10.1586/17434440.2015.1093413
Maybaum J, Henkes H, Aguilar-Pérez M et al (2021) Flow diversion for reconstruction of intradural vertebral artery dissecting aneurysms causing subarachnoid hemorrhage-a retrospective study from four neurovascular centers. Front Neurol 12:700164. https://doi.org/10.3389/fneur.2021.700164
Phillips TJ, Wenderoth JD, Phatouros CC et al (2012) Safety of the pipeline embolization device in treatment of posterior circulation aneurysms. AJNR Am J Neuroradiol 33(7):1225–1231. https://doi.org/10.3174/ajnr.A3166
Siddiqui AH, Monteiro A, Hanel RA et al (2023) Triple therapy versus dual-antiplatelet therapy for dolichoectatic vertebrobasilar fusiform aneurysms treated with flow diverters. J Neurointerv Surg 15(7):655–663. https://doi.org/10.1136/jnis-2022-019151
Davies JM, Lawton MT (2014) Advances in open microsurgery for cerebral aneurysms. Neurosurgery 74(Suppl 1):S7-16. https://doi.org/10.1227/neu.0000000000000193
Yasargil MG, Krayenbuhl HA, Jacobson JH 2nd (1970) Microneurosurgical arterial reconstruction. Surgery 67(1):221–233
Costa M, Baldoncini M, Tataryn ZL et al (2021) Microsurgical clipping of carotid-ophthalmic tandem aneurysms: case report and surgical nuances. Medicina (Kaunas) 57(7):731–740. https://doi.org/10.3390/medicina57070731
Kawashima M, Rhoton AL Jr, Tanriover N, Ulm AJ, Yasuda A, Fujii K (2005) Microsurgical anatomy of cerebral revascularization. Part II: posterior circulation. J Neurosurg 102(1):132–147. https://doi.org/10.3171/jns.2005.102.1.0132
Coert BA, Chang SD, Marks MP, Steinberg GK (2005) Revascularization of the posterior circulation. Skull Base 15(1):43–62. https://doi.org/10.1055/s-2005-868162
Tomizawa M, Hori S, Nishimura N et al (2020) Arterial reconstruction using the donor’s gonadal vein in living renal transplantation with multiple renal arteries: a case report and a literature review. BMC Nephrol 21(1):190. https://doi.org/10.1186/s12882-020-01848-z
Matsukawa H, Tanikawa R, Kamiyama H et al (2017) Graft occlusion and graft size changes in complex internal carotid artery aneurysm treated by extracranial to intracranial bypass using high-flow grafts with therapeutic internal carotid artery occlusion. Neurosurgery 81(4):672–679. https://doi.org/10.1093/neuros/nyx075
Morton RP, Moore AE, Barber J et al (2014) Monitoring flow in extracranial-intracranial bypass grafts using duplex ultrasonography: a single-center experience in 80 grafts over 8 years. Neurosurgery 74(1):62–70. https://doi.org/10.1227/neu.0000000000000198
Eller JL, Sasaki-Adams D, Sweeney JM, Abdulrauf SI (2012) Localization of the internal maxillary artery for extracranial-to-intracranial bypass through the middle cranial fossa: a cadaveric study. J Neurol Surg B Skull Base 73(1):48–53. https://doi.org/10.1055/s-0032-1304556
Krajina A, Chrobok V (2014) Radiological diagnosis and management of epistaxis. Cardiovasc Intervent Radiol 37(1):26–36. https://doi.org/10.1007/s00270-013-0776-y
Sato K, Ogoh S, Hirasawa A, Oue A, Sadamoto T (2011) The distribution of blood flow in the carotid and vertebral arteries during dynamic exercise in humans. J Physiol 589(Pt 11):2847–2856. https://doi.org/10.1113/jphysiol.2010.204461
Lee SH, Ahn JS, Kwun BD, Park W, Park JC, Roh SW (2015) Surgical flow alteration for the treatment of intracranial aneurysms that are unclippable, untrappable, and uncoilable. J Korean Neurosurg Soc 58(6):518–527. https://doi.org/10.3340/jkns.2015.58.6.518
Nussbaum ES, Kallmes KM, Lassig JP, Goddard JK, Madison MT, Nussbaum LA (2018) Cerebral revascularization for the management of complex intracranial aneurysms: a single-center experience. J Neurosurg 131(4):1297–1307. https://doi.org/10.3171/2018.4.Jns172752
Lu X, Huang Y, Zhou P, Zhu W, Wang Z, Chen G (2021) Cerebral revascularization for the management of complex middle cerebral artery aneurysm: a case series. Exp Ther Med 22(2):883. https://doi.org/10.3892/etm.2021.10315
Kurşun B, Uğur L, Keskin G (2018) Hemodynamic effect of bypass geometry on intracranial aneurysm: a numerical investigation. Comput Methods Programs Biomed 158:31–40. https://doi.org/10.1016/j.cmpb.2018.02.008
Spetzler RF, Riina HA, Lemole GM Jr (2001) Giant aneurysms. Neurosurgery 49(4):902–908. https://doi.org/10.1097/00006123-200110000-00022
Drake CG (1969) The surgical treatment of vertebral-basilar aneurysms. Clin Neurosurg 16:114–169. https://doi.org/10.1093/neurosurgery/16.cn_suppl_1.114
Drake CG (1975) Ligation of the vertebral (unilateral or bilateral) or basilar artery in the treatment of large intracranial aneurysms. J Neurosurg 43(3):255–274. https://doi.org/10.3171/jns.1975.43.3.0255
Goehre F, Kamiyama H, Noda K et al (2016) Technical description of the medial and lateral anterior temporal approach for the treatment of complex proximal posterior cerebral artery aneurysms. World Neurosurg 86:490–496. https://doi.org/10.1016/j.wneu.2015.09.068
Nakase H, Kamada Y, Aoki H, Goda K, Morimoto T, Sakaki T (2000) Clinical study on recurrent intracranial aneurysms. Cerebrovasc Dis 10(4):255–260. https://doi.org/10.1159/000016067
Asgari S, Wanke I, Schoch B, Stolke D (2003) Recurrent hemorrhage after initially complete occlusion of intracranial aneurysms. Neurosurg Rev 26(4):269–274. https://doi.org/10.1007/s10143-003-0285-6
Han HJ, Lee W, Kim J et al (2022) Incidence rate and predictors of recurrent aneurysms after clipping: long-term follow-up study of survivors of subarachnoid hemorrhage. Neurosurg Rev 45(5):3209–3217. https://doi.org/10.1007/s10143-022-01828-x
Wang X, Tong X, Liu J, Shi M, Shang Y, Wang H (2023) Petrous carotid to upper posterior circulation bypass for the treatment of basilar trunk aneurysm: a novel high-flow intracranial-intracranial skull base bypass for posterior circulation. Oper Neurosurg (Hagerstown) 24(3):301–309. https://doi.org/10.1227/ons.0000000000000510
Sia SF, Morgan MK (2013) High flow extracranial-to-intracranial brain bypass surgery. J Clin Neurosci 20(1):1–5. https://doi.org/10.1016/j.jocn.2012.05.007
Raper DMS, Rutledge WC, Winkler EA et al (2020) Controversies and advances in adult intracranial bypass surgery in 2020. Oper Neurosurg (Hagerstown) 20(1):1–7. https://doi.org/10.1093/ons/opaa276
Acknowledgements
We thank Hu Wang and Hong Ji for the clinical data support and all the patients treated in our medical center.
Author information
Authors and Affiliations
Contributions
The corresponding author Xiaoguang Tong contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Meng Zhang, Xiangchen Wu, Kaiming Gao, Litian Huang, and Xingdong Wang. The first draft of the manuscript was written by Meng Zhang and all authors commented on the previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This retrospective chart review study involving human participants was in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The Human Investigation Committee (IRB) of Tianjin Huanhu Hospital approved this study. Written informed consent was obtained from all individual participants included in the study and all enrolled patients or their families before conducting this clinical study. We followed the principle of confidentiality and respected any wishes of the patients.
Consent for publication
The authors affirm that human research participants provided written informed consent for publication of the images in Figs. 2 and 4.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, M., Wu, X., Gao, K. et al. External carotid artery-radial artery graft-posterior cerebral artery bypass for complex vertebrobasilar aneurysms: efficacy and analysis of outcome in a single center. Neurosurg Rev 46, 192 (2023). https://doi.org/10.1007/s10143-023-02101-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10143-023-02101-5