Abstract
Cerebrospinal fluid (CSF) leakage is a major complication after elective neurosurgical procedures. The aim of this systematic literature review is to summarize the incidence rates of postoperative cerebrospinal fluid leakage for neurosurgical procedures, classified by surgical approach. The Pubmed, Cochrane, Embase, and Web of Science databases were searched for studies reporting the outcome of patients undergoing elective neurosurgical procedures. The number of patients, surgical approach, and indication for surgery were recorded for each study. Outcomes related to CSF leakage such as clinical manifestation and treatment were reported as well. One hundred and thirteen studies were included, reporting 94,695 cases. Overall, CSF leaks were present in 3.8% of cases. Skull base surgery had the highest rate of CSF leakage with 6.2%. CSF leakage occurred in 5.9% of anterior skull base procedures, 6.4% of middle fossa, and 5.2% of transpetrosal surgeries. 5.8% of reported infratentorial procedures were complicated by CSF leakage versus 2.9% of supratentorial surgeries. CSF leakage remains a common serious adverse event after cranial surgery. There exists a need for standardized procedures to reduce the incidence of postoperative CSF leakage, as this serious adverse event may lead to increased health care costs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cerebrospinal fluid (CSF) acts as an important protector for the central nervous system. Apart from mechanical protection as shock absorber, it provides nutrients to the brain and disposes of waste products. The CSF in the subarachnoid space is surrounded externally by tough dura mater which is formed by a double layer of connective tissue consisting of collagen fibers, elastin filaments, and fibroblasts. The dura mater protects the brain from invasion by infectious agents and supports blood vessels nourishing the central nervous system.
Various neurosurgical procedures require opening of the dura. Postoperative CSF leakage represents a major and challenging complication in skull base surgery and neurosurgery in general. It can manifest as rhinorrhea, otorrhea, or leakage through the operation wound, i.e. incisional leakage. When a subcutaneous fluid collection does not exit the wound, it is referred to as pseudomeningocele. CSF leakage often results in secondary complications including surgical site infection, meningitis, delayed wound healing, or cranial hypotension. A postoperative CSF leak can be treated conservatively, using bed rest and/or pressure dressings. Lumbar punctures or placement of a lumbar drain may resolve the leak, but in about 3% of surgeries, reoperation is needed [23]. The accompanying increased healthcare needs, including prolonged hospitalization or redo surgeries, result in increased healthcare costs, both to the patient and the healthcare system.
Incidence rates of postoperative CSF leakage have been reported in up to 13% of elective neurosurgical procedures [28]. However, no systematic reporting nor definition of CSF leakage exists. Some studies only reported leaks requiring reoperation, while others included spontaneously resolving leaks as well. The incidence of CSF leakage also depends on the surgical approach, location, and size of the lesion as well as patient characteristics as shown in previous studies where a correlation between the incidence of CSF leaks and the age of the patient, comorbidities, and patient-specific risk factors was observed [68, 90].
Given the major consequences of CSF leakage, it is clear that adequate dural closure is of paramount importance. A large variety of methods and techniques for optimization of dural closure has been described. These include the use of grafts or augmentation techniques, based on both autologous as well as synthetic products [13]. However, controversy exists about which method is the most effective.
To our knowledge, no extensive review nor meta-analysis has been performed on postoperative CSF leakage, taking into account the surgical approach, regardless of closure technique. The current review aims to describe the incidence rates of postoperative CSF leakage for neurosurgical procedures, classified per surgical approach.
Methods
This review was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines. Ethical approval was not required to perform this study.
Information sources and search method
The literature search was performed in PubMed, Embase, Cochrane, and Web Of Science Core Collection (WOS) databases. The strategy was based on the population, intervention, control and outcomes (PICO) principle and constructed using MeSH-terms for PubMed and Emtree terms in the Embase search. The 22 documents the search strategy used for all databases.
Study selection
Title and abstract were used to select for relevant publications. Potentially suitable publications for this review were screened for eligibility based on their full text by BC. Additional publications were included by “snowballing” the references in the selected studies.
Articles written in English, Dutch, and French were screened. Case reports, literature reviews, and meta-analyses were excluded. Spinal and maxillofacial procedures were not included in this review. Studies including trauma cases were excluded as well because these cases are generally approached and treated in a distinct way due to diverse pathophysiology. An adult study population (excluding articles with a specific focus on an elderly or pediatric population) with at least 100 subjects per surgical approach or per treatment group was required for inclusion. Studies describing a specific technique or material for dural closure were included unless the aim of the study was to demonstrate efficacy of these products or techniques. Studies were included only if the rate of CSF leakage could be determined. The final decision for inclusion was based on the full text manuscript. An overview of the inclusion and exclusion criteria is summarized in Table 1.
Outcome definition
The primary outcome was the rate of CSF leakage for a specific surgical approach in elective cranial surgery. Secondary outcome was defined as the clinical presentation such as incisional wound leakage, CSF rhinorrhea, CSF otorrhea, middle ear effusion, or pseudomeningocele. In addition, data on the treatment of CSF leaks (conservative, pressure dressing, puncture, lumbar drainage, redo surgery …) were included. A formal meta-analysis was not possible because of the large clinical heterogeneity in the articles regarding surgical indication, surgical approach, and closure techniques.
Studies were subdivided in three groups according to surgical approach: supratentorial, infratentorial, and skull base surgery. Skull base surgeries were further classified into open and endoscopic approaches. Open skull base procedures involved anterior fossa, middle fossa, and transpetrosal procedures. Endoscopic skull base procedures included transsphenoidal and extended endonasal procedures (transcribriform, transplanum, transclival approaches).
Results
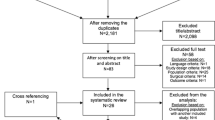
A literature search through the PubMed, Embase, Cochrane Central, and Web of Science databases was performed on May 10, 2021 (search strategies, see 22). The search yielded 10,176 results (Pubmed 4422, Embase 688, Cochrane 306, WOS 2760) of which 2893 duplicates were resolved resulting in 7383 unique hits (Fig. 1). Based on title, 5822 articles were excluded. Abstracts of 782 publications were screened leading to 233 articles eligible for full-text screening. Based on the reference lists of included studies (snowballing), another 15 articles were added. Eventually, 113 publications were included in this review. The majority of included studies were retrospective analyses of single-center or multicenter surgical data. Main reasons for exclusion based on full-text articles included surgical approach not specified (10 studies); less than 100 patients per group or surgical approach (20 studies); CSF leak rate not mentioned (14 studies); other language (13 studies); trauma cases (5 studies); description of a specific surgical technique without comparison (18 studies), or extradural procedures (2 studies).
Level of evidence
This systematic review summarizes data from 94,695 patients in 113 studies. For this analysis, no reviews or meta-analyses were included. Two randomized controlled trials were included, accounting for 562 patients (level I evidence). Nine prospective studies were included with a total of 5267 patients (level II). Eight retrospective reviews of prospectively collected data (level III) and 8 retrospective cohort studies (level IV) accounted for a total of 10,465 and 30,993 patients, respectively. All 86 other included studies were mainly case series, including 47,408 subjects (Table 2).
Supratentorial surgery
Postoperative CSF leakage after elective supratentorial surgery was reported in 16 studies, representing data from 12,803 patients (Table 3) [5, 9, 32, 35, 37, 38, 41, 58, 59, 67, 84, 86, 87, 99, 108]. A total of 376 leaks were reported, corresponding to 2.9% of patients. The incidence rate of CSF leakage in these 16 studies ranged from 1.2% [35] to 10.9% [9], with a calculated mean of 4.1%.
The included studies did not always mention the indication for surgery. Tumors were mentioned in 2321 cases (18.1%), including 1682 meningioma resections. Other indications were epilepsy surgery (1253 cases, 9.8%) and vascular surgery (261 cases, 2.0%). Meningiomas [86, 87, 99] had an average postop CSF leakage rate of 1.9%. In other studies, no CSF leakage rate per indication was mentioned.
The surgical approach was determined as frontal in 378 cases (3.0%), pterional in 152 cases (1.2%), temporal in 821 (6.4%), and parietal in 60 cases (0.5%). For all other cases (88.9%), the surgical approach was not mentioned.
As a consequence of CSF leakage, seventy-three patients needed to return to the operation room. Of these 73, revision surgery was necessary in 45 cases and 28 patients needed CSF shunting. In another 11 reported cases, the wound was resutured with good results [35]. Puncture and/or pressure dressing resolved a CSF collection in 21 patients [109]. Conservative treatment was sufficient in 13 cases [9].
Several risk factors related to CSF leakage were studied. Recurrent surgery and tumor volume were also reported as risk factors for CSF leakage [110]. Another study however [67] found no difference in CSF leak rate between patients with and without previous surgeries. Additionally, Korinek et al. showed that CSF leakage could be a significant risk factor for infection (up to 36.8% leakage rate in infected patients versus 1.4% in noninfected patients) [58, 59].
Skull base surgery
Open anterior fossa approach
Five studies reported postoperative leakage rates after anterior skull base surgery (Table 4) [44, 81, 92, 102, 110]. 5.9% (49 of 827 patients) manifested with a postoperative CSF leak. Incidence rates ranged between 2.2% [44] and 9.5% [102]. While certain authors [44, 81, 92] reported CSF leakage as cases that required surgical revision or CSF shunting, others [102] [110] did not specify the treatment for CSF leakage. Schneider et al. [92] assessed the correlation with extent of tumor resection and found a significantly higher CSF leakage rate (10.1%) in case of radical resection with excision of the dural tail (Simpson grade I) compared to less aggressive Simpson grade II resections (2.3% CSF leakage).
Open middle fossa approach
Six studies described a total of 1616 patients who underwent middle fossa skull base approaches [10, 50, 72, 81, 91, 100] and reported an average of 6.4% postoperative CSF leakage rate (1.3% [81]–20.4% [51]) (Table 4). Of the patients with reported CSF leaks, 5 were specified as incisional leak and 24 presented with rhinorrhea. Conservative or noninvasive treatment was sufficient in 90 cases, of which pressure dressing was exerted for 77 patients. Surgical wound revision was required in 55 cases. CSF diversion with a lumbar drain was performed in 38 patients. The highest reported incidence of CSF leakage was 20.4% [51]. In this study, eight of 32 patients diagnosed with postoperative CSF leakage needed surgical revision representing 5% of the total study population.
Open transpetrosal approach
Eleven distinct publications including 4831 patients discussed the transpetrosal approach (Table 4) [6, 10, 20, 30, 55, 63, 74, 77, 93, 100, 101]. Postoperative CSF leakage was reported in 5.2% of cases (0.85% [6]–20% [101]). Out of the 252 leaks, 58 represented incisional leaks and 90 patients suffered from oto- or rhinorrhea. Reoperation was required in 71 cases (28%), while a lumbar drain was placed in 52 cases (21%). Subcutaneous wound collections occurred in 26 patients (10%). Conservative treatment, including pressure dressing or bed rest sufficed in 22 cases (9%). Other cases of CSF leakage and their treatment were not specified.
Endonasal surgery
The total reported incidence rate of postoperative CSF leakage after transsphenoidal surgery was 2.8% (1136 out of 41,028 subjects), based on 45 studies [1, 2, 7, 8, 11, 14, 17, 18, 24, 26, 29, 34, 40, 42, 43, 48, 52, 53, 56, 60, 66, 69, 76, 78, 80, 83, 85, 88, 94, 96,97,98, 104, 106, 111,112,113,114,115, 117]. Incidence rates ranged between 0.17 and 15.9% (Table 5).
Transsphenoidal surgery using the microscopic endonasal technique was reported in 9 studies, while 24 studies reported results of the endoscopic endonasal transsphenoidal approach. An additional 10 publications did not mention the approach (microscopic/endoscopic). The largest retrospective study [83] mentioned 13,070 patients undergoing transsphenoidal surgery for pituitary neoplasms and found an overall rate of 1.7% (230 of 13,070 patients).
The highest rate of postoperative CSF leakage was reported by Kassam et al. [53], which represents the first large retrospective study on endoscopic endonasal skull base surgery. Of the 800 patients, 127 needed endoscopic repair or lumbar drain placement due to postoperative CSF leakage after endoscopic transsphenoidal surgery. Younus et al. [114] only reported one case in 584 that needed readmission due to CSF leakage (0.17%) after endoscopic transsphenoidal pituitary surgery. However, other interventions related to CSF leakage such as treatment during the initial hospital stay and interventions that did not require readmission were not mentioned here.
Overall, endoscopic procedures (4.1%) tended to have higher leakage rates than microscopic surgery (1.7%). On the other hand, endoscopic surgery has been more extensively described in the literature.
Endonasal extended approach
Four studies described the expanded endonasal approach [31, 61, 70, 118]. Twenty-seven of 506 (5.3%) patients presented with postoperative CSF leak, which resolved with CSF shunting in 16 cases. Other cases of postoperative CSF leakage and their treatment were not specified. An extensive variety of closure material and multilayer reconstructions were used for dural closure and reconstruction of the skull base, including fat, pericranium, or fascia lata grafts; bone fragments; fibrin sealants or glues; gelatin sponges; and nasoseptal flaps. Mascarenhas et al. [70] reported lower CSF leakage rates when introducing gasket sealing (4.2% with fascia lata and fibrin glue gasket seal versus 11% with fat graft and fibrin glue alone), even more so after the additional introduction of the nasoseptal flap (1.8% leakage). Preventive lumbar CSF drainage was not consistently applied, and its effect on postoperative CSF leakage was not assessed.
Infratentorial surgeries
A total of 47 studies reported surgical and postoperative data in infratentorial surgeries [3, 4, 10, 12, 15, 20, 21, 25, 27, 33, 45,46,47, 49, 54, 62, 64, 65, 67, 71, 73, 75, 77, 79, 81, 89, 103, 105, 107, 116, 119, 120] (Table 6). Of 28,078 patients, 1625 were diagnosed with postoperative CSF leakage, which accounts for 5.8%. The most common indications here were microvascular decompression (MVD) and acoustic neuroma resection. Not all studies specified the surgical approach. CSF shunting with a VP shunt or lumbar drainage was performed in 30 patients.
Retrosigmoid approach
The retrosigmoid approach was the most frequently described approach to the posterior fossa. In total, 18,607 patients were included in 20 studies, with a total leak rate of 7.4% (1382 out of 18,607 patients). The largest study [3] describing complications of acoustic neuroma resections discussed 6820 included patients with CSF leakage in 14% of patients. Hospital readmission was required for 3.5% of patients. The lowest leakage rate (0.2%) was reported in a recent series on MVD for hemifacial spasm [49].
Evolution of postoperative CSF leakage over time
In order to establish the evolution of postoperative CSF leakage, studies published during the last 2 years (2019–2020) were compared to studies published before 2005 (Table 7). Before 2005, the reported incidences were systematically higher than those reported since 2019. All publications (before 2005, as well as more recent ones) mention the highest incidence of postoperative CSF leakage after anterior skull base procedures. This incidence was also high for transpetrosal surgeries, where 7.9% of patients had symptoms of CSF leakage before 2005. This approach was not documented in articles published after 2018. The endoscopic approach for transsphenoidal surgeries was not reported before 2005. However, the incidence of postoperative CSF leakage is lower in recent studies for both the microscopic and endoscopic approach compared to older studies, partially due to the introduction of advanced preventive surgical tools and techniques, e.g., the vascularized nasoseptal flap.
Discussion
Cerebrospinal fluid leakage represents a major complication after elective neurosurgical procedures. Specific risk factors for postoperative CSF leakage include recurrent surgery, the condition of the dura, the duration of the surgery, the size of the dural defect, or comorbidities such as arterial hypertension or obesity. Common complications associated with CSF leakage include surgical site infection, meningitis, and hydrocephalus, the latter being important because of the risk for persistent CSF leakage due to a pressure gradient. As CSF leakage can be defined in several ways, there are no accurate numbers on its incidence. This review summarizes the reported incidences of postoperative CSF leakage, by surgical approach.
Overall, CSF leakage was present in 3.8% of cranial surgeries and the incidence of postoperative CSF leakage is higher in infratentorial surgery, namely in 5.8%, compared to 2.9% in supratentorial surgery. Open skull base surgery generally led to the highest complication rates, with CSF leakage in 6.2% of cases (5.9% for anterior skull base, 6.4% for middle fossa, and 5.2% for transpetrosal approaches). Endonasal skull base surgery resulted in an average postoperative CSF leakage rate of 2.8%, with rates of 4.1% for endoscopic procedures, 1.7% for microscopic procedures, and 5.3% for extended procedures. The main indications for supratentorial surgery were tumors. Resection of intracerebral tumors often require large dural openings, which can be associated with a higher risk of reconstruction failure with associated CSF leakage, compared to more limited defects. In meningiomas (more particularly in Simson grade I resections), part of the dura mater is resected and replaced by grafts, which can also lead to CSF leakage. The use of a graft is often as well advocated after long-lasting surgeries: exposure of the dura to air and illumination may dry out the dural edges, which can result in shrinkage. One of the highest incidences of CSF leakage after supratentorial procedures (6.3%) was reported in a study to evaluate the costs associated with postoperative CSF leakage [37]. This high incidence could result from the fact that all events associated with postoperative CSF leakage were reported including spontaneously resolving minor subcutaneous CSF collections, asymptomatic CSF collections observed on postoperative imaging, pseudomeningoceles, subcutaneous collections with a need for puncture, or overt surgical site leakage as well as otorrhea and rhinorrhea. Infratentorial and skull base surgery were associated with higher rates of CSF leakage. The infratentorial approach was found to represent a univariate predictor for CSF leakage (p = 0.015) [108]. This was explained by increased stress on the suture line because of high hydrostatic pressure associated with posterior fossa lesions. Due to the challenging accessibility of the region, skull base surgery resulted in the highest incidence of postoperative CSF leakage. The open approach to the skull base, including anterior fossa and transpetrosal surgeries has high complication rates in general. Endoscopic approaches to the skull base are less invasive and could result in lower complication rates; however, controversy exists between the endoscopic and microscopic approach. Regarding efficacy, for sellar lesions, the endoscopic approach is currently preferred according to a growing amount of evidence, for example, a higher rate of gross total resection in case of pituitary tumors and lower rates of complications [22]. On the other hand, CSF leakage was more common after endoscopic surgery. Additionally, Broersen et al. [16] reviewed the literature on microscopic versus endoscopic transsphenoidal surgery for Cushing’s disease and also found a higher incidence of CSF leakage after endoscopic surgery (12.9%) compared to microscopic surgery (4.0%). According to the authors, this might be explained by the more challenging nature of the cases and the attempt to achieve complete tumor resection. Dural repair after extended endoscopic endonasal skull base procedures remains most challenging and suprasellar extension of the lesion can cause arachnoidal defects with a high chance of intraoperative CSF leak. Intraoperative CSF leakage was found to be a risk factor for postoperative CSF leakage. Shahangian et al. [95] reported an overall CSF leak repair failure in 5.3% of cases. In case of an intraoperative leak, the leak repair failure rate increased to 19.9%. Therefore, the reconstruction of intraoperative leaks needs to be performed adequately, for instance by application of grafts such as fascia lata, the Hadad nasoseptal flap [39], or other closure techniques, e.g., in combination with fibrin glues. The efficacy of the Hadad nasoseptal flap for intraoperative leak reconstruction or as a preventive measure was demonstrated, with decrease in leakage rates from 11 to 2.7% [78]. Additionally, in case of intraoperative CSF leakage, other preventive measures could be taken to minimize the risk for postoperative leakage, including lumbar drain placement or compulsory bed rest. Finally, surgical experience can furthermore influence postoperative CSF leakage. In a survey study among 3172 neurosurgeons across the USA, the CSF leak rate was estimated to be 3.9% [19].
Higher age is often considered a risk factor for surgical and postsurgical complications; in the geriatric population, the dura is prone to tears and adherent to the bone [121]. However, studies comparing elderly versus younger patients are at present limited to underpowered retrospective studies. Phan et al. [82] conducted a systematic review comparing outcomes of microvascular decompression in elderly and younger patients. The results suggested that some complications may be significantly higher in the elderly (such as stroke or death); however, no difference was found in the incidence of CSF leakage between both groups (14 out of 439 elderly cases, i.e., 3.2% versus 28 out of 897 (3.1%) in the younger population).
Dural reinforcement
As primary dural closure is not always sufficient to provide adequate sealing, dural reinforcement is often performed. This can be accomplished with autologous material such as pericranium, fat or muscle grafts, or fascia lata. Commercially available fibrin sealants can also be used for dural reinforcement. While numerous studies assessed the use of augmentation material for dural closure, no clear advantage of these sealants could be found. A systematic review on this topic reported no significant advantage of fibrin sealants, with a postoperative CSF leakage rate of 8.2% when no sealant was used, compared to 8.4% in the sealant group. [57]
Limitations of the study
For this review, we assessed the CSF leakage rate taking into account the type of surgery and approach. A major limitation is that few prospective studies exist on this topic which leads to important gaps and missing data. Most importantly, details on the surgical approach and indication were not always provided. Additionally, most manuscripts did not mention a general description or definition of outcome. Most studies reported postoperative CSF leaks only if reoperation, CSF shunting, or readmission were necessary. Subcutaneous fluid collections, imaging suggestive of CSF leak, or other nonclinically relevant CSF leaks requiring interventions, e.g., bed rest, pressure dressing, or puncture, were rarely considered even though these might alter the duration of hospital admission, patient quality of life, and healthcare costs.
In order to diminish the risk of bias due to large discrepancies in CSF leakage rates described by small studies, we decided to only include studies if at least 100 cases were reported. In case several approaches or surgical techniques were described in the same study, a minimum of 100 patients per group were required for inclusion. It is crucial to emphasize the importance of the learning curve because results and adverse events in surgery are influenced by the surgeon’s experience. It has been reported that the experience of both the surgeon and the institution play a role in the incidence of complications, including CSF leak. However, more experienced surgeons may be involved in more challenging cases, which can have opposite effects [16, 19, 53, 69].
Another limitation is the role of the use of commercial sealants or dural substitutes for obtaining watertight dural closure. In order to minimize bias, we did not include commercial studies aiming to show efficacy of a specific commercial product. However, some of the included studies did use commercially available sealants as part of their standard practice. The use of commercial sealants was already reviewed by Esposito et al. in 2016 [28], who concluded that there was no decrease in incidence of postoperative CSF leakage when commercial fibrin sealants were used. However, these findings were based on only one randomized controlled trial in 139 subjects [36]. Such findings emphasize the importance of further research regarding the safety and efficacy of commercial fibrin sealants, and optimal techniques for dura closure in a more extensive way.
Conclusion
Since postoperative CSF leakage remains a widely reported complication of elective neurosurgical procedures, there is a need for a standardized procedure for meticulous dural closure in order to reduce the incidence of this serious adverse event, especially when taking into account the many secondary complications and their additional costs. These include local infection and meningitis, and all associated risks related to hospital readmission, reoperations or other clinically invasive procedures. Powered large-numbered randomized controlled trials are necessary to determine the optimal treatment for dural closure, minimizing the risk of postoperative complications, and CSF leakage in particular.
Change history
06 May 2022
A Correction to this paper has been published: https://doi.org/10.1007/s10143-022-01797-1
References
Abbassioun K, Amirjamshidi M, Mehrazin A, Khalatbary I, Keynama M, Bokai H, Abdollahi M (2006) A prospective analysis of 151 cases of patients with acromegaly operated by one neurosurgeon: a follow-up of more than 23 years. Surg Neurol 66:26–31; discussion 31. https://doi.org/10.1016/j.surneu.2005.11.063
Agam MS, Wedemeyer MA, Wrobel B, Weiss MH, Carmichael JD, Zada G (2018) Complications associated with microscopic and endoscopic transsphenoidal pituitary surgery: experience of 1153 consecutive cases treated at a single tertiary care pituitary center. J Neurosurg 1–8. https://doi.org/10.3171/2017.12.JNS172318
Alattar AA, Hirshman BR, McCutcheon BA, Chen CC, Alexander T, Harris J, Carter BS (2018) Risk factors for readmission with cerebrospinal fluid leakage within 30 days of vestibular schwannoma surgery. Neurosurgery 82:630–637. https://doi.org/10.1093/neuros/nyx197
Alford EN, Chagoya G, Elsayed GA, Bernstock JD, Bentley JN, Romeo A, Guthrie B (2020) Risk factors for wound-related complications after microvascular decompression. Neurosurg Rev. https://doi.org/10.1007/s10143-020-01296-1
Alwadei A, Almubarak AO, Bafaquh M, Qoqandi O, Alobaid A, Alsubaie F, Alzahrani AS, Alyahya NM, Almalki S, Alyamani M, Orz Y (2019) Supratentorial craniotomies with or without dural closure-a comparison. WORLD Neurosurg 125:E1132–E1137. https://doi.org/10.1016/j.wneu.2019.01.262
Ben Ammar M, Piccirillo E, Topsakal V, Taibah A, Sanna M (2012) Surgical results and technical refinements in translabyrinthine excision of vestibular schwannomas: the Gruppo Otologico experience. Neurosurgery 70:1481–1491. https://doi.org/10.1227/NEU.0b013e31824c010f
Asemota AO, Ishii M, Brem H, Gallia GL (2017) Comparison of complications, trends, and costs in endoscopic vs microscopic pituitary surgery: analysis from a US health claims database. Clin Neurosurg 81:458–471. https://doi.org/10.1093/neuros/nyx350
Azad TD, Lee Y-J, Vail D, Veeravagu A, Hwang PH, Ratliff JK, Li G (2017) Endoscopic vs. microscopic resection of sellar lesions-a matched analysis of clinical and socioeconomic outcomes. Front Surg 4:33. https://doi.org/10.3389/fsurg.2017.00033
Barth M, Tuettenberg J, Thomé C, Weiss C, Vajkoczy P, Schmiedek P (2008) Watertight dural closure: is it necessary? A prospective randomized trial in patients with supratentorial craniotomies. Neurosurgery 63:352–358. https://doi.org/10.1227/01.NEU.0000310696.52302.99
Becker SS, Jackler RK, Pitts LH (2003) Cerebrospinal fluid leak after acoustic neuroma surgery: a comparison of the translabyrinthine, middle fossa, and retrosigmoid approaches. Otol Neurotol Off Publ Am Otol Soc Am Neurotol Soc [and] Eur Acad Otol Neurotol 24:107–112. https://doi.org/10.1097/00129492-200301000-00021
Berker M, Hazer DB, Yücel T, Gürlek A, Cila AAA, Aldur M, Onerci M, Yucel T, Gurlek A, Cila AAA, Aldur M, Onerci M, Yücel T, Gürlek A, Cila AAA, Aldur M, Onerci M (2012) Complications of endoscopic surgery of the pituitary adenomas: analysis of 570 patients and review of the literature. Pituitary 15:288–300. https://doi.org/10.1007/s11102-011-0368-2
Bhimani AD, Esfahani DR, Denyer S, Chiu RG, Rosenberg D, Barks AL, Arnone GD, Mehta AI, Mehta IA, Mehta AI, Mehta IA (2018) Adult Chiari I malformations: an analysis of surgical risk factors and complications using an international database. World Neurosurg 115:e490–e500. https://doi.org/10.1016/j.wneu.2018.04.077
Bi X, Liu B, Mao Z, Wang C, Dunne N, Fan Y, Li X (2020) Applications of materials for dural reconstruction in pre-clinical and clinical studies: advantages and drawbacks, efficacy, and selections. Mater Sci & Eng C-MATERIALS Biol Appl 117:111326. https://doi.org/10.1016/j.msec.2020.111326
Boling CC, Karnezis TT, Baker AB, Lawrence LA, Soler ZM, Vandergrift WA 3rd, Wise SK, DelGaudio JM, Patel ZM, Rereddy SK, Lee JM, Khan MN, Govindaraj S, Chan C, Oue S, Psaltis AJ, Wormald P-JJ, Trosman S, Stokken J, Woodard T, Sindwani R, Schlosser RJ (2016) Multi-institutional study of risk factors for perioperative morbidity following transnasal endoscopic pituitary adenoma surgery. Int Forum Allergy Rhinol 6:101–107. https://doi.org/10.1002/alr.21622
Breun M, Nickl R, Perez J, Hagen R, Löhr M, Vince G, Trautner H, Ernestus R-I, Matthies C (2019) Vestibular schwannoma resection in a consecutive series of 502 cases via the retrosigmoid approach: technical aspects, complications, and functional outcome. World Neurosurg 129:e114–e127. https://doi.org/10.1016/j.wneu.2019.05.056
Broersen LHA, Biermasz NR, van Furth WR, de Vries F, Verstegen MJT, Dekkers OM, Pereira AM (2018) Endoscopic vs. microscopic transsphenoidal surgery for Cushing’s disease: a systematic review and meta-analysis. Pituitary 21:524–534. https://doi.org/10.1007/s11102-018-0893-3
Cavallo LM, Frank G, Cappabianca P, Solari D, Mazzatenta D, Villa A, Zoli M, D’Enza AI, Esposito F, Pasquini E (2014) The endoscopic endonasal approach for the management of craniopharyngiomas: a series of 103 patients. J Neurosurg 121:100–113. https://doi.org/10.3171/2014.3.JNS131521
Cheng Y, Xue F, Wang T-Y, Ji J-F, Chen W, Wang Z-Y, Xu L, Hang C-H, Liu X-F (2017) Analyses and treatments of postoperative nasal complications after endonasal transsphenoidal resection of pituitary neoplasms. Medicine (Baltimore) 96:e6614. https://doi.org/10.1097/MD.0000000000006614
Ciric I, Ragin A, Baumgartner C, Pierce D (1997) Complications of transsphenoidal surgery: results of a national survey, review of the literature, and personal experience. Neurosurgery 40:225–227. https://doi.org/10.1097/00006123-199702000-00001
Copeland WR, Mallory GW, Neff BA, Driscoll CLW, Link MJ (2015) Are there modifiable risk factors to prevent a cerebrospinal fluid leak following vestibular schwannoma surgery? J Neurosurg 122:312–316. https://doi.org/10.3171/2014.10.JNS14432
Cote DJ, Dasenbrock HH, Gormley WB, Smith TR, Dunn IF (2019) Adverse events after microvascular decompression: a national surgical quality improvement program analysis. WORLD Neurosurg 128:E884–E894. https://doi.org/10.1016/j.wneu.2019.05.022
Dai W, Zhuang Z, Ling H, Yang Y, Hang C (2020) Systematic review and network meta-analysis assess the comparative efficacy and safety of transsphenoidal surgery for pituitary tumor. Neurosurg Rev. https://doi.org/10.1007/s10143-020-01240-3
Dasenbrock HH, Yan SC, Chavakula V, Gormley WB, Smith TR, Claus EB, Dunn IF (2017) Unplanned reoperation after craniotomy for tumor: a national surgical quality improvement program analysis. Neurosurgery 81:761–771. https://doi.org/10.1093/neuros/nyx089
Dehdashti AR, Ganna A, Karabatsou K, Gentili F (2008) Pure endoscopic endonasal approach for pituitary adenomas: early surgical results in 200 patients and comparison with previous microsurgical series. Neurosurgery 62:1006–1017. https://doi.org/10.1227/01.NEU.0000297072.75304.89
Dubey A, Sung W-S, Shaya M, Patwardhan R, Willis B, Smith D, Nanda A (2009) Complications of posterior cranial fossa surgery-an institutional experience of 500 patients. Surg Neurol 72:369–375. https://doi.org/10.1016/j.surneu.2009.04.001
Duntze J, Litré CF, Graillon T, Maduri R, Pech-gourg G, Rakotozanany P, Gras R, Dufour H (2012) Rhinorrhée cérébrospinale après chirurgie hypophysaire endoscopique trans-sphénoïdale: Réflexions après 337 patients. Neurochirurgie 58:241–245. https://doi.org/10.1016/j.neuchi.2012.02.005
Duong DH, O’Malley S, Sekhar LN, Wright DG (2000) Postoperative hydrocephalus in cranial base surgery. Skull Base Surg 10:197–200. https://doi.org/10.1055/s-2000-9331
Esposito F, Angileri FF, Kruse P, Cavallo LM, Solari D, Esposito V, Tomasello F, Cappabianca P (2016) Fibrin sealants in dura sealing: a systematic literature review. PLoS ONE 11:e0151533. https://doi.org/10.1371/journal.pone.0151533
Fatemi N, Dusick JR, de Paiva Neto MA, Kelly DF (2008) The endonasal microscopic approach for pituitary adenomas and other parasellar tumors: a 10-year experience. Neurosurgery 63:244–56; discussion 256. https://doi.org/10.1227/01.NEU.0000327025.03975.BA
Fishman AJ, Marrinan MS, Golfinos JG, Cohen NL, Roland JTJ (2004) Prevention and management of cerebrospinal fluid leak following vestibular schwannoma surgery. Laryngoscope 114:501–505. https://doi.org/10.1097/00005537-200403000-00022
Fomichev D, Kalinin P, Kutin M, Sharipov O (2016) Extended transsphenoidal endoscopic endonasal surgery of suprasellar craniopharyngiomas. World Neurosurg 94:181–187. https://doi.org/10.1016/j.wneu.2016.06.124
Giovanni S, Della Pepa GM, La Rocca G, Lofrese G, Albanese A, Maria G, Marchese E (2014) Galea-pericranium dural closure: can we safely avoid sealants? Clin Neurol Neurosurg 123:50–54. https://doi.org/10.1016/j.clineuro.2014.05.005
Go K-O, Hwang K, Han JH (2020) Surgical nuances to reduce and manage cerebrospinal fluid leaks after microvascular decompression. J Clin Med 9. https://doi.org/10.3390/jcm9040902
Gondim JA, Almeida JPC, Albuquerque LAF, Schops M, Gomes E, Ferraz T, Sobreira W, Kretzmann MT (2011) Endoscopic endonasal approach for pituitary adenoma: surgical complications in 301 patients. Pituitary 14:174–183. https://doi.org/10.1007/s11102-010-0280-1
Gooneratne IK, Mannan S, de Tisi J, Gonzalez JC, McEvoy AW, Miserocchi A, Diehl B, Wehner T, Bell GS, Sander JW, Duncan JS (2017) Somatic complications of epilepsy surgery over 25 years at a single center. EPILEPSY Res 132:70–77. https://doi.org/10.1016/j.eplepsyres.2017.02.016
Green AL, Arnaud A, Batiller J, Eljamel S, Gauld J, Jones P, Martin D, Mehdorn M, Ohman J, Weyns F (2015) A multicentre, prospective, randomized, controlled study to evaluate the use of a fibrin sealant as an adjunct to sutured dural repair. Br J Neurosurg 29:11–17. https://doi.org/10.3109/02688697.2014.948808
Grotenhuis JA (2005) Costs of postoperative cerebrospinal fluid leakage: 1-year, retrospective analysis of 412 consecutive nontrauma cases. Surg Neurol 64:490–493. https://doi.org/10.1016/j.surneu.2005.03.041
Guangming Z, Huancong Z, Wenjing Z, Guoqiang C, Xiaosong W (2009) Should epidural drain be recommended after supratentorial craniotomy for epileptic patients? Surg Neurol 72:138–41; discussion 141. https://doi.org/10.1016/j.surneu.2008.06.014
Hadad G, Bassagasteguy L, Carrau RL, Mataza JC, Kassam A, Snyderman CH, Mintz A (2006) A novel reconstructive technique after endoscopic expanded endonasal approaches: vascular pedicle nasoseptal flap. Laryngoscope 116:1882–1886. https://doi.org/10.1097/01.mlg.0000234933.37779.e4
Halvorsen H, Ramm-Pettersen J, Josefsen R, Rønning P, Reinlie S, Meling T, Berg-Johnsen J, Bollerslev J, Helseth E (2014) Surgical complications after transsphenoidal microscopic and endoscopic surgery for pituitary adenoma: a consecutive series of 506 procedures. Acta Neurochir (Wien) 156:441–449. https://doi.org/10.1007/s00701-013-1959-7
Hamou HA, Kotliar K, Tan SK, Weiss C, Christian B, Clusmann H, Schubert GA, Albanna W, Weiß C, Christian B, Clusmann H, Schubert GA, Albanna W (2020) Surgical nuances and placement of subgaleal drains for supratentorial procedures: a prospective analysis of efficacy and outcome in 150 craniotomies. Acta Neurochir (Wien) 162:729–736. https://doi.org/10.1007/s00701-019-04196-6
Han Z-L, He D-S, Mao Z-G, Wang H-J (2008) Cerebrospinal fluid rhinorrhea following trans-sphenoidal pituitary macroadenoma surgery: experience-from 592 patients. Clin Neurol Neurosurg 110:570–579. https://doi.org/10.1016/j.clineuro.2008.02.017
Hannan CJ, Almhanedi H, Al-Mahfoudh R, Bhojak M, Looby S, Javadpour M (2020) Predicting post-operative cerebrospinal fluid (CSF) leak following endoscopic transnasal pituitary and anterior skull base surgery: a multivariate analysis. Acta Neurochir (Wien) 162:1309–1315. https://doi.org/10.1007/s00701-020-04334-5
Horowitz G, Fliss DM, Margalit N, Wasserzug O, Gil Z (2011) Association between cerebrospinal fluid leak and meningitis after skull base surgery. Otolaryngol NECK Surg 145:689–693. https://doi.org/10.1177/0194599811411534
Hyun S-J, Kong D-S, Park K (2010) Microvascular decompression for treating hemifacial spasm: lessons learned from a prospective study of 1,174 operations. Neurosurg Rev 33:325–334. https://doi.org/10.1007/s10143-010-0254-9
Jagannath PM, Venkataramana NK, Bansal A, Ravichandra M (2012) Outcome of microvascular decompression for trigeminal neuralgia using autologous muscle graft: a five-year prospective study. Asian J Neurosurg 7:125–130. https://doi.org/10.4103/1793-5482.103713
Jain VK, Mehrotra N, Sahu RN, Behari S, Banerji D, Chhabra DK (2005) Surgery of vestibular schwannomas: an institutional experience. Neurol India 53:41–45. https://doi.org/10.4103/0028-3886.15052
Jang JH, Kim KH, Lee YM, Kim JS, Kim YZ (2016) Surgical results of pure endoscopic endonasal transsphenoidal surgery for 331 pituitary adenomas: a 15-year experience from a single institution. World Neurosurg 96:545–555. https://doi.org/10.1016/j.wneu.2016.09.051
Jiang C, Liang W, Wang J, Dai Y, Jin W, Sun X, Xu W (2020) Microvascular decompression for hemifacial spasm associated with distinct offending vessels: a retrospective clinical study. Clin Neurol Neurosurg 194:105876. https://doi.org/10.1016/j.clineuro.2020.105876
Kanzaki J, Ogawa K, Tsuchihashi N, Inoue Y, Yamamoto M, Ikeda S (1991) Postoperative complications in acoustic neuroma surgery by the extended middle cranial fossa approach. Acta Otolaryngol Suppl 487:75–79. https://doi.org/10.3109/00016489109130449
Kanzaki J, Ogawa K, Tsuchihashi N, Inoue Y, Yamamoto M, Ikeda S (1991) Postoperative complications in acoustic neuroma surgery by the extended middle cranial fossa approach. Acta Otolaryngol 111:75–79. https://doi.org/10.3109/00016489109130449
Karnezis TT, Baker AB, Soler ZM, Wise SK, Rereddy SK, Patel ZM, Oyesiku NM, DelGaudio JM, Hadjipanayis CG, Woodworth BA, Riley KO, Lee J, Cusimano MD, Govindaraj S, Psaltis A, Wormald PJ, Santoreneos S, Sindwani R, Trosman S, Stokken JK, Woodard TD, Recinos PF, Vandergrift WA 3rd, Schlosser RJ, Vandergrift III WA, Schlosser RJ (2016) Factors impacting cerebrospinal fluid leak rates in endoscopic sellar surgery. Int Forum Allergy \& Rhinol 6:1117–1125. https://doi.org/10.1002/alr.21783
Kassam AB, Preveoello DM, Carrau RL, Snyderman CH, Thomas A, Gardner P, Zanation A, Duz B, Stefko ST, Byers K, Horowitz MB, Prevedello DM, Carrau RL, Snyderman CH, Thomas A, Gardner P, Zanation A, Duz B, Stefko ST, Byers K, Horowitz MB, Preveoello DM, Carrau RL, Snyderman CH, Thomas A, Gardner P, Zanation A, Duz B, Stefko ST, Byers K, Horowitz MB (2011) Endoscopic endonasal skull base surgery: analysis of complications in the authors’ initial 800 patients: a review. J Neurosurg 114:1544–1568. https://doi.org/10.3171/2010.10.JNS09406
Kher Y, Yadav N, Yadav YR, Parihar V, Ratre S, Bajaj J (2017) Endoscopic vascular decompression in trigeminal neuralgia. Turk Neurosurg 27:998–1006. https://doi.org/10.5137/1019-5149.JTN.17046-16.1
Khrais TH, Falcioni M, Taibah A, Agarwal M, Sanna M (2004) Cerebrospinal fluid leak prevention after translabyrinthine removal of vestibular schwannoma. Laryngoscope 114:1015–1020. https://doi.org/10.1097/00005537-200406000-00011
Kim EH, Ku CR, Lee EJ, Kim SH (2015) Extracapsular en bloc resection in pituitary adenoma surgery. Pituitary 18:397–404. https://doi.org/10.1007/s11102-014-0587-4
Kinaci A, Algra A, Heuts S, O’Donnell D, van der Zwan A, van Doormaal T (2018) Effectiveness of dural sealants in prevention of cerebrospinal fluid leakage after craniotomy: a systematic review. World Neurosurg 118:368-376.e1. https://doi.org/10.1016/j.wneu.2018.06.196
Korinek A-M, Golmard J-L, Elcheick A, Bismuth R, van Effenterre R, Coriat P, Puybasset L (2005) Risk factors for neurosurgical site infections after craniotomy: a critical reappraisal of antibiotic prophylaxis on 4,578 patients. Br J Neurosurg 19:155–162. https://doi.org/10.1080/02688690500145639
Korinek A-MM, Baugnon T, Golmard J-LL, van Effenterre R, Coriat P, Puybasset L (2006) Risk factors for adult nosocomial meningitis after craniotomy: role of antibiotic prophylaxis. Neurosurgery 59:126–133. https://doi.org/10.1227/01.NEU.0000220477.47323.92
Koutourousiou M, Vaz Guimaraes Filho F, Fernandez-Miranda JC, Wang EW, Stefko ST, Snyderman CH, Gardner PA (2017) Endoscopic endonasal surgery for tumors of the cavernous sinus: a series of 234 patients. World Neurosurg 103:713–732. https://doi.org/10.1016/j.wneu.2017.04.096
Laedrach K, Lukes A, Raveh J (2007) Reconstruction of skull base and fronto-orbital defects following tumor resection. SKULL BASE-AN Interdiscip APPROACH 17:59–72. https://doi.org/10.1055/s-2006-959336
Lee MH, Jee TK, Lee JA, Park K (2016) Postoperative complications of microvascular decompression for hemifacial spasm: lessons from experience of 2040 cases. Neurosurg Rev 39:151–8; discussion 158. https://doi.org/10.1007/s10143-015-0666-7
Leonetti J, Anderson D, Marzo S, Moynihan G (2001) Cerebrospinal fluid fistula after transtemporal skull base surgery. Otolaryngol neck Surg Off J Am Acad Otolaryngol Neck Surg 124:511–514. https://doi.org/10.1067/mhn.2001.115089
Leonetti JP, Anderson D, Marzo S, Moynihan G (2001) Prevention and management of cerebrospinal fluid fistula after transtemporal skull base surgery. Skull Base 11:87–92. https://doi.org/10.1055/s-2001-14428
Li D, Wang H, Fan ZZ, Fan ZZ (2010) Complications in retrosigmoid cranial nerve surgery. Acta Otolaryngol 130:247–252. https://doi.org/10.3109/00016480903092340
Little AS, Kelly DF, White WL, Gardner PA, Fernandez-Miranda JC, Chicoine MR, Barkhoudarian G, Chandler JP, Prevedello DM, Liebelt BD, Sfondouris J, Mayberg MR (2020) Results of a prospective multicenter controlled study comparing surgical outcomes of microscopic versus fully endoscopic transsphenoidal surgery for nonfunctioning pituitary adenomas: the Transsphenoidal Extent of Resection (TRANSSPHER) Study. J Neurosurg 132:1043–1053. https://doi.org/10.3171/2018.11.JNS181238
Litvack ZN, West GA, Delashaw JB, Burchiel KJ, Anderson VC (2009) Dural augmentation: part I-evaluation of collagen matrix allografts for dural defect after craniotomy. Neurosurgery 65:890–897. https://doi.org/10.1227/01.NEU.0000356970.22315.BC
Lobatto DJ, de Vries F, Zamanipoor Najafabadi AH, Pereira AM, Peul WC, Vliet Vlieland TPMM, Biermasz NR, van Furth WR (2018) Preoperative risk factors for postoperative complications in endoscopic pituitary surgery: a systematic review. Pituitary 21:84–97. https://doi.org/10.1007/s11102-017-0839-1
Magro E, Graillon T, Lassave J, Castinetti F, Boissonneau S, Tabouret E, Fuentes S, Velly L, Gras R, Dufour H (2016) Complications related to the endoscopic endonasal transsphenoidal approach for nonfunctioning pituitary macroadenomas in 300 consecutive patients. World Neurosurg 89:442–453. https://doi.org/10.1016/j.wneu.2016.02.059
Mascarenhas L, Moshel YA, Bayad F, Szentirmai O, Salek AA, Leng LZ, Hofstetter CP, Placantonakis DG, Tsiouris AJ, Anand VK, Schwartz TH (2014) The transplanum transtuberculum approaches for suprasellar and sellar-suprasellar lesions: avoidance of cerebrospinal fluid leak and lessons learned. World Neurosurg 82:186–195. https://doi.org/10.1016/j.wneu.2013.02.032
McLaughlin MR, Jannetta PJ, Clyde BL, Subach BR, Comey CH, Resnick DK (1999) Microvascular decompression of cranial nerves: lessons learned after 4400 operations. J Neurosurg 90:1–8. https://doi.org/10.3171/jns.1999.90.1.0001
Meyer TA, Canty PA, Wilkinson EP, Hansen MR, Rubinstein JT, Gantz BJ (2006) Small acoustic neuromas: surgical outcomes versus observation or radiation. Otol Neurotol Off Publ Am Otol Soc Am Neurotol Soc [and] Eur Acad Otol Neurotol 27:380–392. https://doi.org/10.1097/00129492-200604000-00015
Miyazaki H, Deveze A, Magnan J (2005) Neuro-otologic surgery through minimally invasive retrosigmoid approach: endoscope assisted microvascular decompression, vestibular neurotomy, and tumor removal. Laryngoscope 115:1612–1617. https://doi.org/10.1097/01.mlg.0000172038.22929.63
Moffat DA, Lloyd SKWW, Macfarlane R, Mannion R, King A, Rutherford S, Axon PR, Donnelly N, Freeman S, Tysome JR, Evans DG, Ramsden RT (2013) Outcome of translabyrinthine surgery for vestibular schwannoma in neurofibromatosis type 2. Br J Neurosurg 27:446–453. https://doi.org/10.3109/02688697.2013.771143
Mostafa BE, El Sharnoubi M, Youssef AM (2008) The keyhole retrosigmoid approach to the cerebello-pontine angle: indications, technical modifications, and results. Skull Base 18:371–376. https://doi.org/10.1055/s-0028-1087220
Nishioka H, Haraoka J, Ikeda Y (2005) Risk factors of cerebrospinal fluid rhinorrhea following transsphenoidal surgery. Acta Neurochir (Wien) 147:1163–1166. https://doi.org/10.1007/s00701-005-0586-3
Nonaka Y, Fukushima T, Watanabe K, Friedman AH, Sampson JH, McElveen JTJ, Cunningham CD 3rd, Zomorodi AR (2013) Contemporary surgical management of vestibular schwannomas: analysis of complications and lessons learned over the past decade. Neurosurgery 72:ons103–15; discussion ons115. https://doi.org/10.1227/NEU.0b013e3182752b05
Paluzzi A, Fernandez-Miranda JC, Tonya Stefko S, Challinor S, Snyderman CH, Gardner PA (2014) Endoscopic endonasal approach for pituitary adenomas: a series of 555 patients. Pituitary 17:307–319. https://doi.org/10.1007/s11102-013-0502-4
Park JS, Kong D-S, Lee J-A, Park K (2007) Intraoperative management to prevent cerebrospinal fluid leakage after microvascular decompression: dural closure with a “plugging muscle” method. Neurosurg Rev 30:139–42; discussion 142. https://doi.org/10.1007/s10143-006-0060-6
Patel PN, Stafford AM, Patrinely JR, Smith DK, Turner JH, Russell PT, Weaver KD, Chambless LB, Chandra RK (2018) Risk factors for intraoperative and postoperative cerebrospinal fluid leaks in endoscopic transsphenoidal sellar surgery. Otolaryngol neck Surg Off J Am Acad Otolaryngol Neck Surg 158:952–960. https://doi.org/10.1177/0194599818756272
Perry A, Kerezoudis P, Graffeo CS, Carlstrom LP, Peris-Celda M, Meyer FB, Bydon M, Link MJ (2019) Little insights from big data: cerebrospinal fluid leak after skull base surgery and the limitations of database research. WORLD Neurosurg 127:E561–E569. https://doi.org/10.1016/j.wneu.2019.03.207
Phan K, Rao PJ, Dexter M (2016) Microvascular decompression for elderly patients with trigeminal neuralgia. J Clin Neurosci 29:7–14. https://doi.org/10.1016/j.jocn.2015.11.027
Raikundalia MD, Pines MJ, Svider PF, Baredes S, Folbe AJ, Liu JK, Eloy JA (2015) Characterization of transsphenoidal complications in patients with acromegaly: an analysis of inpatient data in the United States from 2002 to 2010. Int Forum Allergy Rhinol 5:417–422. https://doi.org/10.1002/alr.21498
Reddy M, Schöggl A, Reddy B, Saringer W, Weigel G, Matula C (2002) A clinical study of a fibrinogen-based collagen fleece for dural repair in neurosurgery. Acta Neurochir (Wien) 144:265–9; discussion 269. https://doi.org/10.1007/s007010200034
Riesgo P, Mariño P, Platero A, Tarazona FJ, Fajardo C, Llácer JL, Rovira V, Rodríguez R, Flor-Goikoetxea A, Piquer J (2019) Postoperative CSF leakages after transsphenoidal surgery for pituitary adenomas: analysis of a series of 302 surgical procedures. Neurocirugia (Astur) 30:215–221. https://doi.org/10.1016/j.neucir.2019.03.003
Sade B, Oya S, Lee JH (2011) Non-watertight dural reconstruction in meningioma surgery: results in 439 consecutive patients and a review of the literature: clinical article. J Neurosurg 114:714–718. https://doi.org/10.3171/2010.7.JNS10460
Sanai N, Sughrue ME, Shangari G, Chung K, Berger MS, McDermott MW (2010) Risk profile associated with convexity meningioma resection in the modern neurosurgical era: Clinical article. J Neurosurg 112:913–919. https://doi.org/10.3171/2009.6.JNS081490
Sanders-Taylor C, Anaizi A, Kosty J, Zimmer LA, Theodosopoulos PV (2015) Sellar reconstruction and rates of delayed cerebrospinal fluid leak after endoscopic pituitary surgery. J Neurol Surg PART B-SKULL BASE 76:281–285. https://doi.org/10.1055/s-0034-1544118
Sanna M, Taibah A, Russo A, Falcioni M, Agarwal M (2004) Perioperative complications in acoustic neuroma (vestibular schwannoma) surgery. Otol Neurotol Off Publ Am Otol Soc Am Neurotol Soc [and] Eur Acad Otol Neurotol 25:379–386. https://doi.org/10.1097/00129492-200405000-00029
Sathaporntheera P, Saetia K (2020) Risk factors associated with CSF leakage and complications after retrosigmoid surgery. Interdiscip Neurosurgery-Advanced Tech CASE Manag 22:100865. https://doi.org/10.1016/j.inat.2020.100865
Scheich M, Ginzkey C, Ehrmann-Müller D, Shehata-Dieler W, Hagen R (2016) Management of CSF leakage after microsurgery for vestibular schwannoma via the middle cranial fossa approach. Eur Arch Otorhinolaryngol 273:2975–2981. https://doi.org/10.1007/s00405-015-3891-3
Schneider M, Schuss P, Güresir Á, Wach J, Hamed M, Vatter H, Güresir E (2019) Cranial nerve outcomes after surgery for frontal skull base meningiomas: the eternal quest of the maximum-safe resection with the lowest morbidity. World Neurosurg 125:e790–e796. https://doi.org/10.1016/j.wneu.2019.01.171
Schwartz MS, Lekovic GP, Miller ME, Slattery WH, Wilkinson EP (2018) Translabyrinthine microsurgical resection of small vestibular schwannomas. J Neurosurg 129:128–136. https://doi.org/10.3171/2017.2.JNS162287
Senior BA, Ebert CS, Bednarski KK, Bassim MK, Younes M, Sigounas DDDDD, Ewend MG (2008) CANDIDATE’S THESIS Minimally invasive pituitary surgery. laryngoscope 118:1842–1855. https://doi.org/10.1097/MLG.0b013e31817e2c43
Shahangian A, Soler ZM, Baker A, Wise SK, Rereddy SK, Patel ZM, Oyesiku NM, DelGaudio JM, Hadjipanayis CG, Woodworth BA, Riley KO, Lee J, Cusimano MD, Govindaraj S, Khan MN, Psaltis A, Wormald PJ, Santoreneos S, Sindwani R, Trosman S, Stokken JK, Woodard TD, Recinos PF, Vandergrift WA 3rd, Boling C, Schlosser RJ (2017) Successful repair of intraoperative cerebrospinal fluid leaks improves outcomes in endoscopic skull base surgery. Int Forum Allergy Rhinol 7:80–86. https://doi.org/10.1002/alr.21845
Sharma M, Ambekar S, Sonig A, Nanda A (2013) Factors predicting the development of new onset post-operative hydrocephalus following trans-sphenoidal surgery for pituitary adenoma. Clin Neurol Neurosurg 115:1951–1954. https://doi.org/10.1016/j.clineuro.2013.05.020
Shiley SG, Limonadi F, Delashaw JB, Barnwell SL, Andersen PE, Hwang PH, Wax MK (2003) Incidence, etiology, and management of cerebrospinal fluid leaks following trans-sphenoidal surgery. Laryngoscope 113:1283–1288. https://doi.org/10.1097/00005537-200308000-00003
Shkarubo AN, Koval KV, Chernov IV, Andreev DN, Panteleyev AA (2019) Endoscopic endonasal transclival approach to tumors of the clivus and anterior region of the posterior cranial fossa (results of surgical treatment of 136 patients). World Neurosurg 121:e246–e261. https://doi.org/10.1016/j.wneu.2018.09.090
Sicking J, Voß KM, Spille DC, Schipmann S, Holling M, Paulus W, Hess K, Steinbicker AU, Stummer W, Grauer O, Wölfer J, Brokinkel B (2018) The evolution of cranial meningioma surgery-a single-center 25-year experience. Acta Neurochir (Wien) 160:1801–1812. https://doi.org/10.1007/s00701-018-3617-6
Slattery WH 3rd, Francis S, House KC (2001) Perioperative morbidity of acoustic neuroma surgery. Otol Neurotol 22:895–902. https://doi.org/10.1097/00129492-200111000-00031
Sluyter S, Graamans K, Tulleken CAF, Van Veelen CWM (2001) Analysis of the results obtained in 120 patients with large acoustic neuromas surgically treated via the translabyrinthine-transtentorial approach. J Neurosurg 94:61–66. https://doi.org/10.3171/jns.2001.94.1.0061
Solero CL, DiMeco F, Sampath P, Mattavelli F, Pizzi N, Salvatori P, Cantù G, Cantu G (2000) Combined anterior craniofacial resection for tumors involving the cribriform plate: early postoperative complications and technical considerations. Neurosurgery 47:1296–1304. https://doi.org/10.1097/00006123-200012000-00007
Stieglitz LH, Wrede KH, Gharabaghi A, Gerganov VM, Samii A, Samii M, Luedemann WO (2009) Factors affecting postoperative cerebrospinal fluid leaks after retrosigmoidal craniotomy for vestibular schwannomas Clinical article. J Neurosurg 111:874–883. https://doi.org/10.3171/2009.2.JNS081380
Strickland BA, Lucas J, Harris B, Kulubya E, Bakhsheshian J, Liu C, Wrobel B, Carmichael JD, Weiss M, Zada G (2018) Identification and repair of intraoperative cerebrospinal fluid leaks in endonasal transsphenoidal pituitary surgery: surgical experience in a series of 1002 patients. J Neurosurg 129:425–429. https://doi.org/10.3171/2017.4.JNS162451
Theodros D, Goodwin CRR, Bender MT, Zhou X, Garzon-Muvdi T, De La Garza-Ramos R, Abu-Bonsrah N, Mathios D, Blitz AMAM, Olivi A, Carson B, Bettegowda C, Lim M (2017) Efficacy of primary microvascular decompression versus subsequent microvascular decompression for trigeminal neuralgia. J Neurosurg 126:1691–1697. https://doi.org/10.3171/2016.5.JNS151692
Vengerovich G, Park KW, Antoury L, Wells C, Suh JD, Lee JT, Heaney AP, Bergsneider M, Wang MB (2020) Readmissions after endoscopic skull base surgery: associated risk factors and prevention. Int Forum Allergy Rhinol 10:110–113. https://doi.org/10.1002/alr.22453
De Vlieger J, Dejaegher J, Van Calenbergh F, De Vlieger J, Dejaegher J, Van Calenbergh F (2019) Posterior fossa decompression for Chiari malformation type I: clinical and radiological presentation, outcome and complications in a retrospective series of 105 procedures. Acta Neurol Belg 119:245–252. https://doi.org/10.1007/s13760-019-01086-7
Walcott BP, Neal JB, Sheth SA, Kahle KT, Eskandar EN, Coumans J-V, Nahed BV (2014) The incidence of complications in elective cranial neurosurgery associated with dural closure material. J Neurosurg 120:278–284. https://doi.org/10.3171/2013.8.JNS13703
Wang G, Sun L, Li W, Yu J (2018) Cerebrospinal fluid rhinorrhea in a bilateral frontal decompressive craniectomy patient caused by strenuous activity: a case report. Medicine (Baltimore) 97:e13189. https://doi.org/10.1097/MD.0000000000013189
Wang L, Wu Z, Tian K, Wang K, Li D, Ma J, Jia G, Zhang L, Zhang J (2017) Clinical features and surgical outcomes of patients with skull base chordoma: a retrospective analysis of 238 patients. J Neurosurg 127:1257–1267. https://doi.org/10.3171/2016.9.JNS16559
Woollons AC, Balakrishnan V, Hunn MK, Rajapaske YR (2000) Complications of trans-sphenoidal surgery: the Wellington experience. Aust N Z J Surg 70:405–408. https://doi.org/10.1046/j.1440-1622.2000.01843.x
Xue H, Wang X, Yang Z, Bi Z, Liu P (2020) Risk factors and outcomes of cerebrospinal fluid leak related to endoscopic pituitary adenoma surgery. Br J Neurosurg 34:447–452. https://doi.org/10.1080/02688697.2020.1754336
Yano S, Kawano T, Kudo M, Makino K, Nakamura H, Kai Y, Morioka M, Kuratsu JI (2009) Endoscopic endonasal transsphenoidal approach through the bilateral nostrils for pituitary adenomas. Neurol Med Chir (Tokyo) 49:1–6. https://doi.org/10.2176/nmc.49.1
Younus I, Gerges MM, Dobri GA, Ramakrishna R, Schwartz TH (2019) Readmission after endoscopic transsphenoidal pituitary surgery: analysis of 584 consecutive cases. J Neurosurg 1–6. https://doi.org/10.3171/2019.7.JNS191558
Younus I, Gerges MM, Uribe-Cardenas R, Morgenstern PF, Eljalby M, Tabaee A, Greenfield JP, Kacker A, Anand VK, Schwartz TH (2020) How long is the tail end of the learning curve? Results from 1000 consecutive endoscopic endonasal skull base cases following the initial 200 cases. J Neurosurg 1–11. https://doi.org/10.3171/2019.12.JNS192600
Zeng YJ, Zhang H, Yu S, Zhang W, Sun XC (2018) Efficacy and safety of microvascular decompression and gamma knife surgery treatments for patients with primary trigeminal neuralgia: a prospective study. World Neurosurg 116:e113–e117. https://doi.org/10.1016/j.wneu.2018.04.120
Zhang C, Ding X, Lu Y, Hu L, Hu G (2017) Cerebrospinal fluid rhinorrhoea following transsphenoidal surgery for pituitary adenoma: experience in a Chinese centre. ACTA Otorhinolaryngol Ital 37:303–307. https://doi.org/10.14639/0392-100X-1086
Zhao B, Wei Y-KK, Li G-LL, Li Y-NN, Yao Y, Kang J, Bin M-B, Yang Y, Wang R-ZZ (2010) Extended transsphenoidal approach for pituitary adenomas invading the anterior cranial base, cavernous sinus, and clivus: a single-center experience with 126 consecutive cases. J Neurosurg 112:108–117. https://doi.org/10.3171/2009.3.JNS0929
Zhao H, Zhang X, Tang Y, Zhang Y, Ying T-T, Zhu J, Li S-T (2017) Operative complications of microvascular decompression for hemifacial spasm: experience of 1548 cases. World Neurosurg 107:559–564. https://doi.org/10.1016/j.wneu.2017.08.028
Zuev AA, Pedyash NV, Epifanov DS, Kostenko GV (2016) Results of surgical treatment of syringomyelia associated with Chiari 1 malformation. An analysis of 125 cases. Zh Vopr Neirokhir Im N N Burdenko 80:27–34. https://doi.org/10.17116/neiro201680127-34
Zwirner J, Scholze M, Waddell JN, Ondruschka B, Hammer N (2019) Mechanical Properties of human dura mater in tension–an analysis at an age range of 2 to 94 years. Sci Rep 9:1–11. https://doi.org/10.1038/s41598-019-52836-9
Acknowledgements
This work was supported by a FWO-TBM project grant of the Flemish government, Belgium (T003018N).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: The authors regret that one of the author names in the original article appears incorrectly.
In addition, they also found another mistake that appeared in the original article:
Line 177-179: “While certain authors [44, 81, 92]reported CSF leakage as cases that required surgical revision or CSF shunting, others [102] [110] did not specify the treatment for CSF leakage.”
Was incorrectly shown in the final published article as :
“While certain authors [44, 81, 92] reported CSF leakage asincisional leak and fcases that required surgical revision or CSF shunting, others [102] [110] did not specify the treatment for CSF leakage.”
The original article has been corrected.
Appendix. Search strategy
Appendix. Search strategy
PUBMED
Concept 1: cranial surgery.
((“surgery” [Subheading] OR “Surgical Procedures, Operative”[Mesh:NoExp] OR surgery[tiab] OR surgeries[tiab] OR surgical[tiab] OR operati*[tiab] OR resect*[tiab]) AND (“Skull”[Mesh] OR skull[tiab] OR ‘head skeleton’[tiab] OR Skulls[tiab] OR crania[tiab] OR cranial[tiab] OR cranium[tiab] OR cranii[tiab] OR frontal-bone*[tiab] OR os-frontale [tiab] OR occipital-bone*[tiab] OR os-occipitale[tiab] OR parietal-bone*[tiab] OR os-parietale[tiab] OR temporal-bone*[tiab] OR os-temporale[tiab] OR sphenoid* [tiab] OR sella*[tiab])).
OR “Neurosurgical Procedures”[Mesh:NoExp] OR neurosurg*[tiab] OR skull-surg*[tiab] OR brain-surg*[tiab] OR Craniotomy[Mesh] OR craniotom*[tiab] OR Trephin*[tiab] OR Trepanation*[tiab] OR Trepanning*[tiab] OR “Decompression, Surgical”[Mesh:NoExp] OR decompressi*[tiab] OR decompression-surg*[tiab] OR decompressive-surg*[tiab] OR “Microvascular Decompression Surgery”[Mesh] OR “Dura Mater”[Mesh] OR Dura-Mater[tiab] OR duratom*[tiab] OR Dura-repair[tiab] OR Duraplast*[tiab].
Concept 2: postoperative complication.
‘wound healing’[tiab] OR ‘wound regeneration’[tiab] OR ‘Wound repair’[tiab] OR “Postoperative complications”[Mesh:NoExp] OR “Postoperative Period”[Mesh:NoExp] OR postop*[tiab] OR post-operat*[tiab] OR after-surgery[tiab] OR postsurgery[tiab] OR after-surgical[tiab] OR post-surgical[tiab] OR postsurgery OR complication*[tiab] OR adverse-event*[tiab] OR “adverse effects” [Subheading] OR adverse-effect*[tiab].
Concept 3: CSF leak.
“Cerebrospinal Fluid Leak”[Mesh] OR cerebro-spinal [tiab] OR cerebrospinal[tiab] OR CSF[tiab] OR Liquorrhea[tiab] OR Liquorhea[tiab] OR Liquorrea[tiab] OR Liquorea[tiab] OR Liquorrhoea[tiab] OR Pseudomeningocele*[tiab] OR Pseudomeningocoele*[tiab] OR “Intracranial Hypotension”[Mesh] OR intracranial-hypotension[tiab] OR meningocele*[tiab] OR meningocoele*[tiab].
Concept: study type.
“Clinical Trial” [Publication Type] OR randomized[tiab] OR randomised[tiab] OR ‘RCT’[tiab] OR review*[tiab] OR “case–control”[tiab] OR retrospective[tiab].
OR prospective[tiab] OR clinical-trial*[tiab] OR intervention-stud*[tiab].
OR “Observational Study”[Publication Type] OR observational-stud*[tiab] OR “epidemiologic studies”[Mesh] OR epidemiolog*[tiab] OR “cohort studies”[Mesh] OR cohort* [tiab] OR incidence[tiab] OR epidemiology [subheading] OR etiology [subheading] OR etiolog*[tiab] OR pathogenesis [tiab].
Cochrane Library (CENTRAL)
Concept 1
#1: ([mh ^ “Surgical Procedures, Operative”] OR [mh “General Surgery”] OR [mh /SU]).
#2: (surgery OR surgeries OR surgical OR operati* OR resect*):ti,ab,kw.
#3: #1 OR #2
#4: [mh “Skull”].
#5: (Skull OR skulls OR cranium OR crania OR cranial OR cranii OR “head skeleton” (frontal NEXT bone*) OR (os frontale) OR (occipital NEXT bone*) OR (os occipitale) OR (parietal NEXT bone*) OR (os parietale) OR sphenoid* OR sella* OR (temporal NEXT bone*) OR (os temporale) OR sella*):ti,ab,kw.
#6: #4 OR #5
#7: #3 AND #6
#8: [mh ^ “Neurosurgical Procedures”] OR [mh “Craniotomy”] OR [mh ^ “Decompression, Surgical”] OR [mh “Microvascular Decompression Surgery”] OR [mh “Dura Mater”].
#9: (neurosurg* OR (skull NEXT surg*) OR (brain NEXT surg*) OR craniotom* OR Trephin* OR Trepanation* OR Trepanning* OR decompressi* OR (decompression NEXT surg*) OR (decompressive NEXT surg*) OR (Microvascular NEXT Decompression*) OR “Dura Mater” OR duratom* OR “Dura repair” OR Duraplast*):ti,ab,kw.
#10: #8 OR #9 OR #7
Concept 2
#11: [mh ^ “Postoperative complications”] OR [mh “Postoperative Period”] OR [mh /AE].
#12: (“wound healing” OR “wound regeneration” OR “wound repair” OR postop* OR (post NEXT operat*) OR “after surgery” OR “after surgical” OR complication* OR (adverse NEXT event*) OR (adverse NEXT effect*)):ti,ab,kw.
#13: #11 OR #12
Concept 3
#14: [mh “Cerebrospinal Fluid Leak”] OR [mh “Intracranial Hypotension”].
#15: ((cerebro NEXT spinal) OR cerebrospinal OR CSF OR Liquorrhea OR Liquorhea OR Liquorrea OR Liquorea OR Liquorrhoea OR Pseudomeningocele* OR Pseudomeningocoele* OR “intracranial hypotension” OR meningocele* OR meningocoele*):ti,ab,kw.
#16: #14 OR #15
#17: #10 AND #13 AND #16
EMBASE
Concept 1: cranial surgery.
((‘surgery’ OR ‘surgeries’ OR ‘surgical’ OR ‘operati*’ OR ‘resect*’) NEAR/6 (‘skull’ OR ‘skulls’ OR ‘crania’ OR ‘cranial’ OR ‘cranium’ OR ‘cranii’ OR ‘head skeleton’ OR ‘frontal bone’ OR ‘os frontale’ OR ‘occipital bone’ OR ‘os occipitale’ OR ‘parietal bone’ OR ‘os parietale’ OR ‘temporal bone’ OR ‘os temporale’ OR ‘sphenoid*’ OR ‘sella*’)):ti,ab,kw.
OR ‘skull surgery’/exp OR ‘neurosurgery’/exp OR ‘neurosurg*’:ti,ab,kw OR ‘brain surg*’:ti,ab,kw OR ‘craniotomy’/exp OR ‘craniotom*’:ti,ab,kw OR ‘Trephin*’:ti,ab,kw OR ‘Trepanation*’:ti,ab,kw OR ‘Trepanning*’:ti,ab,kw OR ‘decompression surgery’/de OR decompressi*: ti,ab,kw OR ‘microvascular decompression’/exp OR ‘dura mater’/exp OR ‘dura mater’:ti,ab,kw OR ‘duratom*’:ti,ab,kw OR ‘dura repair’:ti,ab,kw OR ‘duraplast*’:ti,ab,kw OR ‘duraplasty’/exp.
Concept 2: postoperative complication.
‘wound healing’:ti,ab,kw OR ‘wound regeneration’:ti,ab,kw OR ‘Wound repair’:ti,ab,kw OR ‘postoperative complication’/de OR ‘postoperative period’/de OR ‘postop*’:ti,ab,kw OR ‘post-operat*’:ti,ab,kw OR ‘after-surgery’:ti,ab,kw OR ‘postsurgery’:ti,ab,kw OR ‘after-surgical’:ti,ab,kw OR ‘post-surgical’:ti,ab,kw OR ‘complication*’:ti,ab,kw OR ‘adverse effect*’:ti,ab,kw OR ‘adverse-event*’:ti,ab,kw OR ‘adverse event’/de.
Concept 3: CSF leak.
‘liquorrhea’/exp OR ‘cerebro-spinal’:ti,ab,kw OR ‘Cerebrospinal’:ti,ab,kw OR ‘CSF’:ti,ab,kw OR ‘Liquorhea’:ti,ab,kw OR ‘Liquorrea’:ti,ab,kw OR ‘liquorrhea’:ti,ab,kw OR ‘Liquorrhoea’:ti,ab,kw OR ‘Pseudomeningocele*’:ti,ab,kw OR ‘Pseudomeningocoele*’:ti,ab,kw OR ‘Intracranial Hypotension’:ti,ab,kw OR ‘intracranial hypotension’/exp OR ‘meningocele’/exp OR ‘meningocele*’:ti,ab,kw OR ‘meningocoele*’:ti,ab,kw.
Concept study design.
‘randomi?ed’:ti,ab,kw OR ‘clinical trial*’:ti,ab,kw OR ‘clinical trial’/de OR ‘RCT’:ti,ab,kw OR ‘review*’:ti,ab,kw OR ‘case–control’:ti,ab,kw OR ‘controlled clinical trial’/exp OR ‘case control study’/exp OR ‘case–control’:ti,ab,kw OR ‘retrospective’:ti,ab,kw OR ‘prospective’:ti,ab,kw OR ‘observational study’/exp OR ‘intervention$stud*’:ti,ab,kw.
WOS core collection
Concept 1: cranial surgery.
((“surgery” OR “surgeries” OR “surgical” OR “operati*” OR “resect*”) NEAR/6 (“skull” OR “skulls” OR “crania” OR “cranial” OR “cranium” OR “cranii” OR “head skeleton” OR “frontal bone” OR “os frontale” OR “occipital bone” OR “os occipitale” OR “parietal bone” OR “os parietale” OR “temporal bone” OR “os temporale” OR “sphenoid*” OR “sella*”)).
OR “neurosurg*” OR “brain surg*” OR “craniotom*” OR “Trephin*” OR “Trepanation*” OR “Trepanning*” OR “decompressi*” OR “dura mater” OR “duratom*” OR “dura repair” OR “duraplast*”.
Concept 2: postoperative complication.
“wound healing” OR “wound regeneration” OR “Wound repair” OR “postop*” OR “post-operat*” OR “after-surgery” OR “postsurgery” OR “after-surgical” OR “post-surgical” OR “complication*” OR “adverse effect*” OR “adverse-event*”
Concept 3: CSF leak.
“cerebro-spinal” OR “Cerebrospinal” OR “CSF” OR “Liquorhea” OR “Liquorrea” OR “liquorrhea” OR “Liquorrhoea” OR “Pseudomeningocele*” OR “Pseudomeningocoele*” OR “Intracranial Hypotension” OR “meningocele*” OR “meningocoele*”
Concept study design.
“clinical trial*” OR “randomi?ed” OR “RCT” OR “review*” OR “case control study” OR “case-control” OR “retrospective” OR “prospective” OR “observational stud*” OR “intervention stud*” OR “cohort*” OR “incidence*” OR “epidemiolog*” OR “etiolog*” OR “pathogenesis”
Rights and permissions
About this article
Cite this article
Coucke, B., Van Gerven, L., De Vleeschouwer, S. et al. The incidence of postoperative cerebrospinal fluid leakage after elective cranial surgery: a systematic review. Neurosurg Rev 45, 1827–1845 (2022). https://doi.org/10.1007/s10143-021-01641-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-021-01641-y