Abstract
The pharyngeal plexus is an essential anatomical structure, but the contributions from the glossopharyngeal and vagus nerves and the superior cervical ganglion that give rise to the pharyngeal plexus are not fully understood. The pharyngeal plexus is likely to be encountered during various anterior cervical surgical procedures of the neck such as anterior cervical discectomy and fusion. Therefore, a detailed understanding of its anatomy is essential for the surgeon who operates in and around this region. Although the pharyngeal plexus is an anatomical structure that is widely mentioned in literature and anatomy books, detailed descriptions of its structural nuances are scarce; therefore, we provide a comprehensive review that encompasses all the available data from this critical structure. We conducted a narrative review of the current literature using databases like PubMed, Embase, Ovid, and Cochrane. Information was gathered regarding the pharyngeal plexus to improve our understanding of its anatomy to elucidate its involvement in postoperative spine surgery complications such as dysphagia. The neural contributions of the cranial nerves IX, X, and superior sympathetic ganglion intertwine to form the pharyngeal plexus that can be injured during ACDF procedures. Factors like surgical retraction time, postoperative hematoma, surgical hardware materials, and profiles and smoking are related to postoperative dysphagia onset. Thorough anatomical knowledge and lateral approaches to ACDF are the best preventing measures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
General overview
The origin of the pharyngeal plexus (PP) is the junction of the contributions of cranial nerves (CNs) IX, X, and the superior sympathetic ganglion on the posterior aspect of the middle pharyngeal constrictor muscle [16]. Recent research has suggested that its connections could be more complicated than previous descriptions have indicated [29]. For example, several other reports have shown that connections between the lower cranial nerves, including the hypoglossal nerve (CN XII) and nerve plexuses such as the carotid and intercarotid, contribute to the structure and function of the PP.
Focused descriptions of this structure are limited and its functional mechanisms are not yet entirely understood. Therefore, the clinical and surgical implications of lesions in the pharyngeal plexus have not been fully elucidated [9]. In the present manuscript, we review each of the origins and the nerve plexus connections between cranial nerves that give rise to the PP, intending to describe its surgical correlations.
Origins
As previously stated, the primary connections that give rise to the PP are branches from the glossopharyngeal and vagus nerves, and sympathetic trunk branches. In the next section, we will review data on each apparent neural origin individually and then explain their interconnections within the PP and its relationships in terms of their common nuclear and embryological origin. The PP finds its origins in the ambiguous nucleus within the medulla and is responsible for its innervation. The common nuclear origin with the vagus nerves explains how their functions are shared within the PP (Fig. 1).
Posterior view of the right pharyngeal plexus and associated nerves, arteries, and muscles. Medially, the superior (SPC), middle (MPC), and inferior (IPC) muscles are seen. Traveling from lateral to medial, the stylopharyngeus muscle (SP) is seen. For reference, note the hyoid bone (H) at the level of the C3 vertebra and the thyroid cartilage (TC) at the level of C4 and C5 vertebrae. Over, primarily, the posterior surface of the middle pharyngeal constrictor muscle, note the contributions to the pharyngeal plexus from the vagus (A), glossopharyngeal (B), and sympathetic trunk (C). ICA, internal carotid artery; CCA, common carotid artery; SCG, superior cervical ganglion; * = superior laryngeal nerve; C1 = C1 spinal nerve; C2 = C2 spinal nerve, CN IX = glossopharyngeal nerve; CN X = vagus nerve; CN XII = hypoglossal nerve (after Henle)
Glossopharyngeal nerve
The glossopharyngeal nerve extra-axial segments originate from the postolivary sulcus in the cephalic third of the medulla oblongata, rostral to the apparent origin of the vagus and accessory nerves (Figs. 2 and 3) [2]. It then traverses the cerebellomedullary cistern where it sends a branch, the tympanic nerve of Jacobson, to the middle ear. After the relatively short course through the subarachnoid space, it enters the jugular foramen through the pars nervosa. The glossopharyngeal nerve ganglia (the superior and inferior petrosal ganglia) are located within the jugular foramen. They are relevant to the PP as they are the origin of the glossopharyngeal nerve’s contribution to it, especially in the case of the inferior petrosal ganglion. Once the nerve is outside the skull, it forms approximately six branches, including the tympanic nerve, motor branches for stylopharyngeus muscle, Hering’s nerve to the carotid sinus [30], pharyngeal branches, and tonsillar and lingual branches. It must be stressed that the pharyngeal branches are the most important contributions of CN IX to the PP.
The nerve’s pharyngeal and tonsillar branches are responsible for most of the general somatic afferent information to the pharynx and oropharynx [2, 32]. However, it is noteworthy that the motor contribution of the glossopharyngeal nerve is only exclusive to the innervation of the stylopharyngeus and the lateral part of the superior pharyngeal constrictor muscles [27]. The rest of the muscle innervation is shared with the vagus nerve through the pharyngeal plexus.
Vagus nerve
The vagus nerve finds its apparent origins on the postolivary sulcus between the glossopharyngeal nerve rostrally and the accessory nerve caudally (Fig. 2). It accompanies the previously mentioned nerves into the cerebellomedullary cistern to enter the jugular foramen but is separated from the glossopharyngeal nerve as it pierces the foramen on its pars vasculorum or posterior compartment along with CN XI. Once in the foramen, the vagus nerve presents a ganglionic chain that is represented proximally by the superior or jugular ganglion intracranially and the inferior or nodose ganglion extracranially. The latter ganglion is relevant to the origin of the PP as it provides the bed for the neuronal bodies of the pharyngeal branch containing visceral afferents and special sensory afferents [3].
The vagus nerve provides numerous branches along the jugular fossa, neck, thorax, and abdomen. The vagal neck contributions are closely related to the formation of the PP (Fig. 1). Motor innervation by the vagus nerve accounts for most of the pharyngeal muscles [17] including the soft palate and the pharynx, with a few exceptions such as stylopharyngeus and tensor veli palatini muscles, which are supplied by CN IX and V, respectively [3].
The vagus nerve branches in the neck, four of its most essential divisions being the superior laryngeal nerve (Fig. 4) (which subsequently divides into the internal and external laryngeal nerves), the recurrent laryngeal nerves, the superior cardiac branches, and the pharyngeal branches, are released afterward. These neck branches and their interconnections provide the vagus nerve’s contribution to the pharyngeal plexus when they join the pharyngeal branch from the glossopharyngeal nerve and the sympathetic ganglia, as discussed below.
Superior sympathetic ganglia
The superior cervical ganglion (Figs. 1 and 5) (SCG) lies ventral to the transverse process of the second cervical vertebra. It is closely related to the internal carotid artery, internal jugular vein, and the longus capitis muscle. It provides gray rami to four cervical spinal segments, C1-C4, hence, the proposed four fused segment sympathetic ganglia developmental origin for the SCG. It is responsible for autonomous neural information for the head and neck, including vasomotor and sudomotor nerves and sympathetic function in the pupil, eyelids, and orbitalis smooth muscle [38]. Branches from the SCG are usually organized in three groups of bundles: the lateral, medial, and anterior branches.
The lateral branches connect with the first four cervical segments, the inferior vagal ganglion, and the inferior glossopharyngeal ganglion via the jugular nerve. Also, they innervate the jugular glomus and superior jugular bulb [38].
The medial branches can be classed as cardiac and laryngopharyngeal. The cardiac division carries mainly efferent information related to the lower cervical and upper thoracic segments dedicated to autonomous cardiac input, the esophagus, the superior laryngeal nerve, and the ascendant aortic nerve plexus. The laryngopharyngeal bundles of the medial branches contribute to the PP as they provide innervation to the carotid body first and then migrate through the lateral pharynx to join the pharyngeal branches of CN X and CN IX to the PP. [38]
Interconnections among the lower cranial nerves
The anastomoses among cranial nerves are of several types: lower CNs with themselves, lower with upper CNs, CNs with cervical spinal segments, and through nerve plexuses [29]. The PP is one example of lower cranial nerve interconnection with sympathetic innervation input. The intercarotid and carotid plexus sinuses are other well-described connections between the lower CNs that have spatial and functional relationships with the PP; they have been proposed to be a lateral extension of the PP [29].
There are two classes of connections between the vagus and glossopharyngeal nerves [29]: direct communications when the main trunks are involved and indirect links involving only branches. These communications occur intracranially and extracranially. Tubbs et al. proposed a two-type classification for direct intracranial interactions between the vagus and glossopharyngeal nerves [34] depending on the angle taken by the interconnection (vertical or oblique). Other examples of direct communications are between the inferior or Andersch’s ganglion of CN IX and the jugular ganglion of CN X, and the glossopharyngeo-pneumogastric or van Haller’s ansa [5], formed between the contributions from CN IX and the auricular branch of the vagus nerve (Arnold’s nerve) [34].
These connections and those that give rise to the PP develop because the lower cranial nerves have a common placode origin related to the third and fourth pharyngeal arches, which contain the glossopharyngeal-vagal or petrosal-nodose placodes that then migrate ventrally and differentiate during the developmental stage [33]. This migration is completed effectively because of the interactions of the neural crest with the subsequent placodes in the aforementioned pharyngeal arches. There is evidence that lesions of the neural crest or disruption of the adjacent geniculate ectoderm result in the absence of connections in the nodose and petrosal ganglia and even lack of the ganglion itself [33, 41].
Pharyngeal innervation
The pharyngeal branches, described above, derived from the glossopharyngeal nerve, vagus nerve, and the medial branches of SCG, join in supplying the posterolateral wall of the pharynx. They are closely related to the middle pharyngeal constrictor (MPC), the superior pharyngeal constrictor (SPC), and the inferior constrictor (ICM) muscles (Fig. 6) as the plexus reaches them in a dorsolateral, anteroposterior, and lateromedial fashion [21, 27, 28]. The plexus is located in the retropharyngeal space close to the longus capitis and colli muscles, the prevertebral fascia, the vertebral bodies of the second and third cervical vertebrae posteriorly, and the posterior wall of the pharynx, and is closer to the superior and middle constrictor anteriorly [18, 37].
Although a stronger relationship has been described with the SPC, MPC, and the IPC, the PP also provides branches that pierce the spaces between the pharyngeal constrictors to innervate deeper structures within the pharynx [27]. Thus, the PP can be studied on the basis of its branches and their origins, which are predominantly CNs IX, CN X, or both. Also, pharyngeal muscle innervations can be used as topographical landmarks for understanding the different divisions that the PP and the cranial nerves present in their innervation [27].
Predominant glossopharyngeal branches
The superior and more medial branches near the PP are provided principally by the glossopharyngeal nerve. These CN IX contributions can be divided into those that pierce the stylopharyngeus muscle and then innervate the most lateral portion of the SPC, and those that travel between the superior and middle pharyngeal constrictors and are responsible for the somatic afferent information from pharyngeal mucosae. The glossopharyngeal nerve crosses the pharyngeal branch of the vagus nerve in 75% of cases to innervate the pharyngeal wall. In the remaining cases, it runs parallel to them to form the PP and reach their pharyngeal destination so they can function in the initiation of swallowing [31, 36].
Pharyngeal plexus
Mixed predominance branches are at the junction of CNs X, IX, and the SCG, and thus the PP itself. This first mixed origin trunk innervates the central part of the SPC and then travels between the SPC and the MPC to provide the nerve supply to the salpingo- and palatopharyngeus muscles, reaching them from their dorsal aspect. More caudally, the PP then innervates the MPC itself and the IPC. Peng et al. reported that the main branch of the PP is located within the inferior pharyngeal constrictor and at the posterior side of the greater horn of the hyoid [23]. Mu et al. in 2007 [20] proposed that the innervation of the pharyngeal constrictor (PC) muscles was differential. They showed that PCs had a slow inner layer innervated by the pharyngeal branch of CN IX, and a fast outer layer innervated by the pharyngeal branch of CN X [20].
Innervation of the cricopharyngeal muscle (CPM) has been a source of controversy as it is the most prominent component of the superior esophageal sphincter. Hence, its clinical relevance is a significant issue for physicians and patients [11]. Uludag et al. in 2016 [35] reported that the PP contributes functionally to motor innervation of the CPM in up to 100% of cases; the recurrent laryngeal nerve has a motor contribution in 91% of cases, the external branch of the superior laryngeal nerve (EBSLN) in 76%, and both the RLN and the EBSLN in 70% [35].
Predominant vagal branches
After its contribution to the PP, the vagus nerve gives two of the most essential branches related to the neck and the thoracic region. The superior laryngeal nerve (SLN) pierces the space between the MPC and the IPC to divide into its external branch, which is motor and innervates the cricothyroid muscle (CTM), and its internal branch, which is sensory and provides fibers to the pharynx [1]. Then it gives the recurrent laryngeal nerve (RLN), which enters the larynx crossing below the IPC. Sakamoto reported that communicating branches between the laryngeal nerves and the PP sometimes provide twigs to the constrictor from the dorsal surface and vary in their anastomoses [28]. Variations in the muscle innervations between the RLN and SLN are thought to explain the varied muscle innervation of the PP [16].
Esophageal branches of the recurrent laryngeal nerves
In a recent cadaveric study, ten fresh-frozen cadaveric human specimens, meaning twenty sides, were observed. The esophageal branches were successfully identified in all sides. The authors found that the length of those branches oscillated between 0.8 and 2.1 cm (mean 1.5 cm) and their diameter between 0.5 and 2 mm. Moreover, they were able to describe that the esophageal twigs branched from the RLN between the vertebral levels C6 and T1. The contributions of the RLN were then divided among the tracheal and esophageal twigs that could arise from a common trunk in up to 20% of the cases. Both were lying in the ventral aspect of the designated esophageal and tracheal structures [9]. Although those are secondary projections from the PP, they carry neural information necessary for swallowing and, in many cases, are the explanation for post-operative dysphagia which will be discussed below.
Muscle classification based on the innervation of the PP and its branches
Based on the foregoing description of the PP, Sakamoto in 2009 proposed a four-group classification of pharyngeal muscles depending on the portion of the PP that provides their innervation (Table 1). It is noteworthy that we include the superior esophageal sphincter, the mucosae, and the vocal cords as essential structures innervated by the pharyngeal nerves.
Functional and clinical overview
A structural understanding of the PP is only useful if we also aim to understand its overall function. The functions of the PP are prominent, and injury to it can lead to very disabling clinical symptoms. The plexus is responsible for the innervation for swallowing [4, 31], the sensory innervation of the pharyngeal mucosae, and the innervation of the superior esophageal sphincter. We describe its contribution to the vocal cords below.
Role of the PP in swallowing
Swallowing is a very complex and coordinated motor program that depends on central and peripheral neural circuits to allow voluntary and involuntary control. This network is organized in a three-level functional system [10].
Like every executive system, it needs afferent peripheral and central input fibers, an organizing level consistent of an interneuronal network of premotor neurons (PMNs) that configures the central pattern generator (CPG), and an efferent level corresponding to the cranial motor nuclei that command the output and execution of the function. Both afferent and efferent fibers for swallowing are intertwined within the PP. The central medullary nuclei in charge of the afferents and efferents for swallowing are located within the vagal system, which comprises the solitary tract nucleus (STN), ambiguous nucleus, dorsal motor nucleus of the vagal nerve, and the hypoglossal nucleus. Afferent fibers run along with the glossopharyngeal and vagus nerves [19] to terminate in the STN, which then communicates with the CPG [25] to provide efferents through the motor nucleus of CNs V, VII, the ambiguous nucleus, and CN XII [31].
Several diseases can affect swallowing, either mechanically or functionally. For example, activity in the fast outer layer of the pharyngeal muscles is diminished in patients with idiopathic Parkinson’s disease [20]. That means dysfunction of the PP in terms of innervation of the vagus nerve. Tumors of the pulmonary apex can cause Pancoast syndrome associated with Horner’s syndrome. A case report presented in 2012 described dysphagia in a patient with an apical pulmonary tumor compromising the sympathetic trunk and ganglia, causing the presenting symptoms [12]. Chen et al. reported a case of neurosarcoidosis that resulted in pharyngeal dysfunction, represented by intractable hiccoughs, an extremely rare manifestation [7].
Cervical ganglioneuromas have been reported to cause dysphagia in the pediatric population. Such involvement can be explained by compromising the origins of the PP [15]. The cervical sympathetic trunk can be directly injured since this is the origin of the tumor, and CNs IX and X can be compressed.
Surgical overview
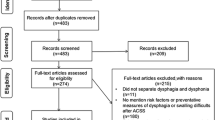
Cervical neurosurgical procedures are not exempt from injuries to the PP [9]. Anterior cervical discectomy with fusion (ACDF) is a surgical treatment for cervical spondylotic disease (CSD) that has shown success and benefits for patients, exhibiting a mortality rate near 0.1% and morbidity rate of 19.3% depending on the clinical center (Fig. 7) [8, 13]. Despite the functional outcomes of the procedure in terms of restoration of spine balance and foraminal space and relief of symptoms, dysphagia is a frequent complication owing to retropharyngeal injuries [8, 9, 26]. Dysphagia has been reported in rates ranging from 0 to 51%, with differential rates between inpatients and outpatients. The risk of dysphagia among outpatients was 113% higher than for inpatients. Although the results of the meta-analysis were not statistically significant, the findings were consistent among studies [9, 39].
Left-sided two level ACDF noting the superficial dissection (left) and deeper dissection (right) with deep to the carotid sheath and trachea/esophagus noting the anterior vertebral bodies and arthroplasties. Pharyngeal plexus branches would not be encountered on the left but would be encountered with a dissection such as seen on the right image
Postoperative dysphagia
Surgical hardware or hematoma in the retropharyngeal space after surgery has been proposed as the mechanism of PP injury. Also, the use of plates and materials such as titanium instrumentation increases the range of anterior longitudinal ligament dissections and consequently increases the risk of dysphagia [9, 26]. Zhu et al. in 2019 compared the incidence of complications of ACDF using self-locking cages vs. cages-with-plate fixation. They found that the risk of dysphagia was significantly higher with cages-with-plate fixation (6–7% vs. 13.2%). Also, they proposed that the mechanism for postoperative dysphagia is explained by the time of the esophageal and pharyngeal retraction and even by the prominence of the plates used [42]. Cases of retropharyngeal granulation, Zenker’s diverticulum, and delayed recurrent laryngeal nerve palsy following ACDF have been reported [8, 14, 40].
Branches of both the origins and outputs of PP can be lesioned during anterior cervical procedures (Fig. 8). The branches of the PP will undoubtedly be encountered during such approaches and vary depending on the level (Fig. 9) [1]. In a study published by Carucci et al. in 2015, the authors proposed that, given the anatomy of the esophageal branches from the RLN, dysphagia was more probable to occur in the mid-cervical spine compared to the upper and lower segments [6]. However, also noteworthy is the fact that ACDF procedures are more commonly performed at the lower cervical segments (C6-T1). Therefore, surgical retraction and hardware placing are only some of the factors that could be responsible for neural irritation; other causes such as hematomas, rare conditions like diffuse idiopathic skeletal hyperostosis (DISH), and osteophytes should be counted as probable causes. Additionally, smoking has been proven to be a risk factor for postoperative dysphagia [22]. Patient-related factors such as age, body mass index, sex, and number of levels have shown little or no correlation in postoperative dysphagia onset [9].
Cadaveric dissections of the left neck without (left) and with (right) the common carotid artery and its branches. Note the pharyngeal branch of the vagus nerve heading toward the posterior pharynx to contribute to the pharyngeal plexus along with a branch derived from the superior cervical ganglion
Thorough knowledge and understanding of the pharyngeal neural anatomy and a lateral ACDF approach are the most important preventive measures to avoid postoperative palsy due to unnecessary injury [9]. Some authors have proposed that the inferior thyroid artery and Berry’s ligament are useful surgical landmarks to identify the RLN intraoperatively to avoid injury risks [24]. Other measures like the use of inflated endotracheal cuff to maintain the tracheal structures in a central position after placement of cervical retractors have been used to reduce RLN palsy. Steroid administration has shown some improvements in prevertebral swelling reduction, although the results are strongly debatable given the heterogenous dosage schemes and follow-up [9].
The PP can also be used as a therapeutic target for treating various pathological states. Pharyngeal constrictor muscle spasms can be treated by neurectomy. Peng et al. in 2000 [23] reported a case series of patients treated with pharyngeal plexus neurectomy, which was successful in 93.7%. Hence, the authors proposed PP neurectomy as a replacement for pharyngeal myotomy to improve voice rehabilitation in those patients.
Conclusions
The PP is a crucial structure responsible for a vast number of vital tasks such as swallowing that importantly affect patient functionality after neurosurgical spine procedures. This structure is likely to be encountered during various anterior cervical surgical procedures of the neck such as anterior cervical discectomy and fusion. Therefore, a detailed understanding of its anatomy and thorough surgical planning are vital factors that the surgeon must take into account to minimize injury risk and iatrogenic disability among spine surgery patients. Having awareness of these branches, using minimal and only short-term retraction, and limiting cautery use might all aid in decreasing iatrogenic injury to this plexus.
References
Anderson SK, Terris DJ (2009) CHAPTER 37 - complications of endoscopic neck surgery. In: Eisele DW, Smith R V (eds) Complications in Head and Neck Surgery (Second Edition), Second Edi. Mosby, Philadelphia, pp 467–475
Binder DK, Sonne DC, Fishbein NJ (2011) Glosophayngeal nerve. In: Cranial nerves: anatomy, pathology, Imaging. Thieme, New York, pp 146–157
Binder DK, Sonne DC, Fishbein NJ (2011) Vagus nerve. In: Cranial nerves: anatomy, pathology, Imaging. Thieme, Ney York, pp 158–171
Broussard DL, Altschuler SM (2000) Brainstem viscerotopic organization of afferents and efferents involved in the control of swallowing. Am J Med 108:79–86. https://doi.org/10.1016/S0002-9343(99)00343-5
Brown H (2002) Anatomy of the spinal accessory nerve plexus: relevance to head and neck cancer and atherosclerosis. Exp Biol Med (Maywood) 227:570–578
Carucci LR, Turner MA, Yeatman CF (2015) Dysphagia secondary to anterior cervical fusion: radiologic evaluation and findings in 74 patients. Am J Roentgenol 204:768–775. https://doi.org/10.2214/AJR.14.13148
Chen X, Ren Z, Huang X (2018) Sarcoidosis of the medulla oblongata causing intractable hiccoughs and numbness of extremities. Medicine (Baltimore) 97:e13667. https://doi.org/10.1097/MD.0000000000013667
Dobran M, Gladi M, Mancini F, Nasi D (2018) Rare case of anterior cervical discectomy and fusion complication in a patient with Zenker’s diverticulum. BMJ Case Rep 11:e226022. https://doi.org/10.1136/bcr-2018-226022
Fisahn C, Yilmaz E, Iwanaga J, Schmidt C, Benca E, Chapman JR, Oskouian RJ, Tubbs RS (2019) Avoiding the esophageal branches of the recurrent laryngeal nerve during retractor placement: precluding postoperative dysphagia during anterior approaches to the cervical spine. Glob Spine J 9:383–387. https://doi.org/10.1177/2192568218810198
Goyal RK, Padmanabhan R, Sang Q (2001) Neural circuits in swallowing and abdominal vagal afferent-mediated lower esophageal sphincter relaxation. Am J Med 111:95–105. https://doi.org/10.1016/S0002-9343(01)00863-4
Hammond CS, Davenport PW, Hutchison A, Otto RA, Smith C, Davenport PW, Otto RA (1997) Motor innervation of the cricopharyngeus muscle by the recurrent laryngeal nerve. J Appl Physiol 83:89–94. https://doi.org/10.1152/jappl.1997.83.1.89
Hermida Pérez JA, Bermejo Hernández A, Hernández Guerra JS, Arroyo Diaz R (2012) Tumor del vértice pulmonar derecho que produce un síndrome de Pancoast. Descripción de un caso clínico Semergen 38:111–114. https://doi.org/10.1016/j.semerg.2011.06.013
Joaquim AF, Makhni MC, Riew KD (2019) Evidence-based use of arthroplasty in cervical degenerative disc disease. Int Orthop Published 43:767–775. https://doi.org/10.1007/s00264-018-04281-y
Kim J-H, Lee S-K, Hong J-H, Moon BJ, Lee J-K (2019) Retropharyngeal granulation: a delayed complication of anterior cervical discectomy and fusion in C2–3. World Neurosurg 125:87–92. https://doi.org/10.1016/j.wneu.2019.01.156
Lima AF, Moreira FC, Menezes A, Dias L (2019) Cervical ganglioneuroma in pediatric age: a case report. Turk Otolarengoloji Arsivi/Turkish Arch Otolaryngol 56:237–240. https://doi.org/10.5152/tao.2018.3690
Matsuzaki H, Paskhover B, Sasaki CT (2014) Contribution of the pharyngeal plexus to vocal cord adduction. Laryngoscope 124:516–521. https://doi.org/10.1002/lary.24345
McHanwell S (2016) Chapter 34: pharynx. In: Standring S (ed) Gray’s anatomy: the anatomical basis of clinical practice, 41st ed. Elsevier, pp 571–586
Mermer RW, Zwillenberg D, Maron A, Brill CB (1990) Unilateral pharyngeal plexus injury following use of an oropharyngeal pack during third-molar surgery. J Oral Maxillofac Surg 48:1102–1104
Mu L, Sanders I (2000) Sensory nerve supply of the human oro- and laryngopharynx: a preliminary study. Anat Rec 258:406–420. https://doi.org/10.1002/(SICI)1097-0185(20000401)258:4<406::AID-AR9>3.0.CO;2-5
Mu L, Sanders I (2007) Neuromuscular specializations within human pharyngeal constrictor muscles. Ann Otol Rhinol Laryngol 116:604–617. https://doi.org/10.1177/000348940711600809
Netter FH (2019) Atlas of human anatomy, 7th Ed. Elsevier
Olsson EC, Jobson M, Lim MR (2015) Risk factors for persistent dysphagia after anterior cervical spine surgery. Orthopedics 38:e319–e323. https://doi.org/10.3928/01477447-20150402-61
Peng Y, Liao J, Fan J, Ye Q (2000) Pharyngeal plexus neurectomy for voice restoration of alaryngeal. Zhonghua Er Bi Yan Hou Ke Za Zhi 35:298–299
Rajabian A, Walsh M, Quraishi NA (2017) Berry’s ligament and the inferior thyroid artery as reliable anatomical landmarks for the recurrent laryngeal nerve (RLN): a fresh-cadaveric study of the cervical spine. The RLN relevant to spine. Spine J 17:S33–S39. https://doi.org/10.1016/j.spinee.2017.01.011
Reed MD, English M, English C, Huff A, Poliacek I, Musselwhite MN, Howland DR, Bolser DC, Pitts T (2019) The role of the cerebellum in control of swallow: evidence of inspiratory activity during swallow. Lung 197:1–6. https://doi.org/10.1007/s00408-018-00192-2
Rong Y, Luo Y, Liu W, Gong F, Tang P, Cai W (2018) Clinical effects of the bridge-type ROI-C interbody fusion cage system in the treatment of cervical spondylosis with osteoporosis. Clin Interv Aging Volume 13:2543–2551. https://doi.org/10.2147/CIA.S182969
Sakamoto Y (2009) Classification of pharyngeal muscles based on innervations from glossopharyngeal and vagus nerves in human. Surg Radiol Anat 31:755–761. https://doi.org/10.1007/s00276-009-0516-9
Sakamoto Y (2013) Interrelationships between the innervations from the laryngeal nerves and the pharyngeal plexus to the inferior pharyngeal constrictor. Surg Radiol Anat 35:721–728. https://doi.org/10.1007/s00276-013-1102-8
Shoja MM, Oyesiku NM, Shokouhi G, Griessenauer CJ, Chern JJ, Rizk EB, Loukas M, Miller JH, Tubbs RS (2014) A comprehensive review with potential significance during skull base and neck operations, part II: glossopharyngeal, vagus, accessory, and hypoglossal nerves and cervical spinal nerves 1-4. Clin Anat 27:131–144. https://doi.org/10.1002/ca.22342
Shoja MM, Rai R, Lachkar S, Iboroma Akobo S, Yilmaz E, Loukas M, Binello E, Gorjaian M, Griessenauer CJ, Iwanaga J, Tubbs RS (2019) The carotid sinus nerve and the first English translation of Hering’s original research on this nerve. Cureus 11:1–7. https://doi.org/10.7759/cureus.3898
Sinclair W (1971) Role of the pharyngeal plexus in initiation of swallowing. Am J Physiol Content 221:1260–1263. https://doi.org/10.1152/ajplegacy.1971.221.5.1260
Staloff RT (2016) Head and neck surgery. Jaypee Brothers medical publishers (P) ltd
Steventon B, Mayor R, Streit A (2014) Neural crest and placode interaction during the development of the cranial sensory system. Dev Biol 389:28–38. https://doi.org/10.1016/j.ydbio.2014.01.021
Tubbs RS, Mortazavi MM, Loukas M, Shoja MM, Cohen-Gadol AA (2011) Intraoperative and anatomical descriptions of intracranial connections between the glossopharyngeal and vagus nerves: clinical implications. J Neurosurg 115:179–181. https://doi.org/10.3171/2011.2.JNS101757
Uludag M, Aygun N, Isgor A (2017) Innervation of the human cricopharyngeal muscle by the recurrent laryngeal nerve and external branch of the superior laryngeal nerve. Langenbeck's Arch Surg 402:683–690. https://doi.org/10.1007/s00423-016-1376-5
Wang C, Kundaria S, Fernandez-Miranda J, Duvvuri U (2016) A description of the anatomy of the glossopharyngeal nerve as encountered in transoral surgery. Laryngoscope 126:2010–2015. https://doi.org/10.1002/lary.25706
Warshafsky D, Goldenberg D, Kanekar SG (2012) Imaging anatomy of deep neck spaces. Otolaryngol Clin N Am 45:1203–1221. https://doi.org/10.1016/j.otc.2012.08.001
Watkinson JC, Gleeson M (2016) Chapter 29: neck. In: Standring S (ed) Gray’s anatomy: the anatomical basis of clinical practice, 41st ed
Yerneni K, Burke JF, Chunduru P, Molinaro AM, Riew KD, Traynelis VC, Tan LA (2019) Safety of outpatient anterior cervical discectomy and fusion: a systematic review and meta-analysis. Neurosurgery 86:1–16. https://doi.org/10.1093/neuros/nyy636
Yerneni K, Burke JF, Nichols N, Tan LA (2019) Delayed recurrent laryngeal nerve palsy following anterior cervical discectomy and fusion. World Neurosurg 122:380–383. https://doi.org/10.1016/j.wneu.2018.11.066
Yntema CL (1944) Experiments on the origin of the sensory ganglia of the facial nerve in the chick. J Comp Neurol 81:147–167. https://doi.org/10.1002/cne.900810204
Zhu D, Zhang D, Liu B, Li C, Zhu J (2019) Can self-locking cages offer the same clinical outcomes as anterior cage-with-plate fixation for 3-level anterior cervical discectomy and fusion (ACDF) in mid-term follow-up? Med Sci Monit 25:547–557. https://doi.org/10.12659/MSM.911234
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The protocol of the study did not require approval by the ethical committees or informed consent. The study followed the Declaration of Helsinki (64th WMA General Assembly, Fortaleza, Brazil, October 2013).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gutierrez, S., Iwanaga, J., Pekala, P. et al. The pharyngeal plexus: an anatomical review for better understanding postoperative dysphagia. Neurosurg Rev 44, 763–772 (2021). https://doi.org/10.1007/s10143-020-01303-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-020-01303-5