Abstract
Cardiac abnormalities (echocardiographic wall motion abnormality (WMA), biomarker elevation of cardiac troponin (cTn), B-type natriuretic peptide (BNP), or N-terminal prohormone of B-type natriuretic peptide (NT-proBNP)) frequently occur after subarachnoid hemorrhage (SAH). The clinical significance of cardiac abnormalities after SAH remains controversial. This meta-analysis was performed to assess the association between cardiac abnormalities and patient outcomes, including delayed cerebral ischemia (DCI), poor outcome, and death in SAH patients. PubMed and Embase were searched for observational studies reporting an association between cardiac abnormalities and outcome after SAH that were published before 31 December 2017. We extracted data regarding patient characteristics, cardiac abnormalities, and outcome measurements (DCI, poor outcome, or death). Risk ratios (RRs) and 95% confidence intervals (CIs) were calculated using a random-effects model. Twenty-six studies involving 3917 patients were included in our data analysis. WMA showed significant associations with higher rates of DCI (RR, 2.03; 95% CI, 0.99–4.15), poor outcome (RR, 1.45; 95% CI, 1.08–1.93), and death (RR, 2.54; 95% CI, 1.59–4.05). cTn elevation was associated with an increased risk of DCI (RR, 1.48; 95% CI, 1.23–1.79), poor outcome (RR, 1.85; 95% CI, 1.49–2.30), and death (RR, 2.68; 95% CI, 2.19–3.27). Elevation of BNP or NT-proBNT was significantly associated with higher rates of DCI (RR, 1.87; 95% CI, 1.16–3.02). WMA and elevation of cTn, BNP, and NT-proBNP in SAH patients are associated with an increased risk of DCI, poor outcome, and death after SAH.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Subarachnoid hemorrhage (SAH) is a devastating cerebrovascular disease that occurs at a relatively young age and threatens brain perfusion and function. SAH is a serious and significant health problem, especially given its poor prognosis, high rates of mortality and disability, and poor clinical outcomes [1]. Cardiac abnormalities after SAH have been described in many reports, and cardiac abnormalities, including echocardiographic wall motion abnormalities (WMA) and elevated biochemical markers of myocardial damage and congestive heart failure, are associated with poor outcomes in some studies [2]. Cerebral autoregulation is disturbed following SAH [3], and cardiac abnormalities may lead to decreased focal and global cerebral perfusion and may contribute to the development of delayed cerebral ischemia (DCI) [4, 5]. DCI is the single most crucial cause of mortality and morbidity after SAH [6]. These findings highlight the clinical importance of cardiac abnormalities for the outcome of SAH.
We conducted a meta-analysis on observational studies to assess the association between cardiac abnormalities (WMA and elevated biochemical markers of myocardial damage and congestive heart failure) and the occurrence of DCI, poor outcome, and death after SAH. Electrocardiographic changes in the articles were heterogeneous [2, 7]; therefore, we did not assess ECG abnormalities in this study.
Materials and methods
We managed this study according to the methods of the Cochrane Handbook for Systematic Review and Meta-Analysis. We reported the findings according to the Preferred Reporting Items for Systematic Review and Meta-Analysis statement.
Search strategy
Two authors (LZ and BZ) independently searched PubMed and Embase for studies reporting associations between cardiac abnormalities and SAH outcome that were published before 31 December 2017. The following keywords were used: “subarachnoid hemorrhage” OR “subarachnoid hemorrhage” OR “subarachnoid blood” OR “subarachnoid bleeding” OR “intracranial bleeding” OR “intracranial aneurysm.” Each of these keywords was combined with the keyword “echocardiography,” “echocardiographic,” “stunning,” “left ventricular dysfunction,” “LV dysfunction,” “apical ballooning,” “takotsubo,” “myocardial damage,” “myocardial necrosis,” “troponin,” “B-type natriuretic peptide,” “BNP,” “N-terminal prohormone of B-type natriuretic peptide,” and “NT-proBNP” in different combinations. We also manually checked the bibliographies of the included studies and previous reviews to identify other potentially eligible studies. We followed this procedure until no additional studies were found.
Study selection
Two authors (LZ and BZ) independently assessed the eligibility of studies. Only articles published in English were included in this study. We included observational studies that examined the association between cardiac abnormalities and outcome after SAH. SAH had to be diagnosed by either computed tomography (CT) scanning or cerebrospinal fluid examination. Cardiac abnormalities were defined as WMA, elevated cardiac troponin (cTn) levels for myocardial damage, and elevated B-type natriuretic peptide (BNP) or N-terminal prohormone BNP (NT-proBNP) levels for congestive heart failure. Outcomes after SAH are defined as DCI, poor outcome, or death. Studies with fewer than ten patients, conference abstracts, reviews, and case reports were excluded.
Studies that included non-consecutive patients were excluded to avoid selection bias. When there were duplicate or overlapping data, only the report with the largest number of patients was eligible for data extraction. When we had disagreements regarding the literature search and eligibility, we resolved the disagreements by reviewing the article in question until a consensus was reached.
Data extraction and quality assessment
We recorded the definition of inclusion or exclusion criteria of each searched article. We extracted the following data from each included article: the first author’s last name, publication year, study design (prospective cohort study or retrospective cohort study), number of included patients, sex, mean age, patients with poor condition on admission, follow-up period, patients with cardiac abnormalities, and patients with DCI, poor outcome, or death. We reviewed the article in question together until a consensus was reached in the case of a disagreement.
The neurological condition on admission in the included articles was defined as poor according to one of the following scoring systems: Hunt-Hess ≥ 3 [8], World Federation of Neurosurgical Societies ≥ 3 [9], and Glasgow Coma Scale < 12 [10]. As determinants, we extracted the incidence of WMA, cTn elevation, and BNP or NT-proBNP elevation. The number of patients with DCI, the number of patients with poor outcome, and the number of deaths from any cause were extracted as outcome measurements. Poor outcome was defined by a handicap scale such as the modified Rankin scale (dichotomized at > 3) or the Glasgow Outcome Scale (dichotomized at ≤ 3).
Among the included studies, there were several varying definitions of DCI. Considering the heterogeneity of DCI definitions, we simply extracted the number of patients with DCI reported by the studies. We did not extract therapy data in the meta-analysis because this information was not present in some of the included studies.
The two authors (LZ and BZ) involved in selecting the studies also evaluated the quality of included studies with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) checklist (https://www.strobe-statement.org). For every included article, the two authors (LZ and BZ) independently assigned a score (either 0 or 1) to each of the 22 STROBE items. Several STROBE items consist of subitems; the subitems of each item were also scored as 0 or 1 and averaged. These scores were then added to generate the STROBE score. The two authors solved disagreements by direct communication.
Data synthesis
Relationships between cardiac abnormalities (WMA, cTn elevation, and BNP or NT-proBNT elevation) and the three outcome measurements were analyzed. The crude proportions of the extracted variables were calculated. Cross-tables were structured to calculate risk ratios (RRs) for each determinant and outcome in each study. We calculated the pooled RRs with their corresponding 95% confidence intervals (CIs) using a random-effects model with Cochrane’s Review Manager version 5.3 (The Cochrane Collaboration, London, UK). We also tested the statistical heterogeneity (I2) of the effects using the same program; a value greater than 50% was considered to indicate significant heterogeneity [11].
Results
Study characteristics
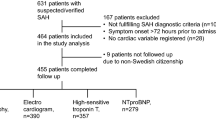
The initial database search identified 2590 studies that reported cardiac abnormalities after SAH, and a search of other sources found no additional studies (Fig. 1). Ninety-seven studies were selected after title screening and detailed abstract evaluation. Twenty-six studies [4, 12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36] were selected after detailed evaluation of the full article. No overlap was found among these 26 studies. No study was excluded due to non-English language alone.
Baseline characteristics
Table 1 shows the baseline characteristics of patients included in the meta-analysis. Twenty-six studies with a total of 3917 patients were included. In the 26 studies in which sex distributions were reported, the mean percentage of men was 34%. The mean age varied from 46 to 70 years, with a weighted mean of 55 years (24 studies). The follow-up duration (for outcome assessment) varied from follow-up during the hospital stay (13 studies) to 12 months of follow-up (1 study). The percentage of patients with poor condition on admission varied from 23 to 74% (mean, 51%; 20 studies).
Table 2 presents the proportions of patients with cardiac abnormalities and the proportions of patient outcomes. WMA was reported in 9 to 71% of patients (mean, 22%; 17 studies), cTn elevation in 11 to 52% (mean, 29%; 15 studies), and BNP or NT-proBNT elevation in 9 to 71% (mean, 36%; 5 studies). The cTn, BNP, and NT-proBNT values were measured during the acute stage of SAH. The percentage of patients with DCI varied from 6 to 54% (mean, 27%; 10 studies). The definitions of DCI were slightly different and included the following: scores of temporary focal neurologic signs [13], neurological deterioration and imaging evidence of spasms or CT evidence of infarction [4, 14, 15, 17, 18, 30, 33], neurological deterioration excluding other causes (using CT) [21], development of a new CT lucency with confirmation of vasospasm by serial transcranial Doppler or angiography [29], or probable and definite DCI as one event [34].
A wide interval of the baseline characteristics is one of the major limitations of this study; however, we may not be able to overcome this limitation.
Relationship between determinants and outcome
The chosen end points are not represented in each included study; thus, only a fraction of the studies could be included in the specific analysis (DCI, poor outcome or death).
Figure 2 shows the pooled RRs and corresponding 95% CIs of WMA for DCI, poor outcome, and death. WMA showed significant associations with higher rates of DCI (RR = 2.03; 95% CI, 0.99–4.15; P = 0.05; I2 = 88%), poor outcome (RR = 1.45; 95% CI, 1.08–1.93; P = 0.01; I2 = 34%), and death (RR = 2.54; 95% CI, 1.59–4.05; P < 0.0001; I2 = 68%).
Figure 3 shows the pooled RRs and corresponding 95% CIs of cTn elevation for DCI, poor outcome, and death. cTn elevation showed significant associations with higher rates of DCI (RR = 1.48; 95% CI, 1.23–1.79; P < 0.0001; I2 = 0%), poor outcome (RR = 1.85; 95% CI, 1.49–2.30; P < 0.00001; I2 = 0%), and death (RR = 2.68; 95% CI, 2.19–3.27; P < 0.00001; I2 = 0%).
Figure 4 shows the pooled RRs and corresponding 95% CIs of BNP and NT-proBNT elevation for DCI, poor outcome, and death. Elevation of BNP and NT-proBNT exhibited a significant association with higher rates of DCI (RR = 1.87; 95% CI, 1.16–3.02; P = 0.01; I2 = 53%).
Discussion
The main finding of this meta-analysis patently indicates that cardiac abnormalities (WMA, cTn elevation, and BNP or NT-proBNT elevation) in a high proportion of SAH patients are predictors for adverse clinical outcome in SAH. Because these cardiac abnormalities are also risk factors for the occurrence of DCI, the relationship between cardiac abnormalities and outcome may therefore, at least in part, be explained by the contribution of cardiac abnormalities to the development of DCI.
The cause of cardiac abnormalities after SAH cannot be answered by the present study. Myocardial ischemia is an unlikely cause of the dysfunction because normal myocardial perfusion was reported [37]. The generally accepted cause is that a catecholamine burst due to massive sympathetic activation causes myocardial dysfunction by microvascular dysfunction, epicardial coronary vasospasm, or hyperdynamic contractility with midventricular or outflow tract obstruction; another explanation is that these abnormalities are caused by the direct effects of catecholamines on cardiomyocytes [38].
On echocardiography, basal and midventricular segments of the anteroseptal and anterior walls are the most frequently affected in neurogenic stunned myocardium [19, 24, 25, 39]. The transient regional WMA of SAH patients typically extends beyond the territory of a single coronary artery [13, 34, 40,41,42]. Thus, the apical-sparing pattern of left ventricular dysfunction is believed to favor a neurally mediated mechanism of injury and argues against obstruction or vasospasm of the coronary arteries [43]. Cardiogenic shock can occasionally accompany neurogenic-stunned myocardium with coexisting left ventricular outflow tract obstruction, systolic anterior motion, or mitral regurgitation [44]. On two-dimensional echocardiography using the velocity-vector technique, left ventricular systolic and diastolic dysfunction occurs not only in the radial direction but also in the longitudinal direction in patients with stress cardiomyopathy [45]. Newer techniques such as two-dimensional speckle tracking echocardiography have also been used to distinguish stress cardiomyopathy from other conditions, such as systolic dysfunction complicated by occlusion of the left anterior descending coronary artery [46].
cTn is the most sensitive and specific marker of myocardial injury after SAH among the various biomarkers [13, 14, 47]. cTn elevation is positively correlated with regional WMA [25, 39, 48]. Three protein subunits (troponins I, T, and C) constitute the troponin complex. Troponins I and T have been shown to be unique cardiac isoforms, whereas troponin C isoforms exist in cardiac and skeletal muscle, rendering troponin C unsuitable for diagnostic use. cTns are complexed with actin in cardiac myofibrils, with a smaller fraction (3–6%) soluble in the cytoplasm [49]. cTnI and cTnT are the most widely used biomarkers for myocardial necrosis in clinical patients with suspected acute coronary syndrome [50]. cTnI and cTnT provide identical information and are widely used as the preferred biomarkers to diagnose myocardial infarction [47] and cardiac damage after SAH [13, 14, 17]. Elevated cTnI level is associated with the severity of SAH (Hunt-Hess scale) [13, 17, 22, 39, 48], which typically peaks within 2 days after ictus and shows a subsequent decay in levels [17, 48]. cTn elevation (categorized into quintiles: undetectable, > 0–0.5, > 0.5–2.0, > 2.0–10.0, and > 10.0 μg/L) was reported to be significantly associated with an increased likelihood of poor outcome (as measured by a modified Rankin scale score of 4–6) at discharge and at 3 months [17]. In this respect, the presence and the degree of the cardiac abnormality may influence the outcome; therefore, the degree of cTn elevation has been associated with poor outcome. However, after adjusting for age, clinical grade, and aneurysm size, this positive association remained significant at discharge but not at 3 months [17].
B-type natriuretic peptide, a vasoactive hormone with natriuretic, diuretic, and vasodilator activity, is predominantly expressed in the ventricles in response to cardiac overload [51]. BNP is mainly synthesized in cardiac tissue as a result of myocyte stretch [52]. The secretion of BNP and NT-proBNP by the cardiac ventricles occurs in response to increased myocardium wall stress when WMA occurs after SAH [20, 29, 33, 35]. This finding is supported by studies showing that levels of BNP/NT-proBNT in stress cardiomyopathy or takotsubo cardiomyopathy following SAH are elevated and correlate with catecholamine increase and the severity of left ventricular dysfunction [37, 53]. NT-proBNP values increased over the first several days after admission [53,54,55,56]. The best subset of variables predicting high NT-proBNP load were female sex, advanced age, high plasma troponin I and T levels at admission, and worse clinical condition at admission [53, 56]. BNP and NT-proBNP appear to have very similar utility in the diagnosis and prognosis of congestive heart failure after SAH and could be used for a screening tool for stress cardiomyopathy after SAH [20, 29, 35, 53,54,55,56].
DCI occurs unpredictably at 4–12 days after the initial hemorrhage in approximately 30% of patients [57] and is an important contributor to poor outcome. Many patients with SAH have hypovolemia and narrowed arteries, and cerebral perfusion autoregulation is disturbed after SAH [58, 59]. The total amount of extravasated blood, the duration of loss of consciousness at the time of ictus [60] and the occurrence of hypovolemia and hypotension [61] are powerful and independent predictors of DCI. The pathogenesis of DCI is usually attributed to vasospasm of the intracranial arteries. However, vasospasm cannot be the only cause of DCI because vasospasm does not exist in one third of patients with DCI, and DCI is not observed in one third of patients with severe vasospasm [62]. Novel pathological mechanisms, including microthrombosis, cortical spreading depression, and damage to cerebral tissue during the first 72 h after aneurysm rupture (“early brain injury”), have been suggested to contribute to DCI [63]. In the current study, we established an association between cardiac abnormalities and DCI after SAH.
These results should be interpreted with caution, as this study has several shortcomings. First, the inclusion of five retrospective studies introduces a high risk of bias in the data collection of retrospective observational studies. Second, the included studies were published over a period of 27 years. During this period, case fatality rates have decreased because the diagnosis and treatment of SAH have improved [1], possibly affecting the prevalence and consequence of cardiac complications on outcomes. Third, the baseline characteristics of the included studies and the prevalence of cardiac abnormalities and outcomes showed large variations, indicating differences in study populations that influence the results. Finally, the heterogeneity of the timing of assessment, the different clinical severity scores and the thresholds used to dichotomize cTn and BNP/NTproBNP may be complicating factors.
Conclusions
WMA and elevation of cTn, BNP, and NT-proBNP are associated with an increased risk of DCI, poor outcome, and death after SAH. Future research should be directed toward elucidating the pathophysiologic mechanisms and potential treatment options for improving outcomes for this vulnerable patient population.
References
Nieuwkamp DJ, Setz LE, Algra A, Linn FH, de Rooij NK, Rinkel GJ (2009) Changes in case fatality of aneurysmal subarachnoid haemorrhage over time, according to age, sex, and region: a meta-analysis. Lancet Neurol 8:635–642
van der Bilt IA, Hasan D, Vandertop WP, Wilde AA, Algra A, Visser FC, Rinkel GJ (2009) Impact of cardiac complications on outcome after aneurysmal subarachnoid hemorrhage: a meta-analysis. Neurology 72:635–642
Jaeger M, Soehle M, Schuhmann MU, Meixensberger J (2012) Clinical significance of impaired cerebrovascular autoregulation after severe aneurysmal subarachnoid hemorrhage. Stroke 43:2097–2101
Mayer SA, Lin J, Homma S, Solomon RA, Lennihan L, Sherman D, Fink ME, Beckford A, Klebanoff LM (1999) Myocardial injury and left ventricular performance after subarachnoid hemorrhage. Stroke 30:780–786
Cremers CH, van der Bilt IA, van der Schaaf IC, Vergouwen MD, Dankbaar JW, Cramer MJ, Wilde AA, Rinkel GJ, Velthuis BK (2016) Relationship between cardiac dysfunction and cerebral perfusion in patients with aneurysmal subarachnoid hemorrhage. Neurocrit Care 24:202–206
van Gijn J, Kerr RS, Rinkel GJ (2007) Subarachnoid haemorrhage. Lancet 369:306–318
Zhang L, Qi S (2016) Electrocardiographic abnormalities predict adverse clinical outcomes in patients with subarachnoid hemorrhage. J Stroke Cerebrovasc Dis 25:2653–2659
Hunt WE, Hess RM (1968) Surgical risk as related to time of intervention in the repair of intracranial aneurysms. J Neurosurg 28:14–20
[No authors listed] (1988) Report of World Federation of Neurological Surgeons Committee on a Universal Subarachnoid Hemorrhage Grading Scale. J Neurosurg 68:985–986
Teasdale G, Jennett B (1974) Assessment of coma and impaired consciousness. A practical scale. Lancet 2:81–84
Higgins JPT, Green S, eds (2011) Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]. The Cochrane Collaboration. Available at: http://www.cochrane-handbook.org. Accessed 10 January 2014
Pollick C, Cujec B, Parker S, Tator C (1988) Left ventricular wall motion abnormalities in subarachnoid hemorrhage: an echocardiographic study. J Am Coll Cardiol 12:600–605
Parekh N, Venkatesh B, Cross D, Leditschke A, Atherton J, Miles W, Winning A, Clague A, Rickard C (2000) Cardiac troponin I predicts myocardial dysfunction in aneurysmal subarachnoid hemorrhage. J Am Coll Cardiol 36:1328–1335
Deibert E, Barzilai B, Braverman AC, Edwards DF, Aiyagari V, Dacey R, Diringer M (2003) Clinical significance of elevated troponin I levels in patients with nontraumatic subarachnoid hemorrhage. J Neurosurg 98:741–746
Sviri GE, Shik V, Raz B, Soustiel JF (2003) Role of brain natriuretic peptide in cerebral vasospasm. Acta Neurochir 145:851–860
Naidech AM, Kreiter KT, Janjua N, Ostapkovich ND, Parra A, Commichau C, Fitzsimmons BF, Connolly ES, Mayer SA (2005) Cardiac troponin elevation, cardiovascular morbidity, and outcome after subarachnoid hemorrhage. Circulation 112:2851–2856
Schuiling WJ, Dennesen PJ, Tans JT, Kingma LM, Algra A, Rinkel GJ (2005) Troponin I in predicting cardiac or pulmonary complications and outcome in subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry 76:1565–1569
Banki N, Kopelnik A, Tung P, Lawton MT, Gress D, Drew B, Dae M, Foster E, Parmley W, Zaroff J (2006) Prospective analysis of prevalence, distribution, and rate of recovery of left ventricular systolic dysfunction in patients with subarachnoid hemorrhage. J Neurosurg 105:15–20
Kothavale A, Banki NM, Kopelnik A, Yarlagadda S, Lawton MT, Ko N, Smith WS, Drew B, Foster E, Zaroff JG (2006) Predictors of left ventricular regional wall motion abnormalities after subarachnoid hemorrhage. Neurocrit Care 4:199–205
Yarlagadda S, Rajendran P, Miss JC, Banki NM, Kopelnik A, Wu AH, Ko N, Gelb AW, Lawton MT, Smith WS, Young WL, Zaroff JG (2006) Cardiovascular predictors of in-patient mortality after subarachnoid hemorrhage. Neurocrit Care 5:102–107
Ramappa P, Thatai D, Coplin W, Gellman S, Carhuapoma JR, Quah R, Atkinson B, Marsh JD (2008) Cardiac troponin-I: a predictor of prognosis in subarachnoid hemorrhage. Neurocrit Care 8:398–403
Sandhu R, Aronow WS, Rajdev A, Sukhija R, Amin H, D'aquila K, Sangha A (2008) Relation of cardiac troponin I levels with in-hospital mortality in patients with ischemic stroke, intracerebral hemorrhage, and subarachnoid hemorrhage. Am J Cardiol 102:632–634
Sugimoto K, Watanabe E, Yamada A, Iwase M, Sano H, Hishida H, Ozaki Y (2008) Prognostic implications of left ventricular wall motion abnormalities associated with subarachnoid hemorrhage. Int Heart J 49:75–85
Hravnak M, Frangiskakis JM, Crago EA, Chang Y, Tanabe M, Gorcsan J 3rd, Horowitz MB (2009) Elevated cardiac troponin I and relationship to persistence of electrocardiographic and echocardiographic abnormalities after aneurysmal subarachnoid hemorrhage. Stroke 40:3478–3484
Miketic JK, Hravnak M, Sereika SM, Crago EA (2010) Elevated cardiac troponin I and functional recovery and disability in patients after aneurysmal subarachnoid hemorrhage. Am J Crit Care 19:522–528
Jyotsna M, Prasad V, Indrani G, Trikamji BV (2010) Importance of detection of segmental wall motion abnormalities of left ventricle in nontraumatic subarachnoid hemorrhage: a prospective study. Echocardiography 27:496–500
Ichinomiya T, Terao Y, Miura K, Higashijima U, Tanise T, Fukusaki M, Sumikawa K (2010) QTc interval and neurological outcomes in aneurysmal subarachnoid hemorrhage. Neurocrit Care 13:347–354
Temes RE, Tessitore E, Schmidt JM, Naidech AM, Fernandez A, Ostapkovich ND, Frontera JA, Wartenberg KE, Di Tullio MR, Badjatia N, Connolly ES, Mayer SA, Parra A (2010) Left ventricular dysfunction and cerebral infarction from vasospasm after subarachnoid hemorrhage. Neurocrit Care 13:359–365
Taub PR, Fields JD, Wu AH, Miss JC, Lawton MT, Smith WS, Young WL, Zaroff JG, Ko NU (2011) Elevated BNP is associated with vasospasm-independent cerebral infarction following aneurysmal subarachnoid hemorrhage. Neurocrit Care 15:13–18
Degos V, Apfel CC, Sanchez P, Colonne C, Renuit I, Clarençon F, Nouet A, Boch AL, Pourmohamad T, Kim H, Gourraud PA, Young WL, Puybasset L (2012) An admission bioclinical score to predict 1-year outcomes in patients undergoing aneurysm coiling. Stroke 43:1253–1259
Gupte M, John S, Prabhakaran S, Lee VH (2013) Troponin elevation in subarachnoid hemorrhage does not impact in-hospital mortality. Neurocrit Care 18:368–373
Kilbourn KJ, Levy S, Staff I, Kureshi I, McCullough L (2013) Clinical characteristics and outcomes of neurogenic stress cadiomyopathy in aneurysmal subarachnoid hemorrhage. Clin Neurol Neurosurg 115:909–914
van der Bilt HD, van den Brink R, Cramer MJ, van der Jagt M, van Kooten F, Meertens J, van den Berg M, Groen R, Ten Cate F, Kamp O, Götte M, Horn J, Groeneveld J, Vandertop P, Algra A, Visser F, Wilde A, Rinkel G (2014) Cardiac dysfunction after aneurysmal subarachnoid hemorrhage: relationship with outcome. Neurology 82:1–8
Kilbourn KJ, Ching G, Silverman DI, McCullough L, Brown RJ (2015) Clinical outcomes after neurogenic stress induced cardiomyopathy in aneurysmal sub-arachnoid hemorrhage: a prospective cohort study. Clin Neurol Neurosurg 128:4–9
Duello KM, Nagel JP, Thomas CS, Blackshear JL, Freeman WD (2015) Relationship of troponin T and age- and sex-adjusted BNP elevation following subarachnoid hemorrhage with 30-day mortality. Neurocrit Care 23:59–65
Banki NM, Kopelnik A, Dae MW, Miss J, Tung P, Lawton MT, Drew BJ, Foster E, Smith W, Parmley WW, Zaroff JG (2005) Acute neurocardiogenic injury after subarachnoid hemorrhage. Circulation 112:3314–3319
Lyon AR, Rees PS, Prasad S, Poole-Wilson PA, Harding SE (2008) Stress (takotsubo) cardiomyopathy: a novel pathophysiological hypothesis to explain catecholamine-induced acute myocardial stunning. Nat Clin Pract Cardiovasc Med 5:22–29
Tanabe M, Crago EA, Suffoletto MS, Hravnak M, Frangiskakis JM, Kassam AB, Horowitz MB, Gorcsan J 3rd (2008) Relation of elevation in cardiac troponin I to clinical severity, cardiac dysfunction, and pulmonary congestion in patients with subarachnoid hemorrhage. Am J Cardiol 102:1545–1550
Kono T, Morita H, Kuroiwa T, Onaka H, Takatsuka H, Fujiwara A (1994) Left ventricular wall motion abnormalities in patients with subarachnoid hemorrhage: neurogenic stunned myocardium. J Am Coll Cardiol 24:636–640
Mayer SA, LiMandri G, Sherman D, Lennihan L, Fink ME, Solomon RA, DiTullio M, Klebanoff LM, Beckford AR, Homma S (1995) Electrocardiographic markers of abnormal left ventricular wall motion in acute subarachnoid hemorrhage. J Neurosurg 83:889–896
Zaroff JG, Rordorf GA, Ogilvy CS, Picard MH (2000) Regional patterns of left ventricular systolic dysfunction after subarachnoid hemorrhage: evidence for neurally mediated cardiac injury. J Am Soc Echocardiogr 13:774–779
Nguyen H, Zaroff JG (2009) Neurogenic stunned myocardium. Curr Neurol Neurosci Rep 9:486–491
Brunetti ND, Ieva R, Rossi G, Barone N, De Gennaro L, Pellegrino PL, Mavilio G, Cuculo A, Di Biase M (2008) Ventricular outflow tract obstruction, systolic anterior motion and acute mitral regurgitation in Tako-Tsubo syndrome. Int J Cardiol 127:152–157
Burri MV, Nanda NC, Lloyd SG, Hsiung MC, Dod HS, Beto RJ, Bhardwaj R, Jain A, Jackson J, Agarwal A, Chaurasia P, Prasad AN, Manda J, Pothineni KR (2008) Assessment of systolic and diastolic left ventricular and left atrial function using vector velocity imaging in Takotsubo cardiomyopathy. Echocardiogr 25:1138–1144
Mansencal N, Abbou N, Pilliere R, El Mahmoud R, Farcot JC, Dubourg O (2009) Usefulness of two-dimensional speckle tracking echocardiography for assessment of Tako-Tsubo cardiomyopathy. Am J Cardiol 103:1020–1024
de Lemos JA (2013) Increasingly sensitive assays for cardiac troponins: a review. JAMA 309:2262–2269
Tung P, Kopelnik A, Banki N, Ong K, Ko N, Lawton MT, Gress D, Drew B, Foster E, Parmley W, Zaroff J (2004) Predictors of neurocardiogenic injury after subarachnoid hemorrhage. Stroke 35:548–551
Apple FS (1999) Tissue specificity of cardiac troponin I, cardiac troponin T and creatine kinase-MB. Clin Chim Acta 284:151–159
Thygesen K, Alpert JS, Jaffe AS et al (2012) Third universal definition of myocardial infarction. Circulation 126:2020–2035
Magga J, Vuolteenaho O, Tokola H, Marttila M, Ruskoaho H (1998) B-type natriuretic peptide: a myocyte-specific marker for characterizing load-induced alterations in cardiac gene expression. Ann Med 30(suppl 1):39–45
Magga J, Marttila M, Mantymaa P, Vuolteenaho O, Ruskoaho H (1994) Brain natriuretic peptide in plasma, atria, and ventricles of vasopressinand phenylephrine-infused conscious rats. Endocrinology 134:2505–2515
Sawada Y, Suda M, Yokoyama H, Kanda T, Sakamaki T, Tanaka S, Nagai R, Abe S, Takeuchi T (1997) Stretch-induced hypertrophic growth of cardiocytes and processing of braintype natriuretic peptide are controlled by proprotein-processing endoprotease furin. J Biol Chem 272:20545–20554
Oras J, Grivans C, Dalla K, Omerovic E, Rydenhag B, Ricksten SE, Seeman-Lodding H (2015) High-sensitive troponin T and N-terminal pro B-type natriuretic peptide for early detection of stress-induced cardiomyopathy in patients with subarachnoid hemorrhage. Neurocrit Care 23:233–242
Espiner EA, Leikis R, Ferch RD, MacFarlane MR, Bonkowski JA, Frampton CM, Richards AM (2002) The neuro-cardio-endocrine response to acute subarachnoid haemorrhage. Clin Endocrinol 56:629–635
Meaudre E, Jego C, Kenane N, Montcriol A, Boret H, Goutorbe P, Habib G, Palmier B (2009) B-type natriuretic peptide release and left ventricular filling pressure assessed by echocardiographic study after subarachnoid hemorrhage: a prospective study in non-cardiac patients. Crit Care 13:R76
Nyberg C, Karlsson T, Ronne-Engström E (2014) Predictors of increased cumulative serum levels of the N-terminal prohormone of brain natriuretic peptide 4 days after acute spontaneous subarachnoid hemorrhage. J Neurosurg 120:599–604
Lang EW, Diehl RR, Mehdorn HM (2001) Cerebral autoregulation testing after aneurysmal subarachnoid hemorrhage: the phase relationship between arterial blood pressure and cerebral blood flow velocity. Crit Care Med 29:158–163
Soehle M, Czosnyka M, Pickard JD, Kirkpatrick PJ (2004) Continuous assessment of cerebral autoregulation in subarachnoid hemorrhage. Anesth Analg 98:1133–1139
Hop JW, Rinkel GJE, Algra A, van Gijn J (1999) Initial loss of consciousness and risk of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. Stroke 30:2268–2271
Chang HS, Hongo K, Nakagawa H (2000) Adverse effects of limited hypotensive anesthesia on the outcome of patients with subarachnoid hemorrhage. J Neurosurg 92:971–975
Uekusa H, Miyazaki C, Kondo K, Harada N, Nomoto J, Sugo N, Nemoto M (2014) Hydroperoxide in internal jugular venous blood reflects occurrence of subarachnoid hemorrhage-induced delayed cerebral vasospasm. J Stroke Cerebrovasc Dis 23:2217–2224
Rabinstein AA, Friedman JA, Weigand SD, McClelland RL, Fulgham JR, Manno EM, Atkinson JL, Wijdicks EF (2004) Predictors of cerebral infarction in aneurysmal subarachnoid hemorrhage. Stroke 35:1862–1866
Rowland MJ, Hadjipavlou G, Kelly M, Westbrook J, Pattinson KT (2012) Delayed cerebral ischaemia after subarachnoid haemorrhage: looking beyond vasospasm. Br J Anaesth 109:315–329
Funding
This work was supported by Harbin Science and Technology Bureau (grant numbers 2013RFQYJ078).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Informed consent
For this type of study, informed consent is not required.
Rights and permissions
About this article
Cite this article
Zhang, L., Zhang, B. & Qi, S. Impact of echocardiographic wall motion abnormality and cardiac biomarker elevation on outcome after subarachnoid hemorrhage: a meta-analysis. Neurosurg Rev 43, 59–68 (2020). https://doi.org/10.1007/s10143-018-0985-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-018-0985-6