Abstract
The aneurysmal subarachnoid hemorrhage is a major public health problem described as a sudden drastic event with no warning symptoms and high morbidity and mortality rates. The role of the endothelial isoform of nitric oxide synthase gene polymorphism in intracranial aneurysms (IAs) is still a matter of controversy with divergent findings among European, American, and Asian populations. Our study purposed to test the association between intracranial aneurysms formation and nitric oxide gene polymorphisms through a systematic review and meta-analysis. Systematic search on Medline, Lilacs, and EMBASE was performed. The primary search resulted in 139 papers, out of which 9 met our inclusion criteria after a full text analysis. The dominant T786C model found a significant association with IA (OR 1.22, 95 % CI 1.04–1.44, p = 0.01), so did studies of the recessive T786C model (OR 0.37, 95 % CI 0.30–0.45, p < 0.0001) but with opposite effect. Our findings support the presence of the T786C polymorphism as a predictor for the development of intracranial aneurysm in the cerebral vascular system. More studies are necessary in order to elucidate the pathways of the endothelial nitric oxide synthase (eNOS) in cerebrovascular diseases and in defining how different allelic combinations of the eNOS gene single-nucleotide polymorphism (SNP) could favor this pathological process.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The aneurysmal subarachnoid hemorrhage (aSAH) is a major public health problem characterized by a sudden drastic event, which is not preceded by warning signs, and withholds high morbidity and mortality rates [36]. The prevalence of an aSAH is 10 to 11 per 100.000, with a rupture rate varying from 0.05 to 6 % per year [10, 11, 13]. Half of these patients die or become disabled with severe neurological deficits [18].
The sudden and unexpected onset of this condition, as well as the high costs of treatment and screening, have encouraged the search of susceptible elements that can possibly predict an aneurysmal rupture. With this purpose, the International Study of Unruptured Intracranial Aneurysm (ISUIA) [11] first substantiated a higher rupture rate in aneurysms over 10 mm in diameter and located in the posterior circulation. In same manner, several reports identified environmental insults (such as risk factors sex, age, smoking, and hypertension) and genetic factors to influence in the formation and rupture of intracranial aneurysms (IA) [11, 32].
In 2003, Khurana et al. questioned, “Can brain aneurysms that are more prone to rupture be identified genetically?” This was the first report that suggested genetic markers for patients with ruptured IA, which mainly targeted the genetic polymorphisms of the endothelial isoform of the nitric oxide synthase (eNOS) [14–16].
Endothelial cells express eNOS to serve as an important source of nitric oxide (NO), a potent vasodilator, inflammation inhibitor, an important element in smooth muscle cell proliferation, and platelet aggregation [3, 6, 7, 12, 13]. The eNOS gene is known as functionally polymorphic, which can result in a decreased expression of the enzyme and subsequently a decreased production of NO.
Experimental studies with the knockout of the eNOS gene in mice report a higher susceptibility for several vascular diseases [13]. Additionally, various clinical reports have shown an association between the intimate eNOS polymorphisms with cardiovascular diseases such as carotid atherosclerosis [24], coronary vasospasms [28], hypertension, and acute myocardial infarction (AMI) [9].
The following three types of eNOS gene polymorphism are described in literature: the T786C, the G894T, and the 27 VNTR, and each one is related to a vascular disease. The T786C is a single-nucleotide polymorphism (SNP) located on the gene promoter region, characterized by the substitution of thiamine (T) for citosyne (C) at the 786th paired base upstream of the eNOS gene. This SNP has been described in association to coronary vasospasms [28]. The G897T is an SNP located on exon-7 of the eNOS gene and is related to AMI and carotid atheroma [9, 24]. The last polymorphism is a variable number tandem repeat (VNTR) located in intron-4 of the gene and consists of either four or five 27-base pair (bp) repetitions. This one is associated with AMI [37] and aortic aneurysm [21].
The role of eNOS gene polymorphisms in IA is still a matter of controversy with divergent findings among European, American, and Asian populations. Therefore, the purpose of this study was to study the relationship between the formation of IA and eNOS gene polymorphisms by performing a systematic literature search and a quantitative analysis of the available scientific studies.
Materials and methods
Data sources
Electronic searches using Medline, Lilacs, Cochrane, and EMBASE were conducted to identify all published case-control studies reporting genetic polymorphisms of eNOS in IAs and subarachnoid hemorrhage (SAH) in humans published since and including January 2000. Letters and abstracts were included in the searches. The Medical Subject Headings and text words used for the search were “intracranial aneurysm,” “saccular aneurysm,” or “subarachnoid hemorrhage” in combination with “genetics,” “endothelial nitric oxide synthase,” “gene,” “single-nucleotide polymorphism,” “oxide nitric synthase,” “polymorphisms,” or “genetic linkage.” Search results were limited to humans. Studies in English, Portuguese, Spanish, German, and Dutch languages were subsumed and searched. The references of all identified publications were hand-searched for additional studies, and the “related articles” option in Medline and Lilacs was used to examine all additional relevant articles.
Study selection
Selection criteria included case-control studies in which the aneurysm was characterized as a dichotomous trait. Studies were only selected if an unruptured aneurysm was diagnosed using conventional angiography, 3D CT scanning, MR imaging, or MR angiography. The SAH studies were included if the hemorrhage was verified by lumbar puncture, CT scanning, or during surgical repair. Studies were excluded if (i) patients had a history of connective tissue disorder or polycystic disease, (ii) the genotype frequency was not reported and could not be obtained by contacting authors, and (iii) quantitative traits or intermediate phenotypes were investigated. For studies with >1 publication describing results among the same or overlapping groups of patients or controls, only the largest of the available published data sets were included.
Statistical Analysis
Data was analyzed using software for preparing and maintaining Cochrane reviews (Review Manager version 5.0, Cochrane Collaboration, http://www.cc-ims.net/RevMan) and the software BioEstat© 5.3 (Instituto Mamirauá, Brazil, http://www.mamiraua.org.br/pt-br/downloads/programas/). To determine the strength of genetic association, a pooled OR was calculated for each eNOS gene variant by using fixed- and random-effect models, in addition to the calculation of 95 % CIs. Fixed-effect summary ORs were calculated using the Mantel-Haenszel method, and the Der Simonian and Laird method was used to calculate random-effect summary ORs. The frequencies of at-risk genotypes were compared between cases and controls for each single-nucleotide polymorphism analyzed. Tests for heterogeneity were performed for each polymorphic meta-analysis with significance set at p ≤ 0.05.
Results
Number of studies retrieved
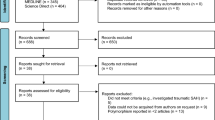
The primary search resulted in 139 papers, out of which 22 met the initial inclusion criteria. After complete text analysis, only nine met the inclusion and exclusion criteria (Fig. 1). Country, year of publication, number of cases and controls, and the mean age of the subjects are summarized in Table 1.
Characteristics of the studied population
Seven studies associated gender and the occurrence of aneurysm rupture. These studies included 799 women and 475 men in the case group and 460 women and 525 men in the control group. The groups possessed a significant statistical difference, which affirms a higher risk in females (OR 1.59, 95 % CI 1.34–1.89, p < 0.0001). The average age in these seven studies suggests that aneurysm rupture is associated with a higher mean age in comparison to the control group (OR 4.89, 95 % CI 3.92–5.87, p < 0.0001).
Four studies associated smoking and aneurysm rupture, which identified 162 smokers among 376 cases and 92 smokers among the 495 controls. A significant difference was found regarding this risk factor (OR 3.83, 95 % CI 2.28–6.43, p < 0.0001), which affirms smokers to have a higher risk of aneurysm rupture.
Four studies associated hypertension and aneurysm rupture, which identified 163 hypertensive patients among the 376 cases and 132 among the 495 controls. However, there was no significant difference regarding this risk factor (OR 1.85, 95 % CI 0.80–4.27, p = 0.14).
Four studies associated diabetes and aneurysm rupture, which identified 31 diabetics among the 376 cases and 68 among the 495 controls. A significant difference between the two groups (OR 0.52, 95 %, CI 0.35–0.78, p = 0.0018) suggests that diabetics are at lower risk of developing aneurysms.
Seven studies evaluated the T786C polymorphism and its association with ruptured/unruptured IA. In assessing the dominant T786C model, there was a significant association with IA (OR 1.21, 95 % CI 1.12–1.30, p < 0.0001). However, the recessive T786C model showed no significant association (OR 0.93, 95 % CI 0.54–1.60, p = 0.80).
For the G894T polymorphism, six studies associated it with ruptured/unruptured IA. No significant association was found in either the dominant model (OR 0.92, 95 % CI 0.69–1.22, p = 0.57) or the recessive model (OR 0.88, 95 % CI 0.45–1.69, p = 0.70).
Five studies evaluated the 27VNTR eNOS polymorphism and its association with ruptured/unruptured IA. No significant association was observed in the recessive model (OR 1.16, 95 % CI 0.58–2.31, p = 0.66); in counterpart, in the dominant model, a significant association was observed (OR 1.17, 95 % CI 0.78–1.74, p = 0.42), suggesting the mutation to serve as a protective factor for ruptured IA.
Four studies evaluated the T786C polymorphism and its association with aSAH. In assessing the dominant T786C model, there was a significant association with IA (OR 1.22, 95 % CI 1.04–1.44, p = 0.01). The recessive T786C model showed significant association (OR 0.37, 95 % CI 0.30–0.45, p < 0.0001), however, with an opposite effect.
For the G894T polymorphism, four studies evaluated its association with aSAH. There was no significant association found in either the dominant model (OR 0.85, 95 % CI 0.57–1.27, p = 0.44) or the recessive model (OR 0.93, 95 % CI 0.37–2.29, p = 0.88).
Three studies evaluated the 27VNTR eNOS polymorphism and its association with aSAH. No significant association was observed in the recessive model (OR 1.41, 95 % CI 0.80–2.47, p = 0.23), neither in the dominant model (OR 1.47, 95 % CI 0.75–2.86, p = 0.25).
The meta-analysis outcomes are summarized in Table 2.
Discussion
This comprehensive meta-analysis provides a detailed study about the role of the T786C, G894T, and 27VNTR eNOS polymorphisms related to an increased risk of IA. After critical analysis of the retrieved studies, we identified significant evidence regarding the T786C polymorphism and the occurrence of intracranial aneurysms and evolution to subarachnoid hemorrhage.
Recent advances in genetic disorders have revealed several alterations in genes and its protein products to be involved in IA formation and rupture [16, 18]. The main advantages of a genetic screening diagnostic tool would be the non-invasiveness, low complexity, and low costs, when compared to current imaging techniques used, such as CT angiography or MR angiography [18].
There are several case-control studies using an allelic-association model in IA that have identified gene polymorphisms, such as eNOS, ACE, endoglin, APOE, IL-6, and MMP-3, related to cerebral aneurysms [2, 25, 27, 30]. However, none have led to definitive findings due to the limited number of studies in the recent database.
The genetic eNOS polymorphism has been extensively studied, since 2003, based on the following three types of genetic abnormalities: T786C, G894T, and 27 VNTR. These studies reported the association between these polymorphisms with the following four events: aneurysm formation, aneurysm size, subarachnoid hemorrhage, and vasospasm following aSAH.
Genetic eNOS polymorphism X aneurysm formation
Eight studies evaluated the relation between eNOS gene polymorphisms and aneurysm formation. These studies comprised patients with ruptured/unruptured IA and healthy volunteers from six different countries (USA, Japan, Korea, India, Germany, and Turkey). In addition to the known functions of NO in vascular tone, platelet regulation, and cerebral blood flow [8, 26], experimental studies recently found NO signaling impairment preceding induced SAH episode in rats and that NO knocked-out mice are more prone to various vascular diseases [13]. Furthermore, increased NO levels in the cerebrospinal fluid have been reported in patients after SAH [22, 31].
Ozum et al. (2008) [29] and Khurana et al. (2004) [17], respectively, described a statistical association of the G984T and the 27VNTR polymorphisms with the presence of intracranial aneurysms. However, in both studies, the patients presented ruptured intracranial aneurysms and the controls were healthy individuals.
Recently, a meta-analysis [25] evaluating the genetics of ruptured and unruptured IA was published. Significant findings support the relation of the dominant T786C polymorphism model (T/T vs T/C and C/C; OR 1.24, 95 % CI 1.0–1.54, p = 0.05) with the development of IA. Alternatively, in the recessive model (T/T and T/C vs C/C), no statistical difference was found (OR 0.83, 95 % CI 0.38–1.83, p = 0.64). Although the number of studies in this meta-analysis was low, the average number of cases (1134) and controls (968) were suitable and there was no evidence of publication bias.
Our results are consistent with this previous meta-analysis demonstrating a positive relation between the T786C polymorphism (dominant model) and the occurrence of IA (OR 1.24, 95 % CI 1.21–1.28, p < 0.0001). Our meta-analysis included 1416 cases and 1289 controls providing a more accurate estimate of this genetic association compared to previously published studies (Figs. 1 and 2).
eNOS polymorphism X aneurism size
Studies about the natural history of intracranial aneurysms have suggested multiple risk factors that may determine which aneurysms are more prone to rupture. Among these factors, the ISUIA results indicated an aneurysm diameter ≥10 mm in patients with no previous SAH and the posterior location as independent predictors of rupture [11].
However, Forget et al (2001) [5] reported that the ISUIA findings are contended by the fact that the majority of ruptured aneurysms are <10 mm in diameter and proposed to drop the aneurysm size out as a sole criterion to guide decisions about unruptured IA treatment. These authors hypothesized about two subpopulations of intracranial aneurysms: (1) aneurysms with a fast development and rupture when >10 mm in size and (2) aneurysm with a slow growth, which are amenable to being studied over months or years by serial imaging, and are more prone to rupture when <10 mm [5, 16].
Based on this controversial issue, Khurana et al. (2003) [16] suggested a genetic contribution as the answer to the different subpopulations of IA. A genetic distinction between large and small ruptured aneurysms could be a factor influencing the size at which rupture occurs. Although they found no significant difference in the distribution of eNOS T786C SNP genotypes between cases and controls, their data suggested that homozygosity vs heterozygosity for this polymorphic gene could differentiate between small- and large-diameter ruptured aneurysms. The eNOS heterozygous genotype remained significantly associated with aneurysm size >10 mm in diameter (p = 0.03) and presented aneurysm mean diameter (8.5 ± 5.2) significantly larger than those of T/T (4.7 ± 1.8 mm) and C/C (6.0 ± 2.3 mm) homozygotes [16].
These authors [16] suggested that in heterozygous genotypes, some interactions between normal and mutant alleles could result in abnormal expression of the eNOS. This difference in the eNOS function may affect the response of the vessel wall to withstand aneurysmal dilatation, expressing the aneurysmal rupture at markedly different aneurysm sizes. However, this suggestion was made based on a relatively small size study (case group n = 52).
On the other hand, these results were not confirmed by Krex et al. (2006) [22] and by Akagawa et al. (2005) [1], who showed an insignificant tendency for the mutant allele (C) to be more frequent in patients with IA >10 mm in size, with no difference between homozygous and heterozygous individuals.
Akagawa et al. (2005) [1] studied the T786C SNP in two different populations, Japanese and Koreans, and was careful to select homogenous cases and controls. Nevertheless, there was a difference regarding the genotype frequency between Asian control population and control populations of Caucasian origin (European—Krex et al. 2006 [22] and North American—Khurana et al. 2003 [16]), with a higher frequency on the C allele in Caucasians. This data may be attributable to ethnically related differences in allele frequencies.
This same negative association between eNOS gene SNP and aneurysm size was reported by Krex et al. (2006) [22] in a European population analysis. In the comparison between Khurana and Krex findings, the difference related to the ethnic background owing that both were performed in Caucasian origin populations can be excluded. On the other hand, the difference in genotype distribution could be influenced by environmental factors such as smoking, hypertension, and gender and by the studied population size.
Due to the limited amount of studies and to the methodological differences, a statistical assessment was not feasible.
Genetic eNOS polymorphism X aneurysmal subarachnoid hemorrhage
The unexpected and sudden occurrence of a SAH has motivated the recent efforts toward unraveling a genetic marker associated with an increased risk of IA rupture. Due to its several functions in blood vessels, the eNOS is intensively studied, under the hypothesis that an abnormal enzymatic expression could be determinant to a genetic susceptibility to aneurysmal rupture.
The most promising data was described by Khurana et al. (2004) [17], which identified an increased susceptibility of rupture in the presence of the eNOS 27VNTR, notably related to the presence of the “4a” allele. This study found a significant difference in the distribution of genotypes between SAH cases and controls (p = 0.002) and the OR between cases and controls for those with at least one 4a allele was 3.95 (95 % CI 1.45–10.56, p = 0.007).
This association was also statistically significant in Ozum et al. (2008) data [29], which investigated the G894T polymorphism in patients with ruptured IA in a Turkish population. There was a significant relationship of the eNOS recessive model (TT vs TG and GG) and the presence of aneurysmal SAH (OR 2.51, 95 % CI 1.12–5.65, p = 0.02, as well an increased frequency of the T allele in the cases group (OR 1.78, 95 % IC 1.04–3.05, p = 0.02).
Krischek et al. (2006) [23] tried to replicate these findings in a Japanese population submitting 297 patients with SAH, 108 patients with UA, and 176 unrelated volunteers. However, they did not find, for the three polymorphisms (T786C, G894T, 27VNTR), any statistical difference within the alleles frequency between the groups. Although the ethnic background cannot be excluded, several clinical differences between both populations may have contributed to these divergent results. Among the Japanese population included a lower percentage of women, a higher number of patients with familial history of IA or SAH and a lower number of patients with multiple aneurysms.
Other studies carried out in distinct populations (Korean, Indian, Japanese) could also not predict the susceptibility to SAH caused by eNOS gene polymorphism.
A single interestingly finding reported by Song et al. (2006) [34] presented an unfavorable outcome (Glasgow outcome scale 3–5) after SAH for patients with the eNOS T786C SNP T/C genotype (heterozygous) with an OR = 4.27 (95 % CI 1.12–16.11, p = 0.032) working as a prognostic marker in individuals with SAH.
In 2008, a meta-analysis analyzed four of these studies above to evaluate the relationship between eNOS gene T786C polymorphism and aSAH and found no association. The OR for the dominant carriers was 1.27 (95 % CI 0.99–1.62, p = 0.06) [30].
On the other hand, our findings showed a significant association between the T786C polymorphism and aSAH in assessing the polymorphism dominant model (OR 1.22, 95 % CI 1.04–1.44, p = 0.01). Our meta-analysis included a larger number of patient from five studies, with a total of 602 aSAH individuals and 603 controls. This positive correlation was not found by Peck et al (2008) [30] with a p value quite distant to the significant rates, whereas our significant results were reached with the sample addition.
Genetic eNOS polymorphism X Pós-SAH cerebral vasospasm
Despite the many advances in understanding the pathophysiology of the delayed cerebral vasospasm after SAH, this event is still one of the most mysterious vascular events in regard its development and role in neurological deficits [17, 19, 35]. Until now, the only predictor for the occurrence of cerebral vasospasm following SAH is the amount of blood in the subarachnoid space in early head CT after bleeding. However, the clinical neurological consequences following this event vary markedly between patients with similar radiological grades of SAH [4].
Since the role of nitric oxide in cerebral vasospasm (after SAH) was discovered, this turned into a subject of study for neuroscientists [9, 21, 33, 37]. Therefore, genes encoding NO isoforms may exhibit aberrations that could account for individual differences in vasomotor responses following aneurysmal rupture.
The first study regarding this issue was designed by Khurana et al. (2004) [17], in which they found a significant association between Fisher’s grade 3 SAH with the development of cerebral vasospasm, and also that the majority of these patients were heterozygous for the eNOS T786C SNP. In addition, the prevalence of the C allele showed a significant association with the occurrence and severity of cerebral vasospasm, represented in 80 % of the patients with asymptomatic vasospasm and in 100 % of the patients with symptomatic vasospasm.
The next published data also confirmed a role of the T786C SNP in the cerebral vasospasm pathophysiology. Ko et al. (2008) [19] showed a strong association of the CC genotype with high-risk patients to develop cerebral vasospasm, when compared with any T genotype—CT or TT (OR 2.07, 95 % CI 1.32–6.67, p = 0.008). In contrast, the previous study with Caucasians as well reported the highest risk for heterozygous patients (CT genotype) [17]. Even though the study by Ko et al. presented some strengths such as a large population (n = 319), a prospective cohort design, and a detailed radiographic outcome assessment, future studies are required to elucidate these findings.
Starke et al. (2008) [35] did a multivariate logistic regression analysis, which denoted, in contrary to previous data, the T allele as a significant predictor of vasospasm. This hypothesis was affirmative in general vasospasm (OR 3.04, 95 % CI 1.3–9.0, p = 0.013), in symptomatic vasospasm (OR 3.3, 95 % CI 1.1–10.0, p = 0.034); in angiographic vasospasm (OR 3.6, 95 % CI 1.3–9.6, p = 0.013), and in severe cerebral vasospasm requiring endovascular intervention (OR 3.5, 95 % CI 1.3–9.5, p = 0.016).
The main published articles about the genetic prediction of cerebral vasospasm following SAH provided such controversial findings. While Khurana et al. (2004) [17] presented higher risk in heterozygous (C/T) genotype patients, Ko et al. (2008) [19] found a significant relation with C homozygous (C/C) patients, and Starke et al. (2008) [35] proposed the T allele as a powerful predictor of vasospasm and its severity. These different findings may be due to inadequate power, different incidences of alleles, or prevalence of vasospasm; however, the ethnicity variable was excluded since all these studies included only Caucasian patients in the sample analysis.
The clinical suspicion and confirmation of cerebral vasospasm through neurologic presentation elevated transcranial Doppler velocities and ratios, and angiography are not uniformly recorded in the reviewed studies. Khurana et al. (2004) [17] included and distributed the patients in groups by positive predictors in neurological examination, radiological findings, and/or increased flow in the transcranial Doppler ultrasonography. In the other two studies, the inclusion criteria were more strict excluding other causes of clinical deterioration (hydrocephalus, seizures, rebleeding, or metabolic disturbances). Adding to that, these patients were managed in a neurological intensive care unit and the follow-up with cerebral angiography was just performed to document the vasospasm if the clinical status persisted for a determined period of time.
Furthermore, another critical divergence detected was the difference in methods employed for the genetic analysis, with Starke et al. (2008) [35] using genomic DNA extracted from oral swabs and other studies using DNA extracted from peripheral blood lymphocytes.
Therefore, due to these different research strategies and different ways to assign participants into groups, this meta-analysis did not include these studies in the statistical evaluation to avoid select bias.
Participant ethnicity
The well-known influence of different ethnicity on genotype expression is reflected in the divergent findings of the presented studies in this meta-analysis. The majority of the studies analyzed (55 %) were performed with Asian descendant participants, which tend to have a clear high prevalence of C/C genotype. Caucasians, on the other hand, showed a more homogenous distribution, with high frequencies of heterozygous (T/C) genotypes. Therefore, the ethnicity should be taken into account before conclusions can be made about how genetics influence in such a complex disease.
Meta-analysis limitations
Even though the number of included studies was limited, the pool of studied population summed over 2000, which allows a more precise estimate to be made of the effect of these genes in comparison to a single study.
In fact, the interpretation of any meta-analysis must be made within the context of its limitations, including study selection, publication bias, variability in the methods, and quality of the chosen articles. Egger symmetry tests and funnel plots showed no substantial evidence of publication bias. Although the design of the participant groups varied considerably between the studies, this was overridden with separate analysis according to a specific outcome (aneurysmal formation, aneurysm size, and SAH).
Our findings support the presence of the T786C SNP as a predictor for the development of intracranial aneurysm in the cerebral vascular system. However, this comprehensive meta-analysis could not come to any conclusion about an effect of an eNOS gene SNP in cerebral vasospasm. Therefore, more studies are necessary in order to elucidate the pathways of the eNOS in cerebrovascular diseases and in defining how different allelic combinations of the eNOS gene SNP could favor this pathological process. These findings will allow clinicians to be the forerunners in optimizing the early treatment for patients with a high genetic risk.
References
Akagawa H, Kasuya H, Onda H, Yoneyama T, Sasahara A et al (2005) Influence of endothelial nitric oxide synthase T-786C single nucleotide polymorphism on aneurysm size. J Neurosurg 102:68–71
Caranci F, Briganti F, Cirillo L, Leonardi M, Muto M (2013) Epidemiology and genetics of intracranial aneurysms. Eur J Radiol 82:1598–1605
Chiang TM, Cole F, Woo-Rasberry V, Kang ES (2001) Role of nitric oxide synthase in collagen-platelet interaction—involvement of platelet nonintegrin collagen receptor nitrotyrosylation. Thromb Res 102:343–352
Fisher CM, Kistler JP, Davis JM (1980) Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by CT scanning. Neurosurgery 6:1–9
Forget TR Jr, Benitez R, Veznedaroglu E, Sharan A, Mitchell W, Silva M, Rosenwasser RH (2001) A review of size and location of ruptured intracranial aneurysms. Neurosurgery 49:1322–1326
Garg UC, Hassid A (1989) Nitric oxide-generating vasodilators and 8-bromo-cyclic guanosine monophosphate inhibit mitogenesis and proliferation of cultured rat vascular smooth muscle cells. J Clin Invest 83:1774–1777
Gurjar MV, Sharma RV, Bhalla RC (1999) eNOS gene transfer inhibits smooth muscle cell migration and MMP-2 and MMP-9 activity. Arterioscler Thromb Vasc Bio 19:2871–2877
Guzik TJ, West NE, Pillai R, Taggart DP, Channon KM (2002) Nitric oxide modulates superoxide release and peroxynitrite formation in human blood vessels. Hypertension 39:1088–1094
Hingorani AD (2003) Endothelial nitric oxide synthase polymorphisms and hypertension. Curr Hypertens Rep 5:19–25
Ingall TJ, Whisnant JP, Wiebers DO, O’Fallon WM (1989) Has there been a decline in subarachnoid hemorrhage mortality? Stroke 20:718–724
ISUIA Wiebers, DO, International Study of Unruptured Intracranial Aneurysms Investigators (2003) Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet 362:103–110
Jellinger K (1977) Pathology of intracerebral hemorrhage. Zentralbl Neurochir 38:29–42
Kuhlencordt PJ, Gyurko R, Han F, Scherrer-Crosbie M, Aretz TH et al (2001) Accelerated atherosclerosis, aortic aneurysm formation, and ischemic heart disease in apolipoprotein E/endothelial nitric oxide synthase double-knockout mice. Circulation 104:448–454
Khurana VG, Besser M (1997) The pathophysiological basis of cerebral vasospasm following aneurysmal subarachnoid haemorrhage. J Clin Neurosci 4:122–131
Khurana VG, Smith LA, Baker TA, Eguchi D, O’Brien T, Katusic ZS (2002) Protective vasomotor effects of in vivo recombinant endothelial nitric oxide synthase gene expression in a canine model of cerebral vasospasm. Stroke 33:782–789
Khurana VG, Sohni YR, Mangrum WI, McClelland RL, O’Kane DJ et al (2003) Endothelial nitric oxide synthase T-786C single nucleotide polymorphism: a putative genetic marker differentiating small versus large ruptured intracranial aneurysms. Stroke 34:2555–2559
Khurana VG, Sohni YG, Mangrum WI, McClelland RL, O’Kane DJ et al (2004) Endothelial nitric oxide synthase gene polymorphisms predict susceptibility to aneurysmal subarachnoid hemorrhage and cerebral vasospasm. J Cereb Blood Flow Metab 24:291–297
Kim TG, Kim NK, Baek MJ, Huh R, Chung SS, Choi JU et al (2011) The relationships between endothelial nitric oxide synthase polymorphisms and the formation of intracranial aneurysms in the Korean population. Neurosurg Focus 30, E23
Ko NU, Rajendran P, Kim H, Rutkowski M, Pawlikowska L, Kwok PY, Young WL (2008) Endothelial nitric oxide synthase polymorphism (−786T → C) and increased risk of angiographic vasospasm after aneurysmal subarachnoid hemorrhage. Stroke 39(4):1103–1108
Koshy L, Easwer HV, Neetha NV, Natarajan C, Bhattacharya RN, Banerjee M (2008) Role of endothelial nitric oxide synthase gene polymorphisms in predicting aneurysmal subarachnoid hemorrhage in South Indian patients. Dis Markers 24:333–339
Kotani K, Shimomura T, Murakami F, Ikawa S, Kanaoka Y, Ohgi S et al (2000) Allele frequency of human endothelial nitric oxide synthase gene polymorphism in abdominal aortic aneurysm. Intern Med 39:537–539
Krex D, Fortun S, Kuhlisch E, Schackert HK, Schackert G (2006) The role of endothelial nitric oxide synthase (eNOS) genetic variants in European patients with intracranial aneurysms. J Cereb Blood Flow Metab 26:1250–1255
Krischek B, Kasuya H, Akagawa H, Tajima A, Narita A et al (2006) Using endothelial nitric oxide synthase gene polymorphisms to identify intracranial aneurysms more prone to rupture in Japanese patients. J Neurosurg 105:717–722
Lembo G, De Luca N, Battagli C, Iovino G, Aretini A, Musicco M et al (2001) A common variant of endothelial nitric oxide synthase (Glu298Asp) is an independent risk factor for carotid atherosclerosis. Stroke 32:735–740
McColgan P, Thant KZ, Sharma P (2010) The genetics of sporadic ruptured and unruptured intracranial aneurysms: a genetic meta-analysis of 8 genes and 13 polymorphisms in approximately 20,000 individuals: clinical article. J Neurosurg 112:714–721
Moncada S, Palmer RMJ, Higgs EA (1991) Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev 43:109–142
Nahed BV, Bydon M, Ozturk AK, Bilguvar K, Bayrakli F, Gunel M (2007) Genetics of intracranial aneurysms. Neurosurgery 60:213–226
Nakayama M, Yasue H, Yoshimura M, Shimasaki Y, Kugiyama K, Ogawa H et al (1999) T-786C mutation in the 5-flanking region of the endothelial nitric oxide synthase gene is associated with coronary vasospasm. Circulation 99:2864–2870
Özüm Ü, Bolat N, Gül E, Özdemir Ö (2008) Endothelial nitric oxide synthase gene [G894T] polymorphism as a possible risk factor in aneurysmal subarachnoid haemorrhage. Acta Neurochir 150:57–62
Peck G, Smeeth L, Whittaker J, Casas JP, Hingorani A, Sharma P (2008) The genetics of primary haemorrhagic stroke, subarachnoid haemorrhage and ruptured intracranial aneurysms in adults. PLoS One 3(11), e3691
Pluta RM (2005) Delayed cerebral vasospasm and nitric oxide—review, new hypothesis, and proposed treatment. Pharmacol Ther 105:23–56
Rinkel GJ, Djibuti M, Algra A, van Gijn J (1998) Prevalence and risk of rupture of intracranial aneurysms: a systematic review. Stroke 29:251–256
Rudic RD, Shesely EG, Maeda N, Smithies O, Segal SS, Sessa WC (1998) Direct evidence for the importance of endothelium-derived nitric oxide in vascular remodeling. J Clin Invest 101:731–736
Song MK, Kim MK, Kim TS, Joo SP, Park MS et al (2006) Endothelial nitric oxide gene T-786C polymorphism and subarachnoid hemorrhage in Korean population. J Korean Med Sci 21:922–926
Starke RM, Kim GH, Komotar RJ, Hickman ZL, Black EM et al (2008) Endothelial nitric oxide synthase gene single-nucleotide polymorphism predicts cerebral vasospasm after aneurysmal subarachnoid hemorrhage. J Cereb Blood Flow Metab 28:1204–1211
Sundt TM Jr, Whisnant JP (1978) Subarachnoid hemorrhage from intracranial aneurysms: surgical management and natural history of disease. N Engl J Med 299:116–122
Wang XL, Wang J (2000) Endothelial nitric oxide synthase gene sequence variations and vascular disease. Mol Genet Metab 70:241–251
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
eNOS and Intracranial Aneurysms
Rights and permissions
About this article
Cite this article
Paschoal, E.H.A., Yamaki, V.N., Teixeira, R.K.C. et al. Relationship between endothelial nitric oxide synthase (eNOS) and natural history of intracranial aneurysms: meta-analysis. Neurosurg Rev 41, 87–94 (2018). https://doi.org/10.1007/s10143-016-0761-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-016-0761-4