Abstract
The treatment for patients with near occlusion of the cervical internal carotid artery (ICA) is controversial. The aim of this study was to examine the results of carotid artery stenting (CAS) as a surgical treatment for ICA near occlusion. Between April 2008 and September 2012, 14 patients (all men; mean age, 75.4 years) with ICA near occlusion were treated with CAS. This represents 5.2 % of a total of 267 patients treated with CAS during the study period. All patients were treated with CAS using an embolic protection device. The proximal balloon protection method was performed in five patients, and the dual protection method using a proximal balloon and distal filter protection was used in nine patients. We examined the change of stenotic lesion, hyperintensity spot in diffusion-weighted imaging (DWI), and perioperative complications after CAS. All near occlusions were successfully dilated. Among 2 of 14 patients, DWI showed 1 and 4 hyperintensity spots. Transient and persistent complications, including neurological deficits, did not occur in any patients. In this small number of cases, CAS using the proximal or dual embolic protection method seems to be a safe and beneficial treatment for ICA near occlusion.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Near occlusion is defined as a severe stenosis of the internal carotid artery (ICA) with a narrow residual lumen and a collapsed distal portion induced by hypoperfusion [7]. The management of patients with near occlusion remains obscure. From analyses of the North American Symptomatic Carotid Endarterectomy Trial (NASCET) [15] and European Carotid Surgery Trial (ECST) data [2], Rothwell et al. [17] reported that the rate of stroke in patients with near occlusion was low, and carotid endarterecomy (CEA) was of little benefit in patients with near occlusion. However, in another analysis of NASCET, Morgenstern et al. [13] reported that the 1-year stroke risk in patients with near occlusion with string treated medically and surgically were 11.1 % and 6.7 %, respectively, and CEA reduced the stroke risk, regardless of the degree of stenosis or the subcategory of carotid near occlusion. Therefore, surgical treatment for patients with near occlusion remains controversial. However, it has been shown that carotid artery stenting (CAS) with embolic protection devices (EPDs) is not inferior to CEA as a surgical treatment for patients with symptomatic or asymptomatic extracranial ICA stenosis [1, 20]. Thus far, there are only three studies of patients with ICA near occlusion treated by CAS [5, 14, 19]. The aim of this study is to examine the results of CAS as a surgical treatment for the ICA near occlusion.
Material and methods
Patients
Our institutional review board approved this study; written informed consent was obtained from all patients. Near occlusion was defined as severe ICA stenosis with distal ICA narrowing: an ICA/common carotid artery (CCA) ratio of <0.40 in men and <0.45 in women, as defined by Rothwell et al. [16]. Between April 2008 and September 2012, 14 patients with the ICA near occlusion were treated with CAS. All the patients were men, and had a mean age of 75.4 years (range, 60–84 years). These patients were 5.2 % of the total 267 CAS procedures performed during the study period. Ten patients were symptomatic and 4 were asymptomatic, 7 had minor stroke, and 3 had transient ischemic attack (TIA). In symptomatic patients, the time interval between the ischemic event and CAS was a mean of 29.3 days (range, 17–45 days). Diagnostic angiography was performed in all patients. In all patients, affected common carotid angiograms showed delayed arrival of contrast medium in the ICA to the head compared to the external carotid artery (ECA). Contralateral common carotid angiograms showed collateral flow with cross filling the contralateral carotid artery in all patients. The clinical characteristics of the patients are shown in Table 1.
Procedures
Dual (10 patients) or triple (4 patients) antiplatelet drugs (clopidogrel, 75 mg; aspirin, 100 mg; and cilostazol, 200 mg) were administered at least 1 week before CAS. All CAS procedures were performed by the same neurointerventionist under local anesthesia. A 9-Fr sheath was placed into the femoral artery, and a 4-Fr sheath was placed into the femoral vein. After placement of the 2 sheaths, the activated clotting time was kept between 300 and 350 s during the procedure by the intravenous administration of heparin. A 9-Fr occlusion balloon-guiding catheter (OPTIMO; Tokai Medical Products, Aichi, Japan) was introduced into the CCA. The proximal end of the 9-Fr occlusion balloon-guiding catheter was connected with the 4-Fr sheath inserted into the femoral vein via the filter device to eliminate debris. Next, a balloon wire system (GuardWire; Medtronic, Minneapolis, MN) was introduced into the ECA. Both the CCA and ECA were occluded, and a contrast medium was gently injected. It was confirmed to reverse to the lumen of the balloon-guiding catheter without the contrast medium flowing to the distal ICA (reversed flow condition). The reversed arterial blood flow was returned to the venous circulation via a 4-Fr sheath placed in the femoral vein via the filter device to eliminate debris. Under the reversed flow conditions by CCA and ECA balloon occlusion (proximal balloon protection), a 0.014-in. microguidewire crossed the stenotic lesion and was introduced into the high cervical ICA. Predilation was performed with a 3.0-mm PTA balloon catheter. Furthermore, a filter wire was used simultaneously under proximal balloon protection if the diameter of ICA was recovered after predilation (dual protection). CAS procedures were performed using two types of embolic protection methods, as shown in Table 1. In five patients, a proximal balloon protection method was used. In nine patients, a dual protection method with a proximal balloon and distal filter protection was used.
Proximal balloon protection method
Under reversed flow conditions by CCA and ECA balloon occlusion, a 0.014-in. microguidewire crossed the stenotic lesion and was introduced into the high cervical ICA (Fig. 1a, b). Predilation was performed using a 3.0-mm PTA balloon catheter. A self-expanding stent (PRECISE; Johnson & Johnson, Miami Lakes, FL or Carotid Wallstent; Boston Scientific, Natick, MA) larger than the diameter of the CCA was deployed from the distal portion of the stenotic ICA to the CCA. If the ICA was very tortuous, an open cell stent (PRECISE) was chosen; otherwise, a closed cell stent (Carotid Wallstent) was chosen. Postdilation was performed, and intravascular ultrasound (IVUS) was performed in all patients for the presence of eventual in-stent prolapse. If IVUS did not show the presence of eventual in-stent prolapse, the proximal balloons were then deflated.
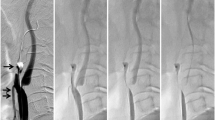
a Lateral unsubtracted angiographic view using the proximal balloon protection method. The ECA was occluded with the balloon (arrow) and the CCA was occluded with the balloon (double arrows). b Diagram showing the proximal balloon protection method. The ECA was occluded with the balloon (arrow) and the CCA was occluded with the balloon (double arrows). The proximal end of the balloon-guiding catheter was connected with the 4-Fr sheath inserted into the femoral vein via the filter device. c Lateral unsubtracted angiographic view in dual protection using the proximal balloon and distal filter protection method. The ECA was occluded with the balloon (arrow) and the CCA was occluded with the balloon (double arrows) and the filter wire (triple arrows) was introduced into the high cervical ICA. d Diagram showing dual protection with the proximal balloon and distal filter protection method. The ECA was occluded with the balloon (arrow) and the CCA was occluded with the balloon (double arrows) and the filter wire (triple arrows) was introduced into the high cervical ICA. The proximal end of the balloon-guiding catheter was connected with the 4-Fr sheath inserted into the femoral vein via the filter device
Dual protection method with proximal balloon and distal filter protection
Under the reversed flow conditions by CCA and ECA balloon occlusion, a 0.014-in. microguidewire crossed the stenotic lesion and was introduced into the high cervical ICA. Predilation was performed using a 3.0-mm PTA balloon catheter. Next, a filter-wire (Angioguard; Johnson & Johnson, Miami Lakes, FL or FilterWire EZ; Boston Scientific, Natick, MA) crossed the stenotic lesion and was introduced into the high cervical ICA, and the 0.014-in. microguidewire was withdrawn. The following procedure was performed with the dual protection method using the proximal balloon and the distal filter protection devices simultaneously (Fig. 1c, d). A self-expanding stent (PRECISE or Carotid Wallstent) larger than the diameter of the CCA was deployed from the distal portion of the stenotic ICA to the CCA. If the ICA was very tortuous, an open cell stent (PRECISE) was chosen; otherwise, a closed cell stent (Carotid Wallstent) was chosen. Postdilation was performed, and IVUS was performed in all patients for the presence of eventual in-stent prolapse. If IVUS did not show the presence of eventual in-stent prolapse, after withdrawing of the filter wire, the proximal balloons were deflated.
After stent deployment, argatroban (2.5 mg/h) was continued for 24 h. The day after CAS, dual antiplatelet drugs were administered for 3 months and a single antiplatelet drug was prescribed indefinitely.
Postoperative evaluation
We examined a change of stenotic lesion, perioperative complications after CAS, and hyperintensity spots on diffusion-weighted imaging (DWI). Baseline magnetic resonance imaging (MRI) was performed in all patients within 2 days before CAS. MRI after CAS was performed within 2 days in all patients to evaluate the ischemic lesions related to the CAS procedure. By comparing the preoperative and postoperative DWI-MRI, the occurrence of newly appearing hyperintensity spots was counted. We then performed neck computed tomography (CT) angiographic and/or duplex sonographic evaluation the day after the procedure and after 1, 3, 6, and 12 months for the first year and then annually to determine clinical outcome, vessel patency, or possible restenosis.
Results
Because all patients were men, the ICA/CCA ratio of all patients was smaller than 0.40. During the procedure, predilation of the stenosis was required in all patients to allow deployment of the stent. IVUS did not show the presence of eventual in-stent prolapse in any patient. All patients were successfully treated and showed satisfactory dilation. No new neurological deficits related to the treated lesion appeared during or after the procedure during the follow-up periods (mean, 16.7 months; range, 5–29 months). By comparing the preoperative and postoperative DWI-MRI, newly appearing hyperintensity spots were observed in 2 patients (14 %), as shown in Table 1. The number of hyperintensity spots in these patients was 1 and 4, and the diameter of each spot was 2 mm for both patients.
Illustrative cases
Patient 7
A 73-year-old man presented with right hemiparesis. DWI-MRI showed a scattered hyperintensity lesion in the left cerebral hemisphere, and MR angiography showed signal declines from the supraclinoid to cervical portion of left ICA. Neck CT angiography showed collapse of the left distal ICA in comparison to the normal caliber right distal ICA (Fig. 2a). These arteries were continuous from the proximal carotid bulb and were headed toward the carotid canal. We diagnosed acute brain infarction as artery-to-artery embolism due to ICA stenosis. The patient received clopidogrel (75 mg/day) and aspirin (100 mg/day), and CAS was planned to prevent recurrent attacks by symptomatic ICA severe stenosis. Cilostazol (200 mg/day) was added 1 week before CAS. Four weeks after onset, the CAS procedure was performed under local anesthesia. The right angiogram showed collateral flow with cross filling of contralateral anterior circulation via the anterior communicating artery (Fig. 2b). The left angiogram showed near occlusion of the left cervical ICA with the ICA/CCA ratio of 0.20 (Fig. 2c). CAS was performed using a dual protection method with proximal balloon and distal filter protection. Under CCA and ECA balloon occlusion, a microguidewire crossed the stenotic lesion and predilation was performed with a 3.0 × 40-mm PTA balloon catheter. Next, a filter-wire crossed the stenotic lesion and was introduced into the high cervical ICA. The following procedure was performed with dual protection using the proximal balloon and distal filter protection (dual protection). An 8 × 21-mm self-expanding stent (Carotid Wallstent) was deployed from the distal portion of the stenotic ICA to the CCA. Postdilation was performed using a 4.0 × 30-mm balloon catheter. There was no residual stenosis of the lesion after CAS (Fig. 2d). Postoperative DWI showed no hyperintensity lesions. The patient was discharged with no new neurological deficits.
Patient 7: Patient with symptomatic cervical ICA near occlusion. a CT angiography showed collapse of the left distal ICA (arrowhead) compared to the normal caliber right distal ICA (arrow). b The right angiogram showed collateral flow with cross filling of contralateral anterior circulation via the anterior communicating artery. c Preoperative lateral view of the digital subtraction angiogram showed that the left ICA was faintly opacified, but that the distal portion was not identified in the arterial phase. The ICA/CCA ratio was 0.20. d Postoperative lateral view of the digital subtraction angiogram showed that the stenotic lesion was well dilated after stenting
Patient 12
An 83-year-old man presented with transient hemiparesis. DWI showed no abnormal lesions. MR angiography showed signal declines from the supraclinoid to cervical portion of the left ICA. Neck CT angiography showed collapse of the left distal ICA in comparison to the normal caliber right distal ICA (Fig. 3a). These arteries were continuous from the proximal carotid bulb and were headed toward the carotid canal. We diagnosed the patients with TIA due to ICA severe stenosis. The patient received clopidogrel (75 mg/day) and aspirin (100 mg/day), and CAS was planned to prevent recurrent attacks by symptomatic ICA severe stenosis. Four weeks after onset, the CAS procedure was performed under local anesthesia. The right angiogram showed collateral flow with cross filling of contralateral anterior circulation via the anterior communicating artery (Fig. 3b). The left angiogram showed the near occlusion of the left cervical ICA with the ICA/CCA ratio of 0.12 (Fig. 3c). CAS was performed using a proximal balloon protection method. Under CCA and ECA balloon occlusion (proximal balloon protection), a microguidewire crossed the stenotic lesion and predilation was performed with a 3.0 × 40-mm PTA balloon catheter (Fig. 3d). A 9 × 40-mm self-expanding stent (PRECISE) was deployed from the distal portion of the stenotic ICA to the CCA. Postdilation was performed with a 5.0 × 20-mm balloon catheter. There was no residual stenosis of the lesion after CAS (Fig. 3e). Postoperative DWI showed no hyperintensity lesion. Four days after CAS, the patient was discharged with no neurological deficits.
Patient 12: Patient with symptomatic cervical ICA near occlusion. a CT angiography showed collapse of the left distal ICA (arrowhead) compared to the normal caliber right distal ICA (arrow). b The right angiogram showed collateral flow with cross filling of contralateral anterior circulation via the anterior communicating artery. c Preoperative lateral view of the digital subtraction angiogram showed that left ICA was faintly opacified, but that the distal portion was not identified in the arterial phase. The ICA/CCA ratio was 0.12. d Lateral unsubtracted angiographic view in proximal balloon protection method showed that the ECA and CCA were occluded with the balloon, and the stenotic lesion was dilated with the PTA balloon. e Postoperative lateral view of the digital subtraction angiogram showed that the stenotic lesion was well dilated after stenting
Discussion
Near occlusion of the cervical ICA is the appearance of partial luminal diameter decrease or virtual luminal collapse of an otherwise normal-appearing artery beyond a prominent carotid bulb stenosis [3, 4]. This disease is uncommon and is referred to by a number of phrases: pseudoocclusion [10, 18], angiographic string sign [12], and the slim sign [10]. The natural history of ICA near occlusion is obscure, and the treatment for patients with near occlusion remains controversial. From the NASCET data, Morgenstern et al. [13] reported that the 1-year-stroke risk after medical treatment was 11.1 % in patients with near occlusion with string versus 35.1 % in patients with 90 % to 94 % stenosis. From ECST data, Rothwell et al. [16] defined poststenotic narrowing of the ICA (near occlusion) as an ICA/CCA ratio of <0.40 in men and <0.45 in women, and reported that the 5-year stroke risk after medical treatment was 8 % in patients with 70 % to 99 % stenosis and ICA narrowing versus 25 % in patients without narrowing. They concluded that poststenotic narrowing of the ICA (near occlusion) was associated with a low risk of stroke after medical treatment. Although the NASCET and ECST data have demonstrated that the stroke rate of near occlusion is not high compared to severe stenosis without narrowing of the ICA, stroke risk of near occlusion after medical treatment only is not low.
The benefit of surgical treatment for patients with near occlusion is controversial. Rothwell et al. [17] remeasured the prerandomization ECST carotid angiograms and redefined the outcome events in the same manner as NASCET. They concluded that CEA was of no benefit in patients with near occlusion. On the other hand, Morgenstern et al. [13] reported that CEA reduced the 1-year stroke risk from 11.1 % to 6.7 %, and that CEA was beneficial for near occlusion and no more dangerous than in patients with ICA severe stenosis without distal narrowing. From these results, we believe that surgical treatment is a beneficial treatment for patients with near occlusion.
CAS has been increasing since the introduction of devices to protect against embolic complications during the procedure because endovascular therapy is less invasive than CEA [19, 20]. In a randomized trial comparing CAS with the use of an EPD to CEA in patients with coexisting conditions that potentially increased the risk posed by CEA, CAS with the use of an EPD is not inferior to CEA [20]. Furthermore, no significant differences could be shown in long-term outcomes between patients who underwent CAS with an EPD and those who underwent CEA [6]. In a randomized trial comparing CAS with the use of an EPD to CEA in patients with symptomatic or asymptomatic carotid stenosis, the risk of stroke, myocardial infarction, or death did not differ significantly in the group undergoing CAS and the group undergoing CEA [1]. CAS with the use of an EPD does not seem to be inferior or equal to CEA.
There are three studies of patients with ICA near occlusion treated by CAS [5, 14, 19]. Terada et al. [19] examined the clinical results and complications of 20 CAS procedures in patients with ICA near occlusion. EPD was used in 19 of 20 procedures (15 distal balloon protection and 4 proximal balloon protection). The clinical results of CAS for ICA near occlusion under embolic protection were fairly good results from the viewpoint of periprocedural neurological morbidity, angiographic follow-up results, and stroke prevention. They concluded that CAS could be considered an alternative to CEA in patients with ICA near occlusion. Nikas et al. [14] examined the safety and effectiveness of 25 CAS procedures in patients with ICA near occlusion. EPD was used in all procedures (1 distal filter protection and 24 proximal protection). They concluded that CAS under proximal cerebral protection seemed to be a feasible and safe procedure to manage patients with ICA near occlusion. González et al. [5] examined the results and complications of 116 CAS procedures in patients with ICA near occlusion. EPD was used in 92 patients (79.3 %) and all of the protection methods were distal balloon protection. They concluded that CAS would be beneficial when performed by an experienced neurointerventional team. CAS with the use of EPD seems to be a safe and effective procedure for patients with ICA near occlusion.
In this study, we performed CAS in patients with ICA near occlusion. For a periprocedural antiplatelet agent, we used triple antiplatelet agents before CAS. The usefulness of triple antiplatelet therapy has been already reported in comparison with dual antiplatelet therapy in patients undergoing coronary stenting [8, 9]. Furthermore, we investigated the radiographic characteristics and outcome of DWI changes in the coiling of unruptured cerebral aneurysm by analyzing the correlation of antiplatelet therapy. We reported that the periprocedural use of multiple (dual or triple) antiplatelet drugs was expected to reduce the volume of thromboembolism and permanent tissue damage [11]. Therefore, we believe that the use of triple antiplatelet drugs during the periprocedural period is useful. In the protection method, we performed CAS using dual protection method with proximal balloon and distal filter protection. If proximal balloon protection was well applied, filter device may be not needed. However, we thought that reversed flow condition may not be achieved during delivery of a PTA balloon catheter or stent through the lumen of the balloon-guiding catheter. If reversed flow condition may not be achieved, there was the risk of the debris migration into the cerebral arteries. Therefore, we performed CAS using dual protection method with proximal balloon and distal filter protection to ensure debris capture. As the result of CAS using proximal or dual embolic protection method, all near occlusions were successfully dilated, and the stenotic rate improved. Transient and persistent complications, including neurological deficits, did not occur in any patient. CAS using the proximal or dual embolic protection method as a surgical treatment was safe and beneficial for an ICA near occlusion. However, our study is limited by the small number of cases. Furthermore, there are few studies of CAS for patients with ICA near occlusion [5, 12, 17]. Therefore, a larger number of CAS patients with ICA near occlusion are necessary.
In conclusion, our study showed that CAS using the proximal or dual embolic protection method as a surgical treatment is safe and beneficial for the ICA near occlusion.
References
Brott TG, Hobson RW 2nd, Howard G, Roubin GS, Clark WM, Brooks W, Mackey A, Hill MD, Leimgruber PP, Sheffet AJ, Howard VJ, Moore WS, Voeks JH, Hopkins LN, Cutlip DE, Cohen DJ, Popma JJ, Ferguson RD, Cohen SN, Blackshear JL, Silver FL, Mohr JP, Lal BK, Meschia JF, Investigators CREST (2010) Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med 363:11–23
European Carotid Surgery Trialists' Collaborative Group (1998) Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST). Lancet 351:1379–1387
Fox AJ (1993) How to measure carotid stenosis. Radiology 186:316–318
Fox AJ, Eliasziw M, Rothwell PM, Schmidt MH, Warlow CP, Barnett HJ (2005) Identification, prognosis, and management of patients with carotid artery near occlusion. AJNR Am J Neuroradiol 26:2086–2094
González A, Gil-Peralta A, Mayol A, Gonzalez-Marcos JR, Moniche F, Aguilar M, Gutierrez I (2011) Internal carotid artery stenting in patients with near occlusion: 30-day and long-term outcome. AJNR Am J Neuroradiol 32:252–258
Gurm HS, Yadav JS, Fayad P, Katzen BT, Mishkel GJ, Bajwa TK, Ansel G, Strickman NE, Wang H, Cohen SA, Massaro JM, Cutlip DE, Investigators SAPPHIRE (2008) Long-term results of carotid stenting versus endarterectomy in high-risk patients. N Engl J Med 358:1572–1579
Hirata Y, Sakata N, Inoue T, Yasumori K, Yasaka M, Okada Y (2011) Histopathological features with angiographic correlates of internal carotid artery pseudo-occlusion: impact of plaque compositions. Clinical article. J Neurosurg 115:350–358
Jeong YH, Hwang JY, Kim IS, Park Y, Hwang SJ, Lee SW, Kwak CH, Park SW (2010) Adding cilostazol to dual antiplatelet therapy achieves greater platelet inhibition than high maintenance dose clopidogrel in patients with acute myocardial infarction: Results of the adjunctive cilostazol versus high maintenance dose clopidogrel in patients with AMI (ACCEL-AMI) study. Circ Cardiovasc Interv 3:17–26
Lee SW, Park SW, Yun SC, Kim YH, Park DW, Kim WJ, Lee JY, Lee CW, Hong MK, Kim JJ, Park SJ (2010) Triple antiplatelet therapy reduces ischemic events after drug-eluting stent implantation: Drug-Eluting stenting followed by Cilostazol treatment REduces Adverse Serious cardiac Events (DECREASE registry). Am Heart J 159:284–291
Lippman HH, Sundt TM Jr, Holman CB (1970) The poststenotic carotid slim sign: supurious internal carotid hypolasia. Mayo Clin Proc 45:762–767
Matsushige T, Kiura Y, Sakamoto S, Okazaki T, Shinagawa K, Ichinose N, Takasu M, Akiyama Y, Sugiyama K, Kurisu K (2013) Multiple antiplatelet therapy contributes to the reversible high signal spots on diffusion-weighted imaging in elective coiling of unruptured cerebral aneurysm. Neuroradiology 55:449–457
Mehigan JT, Olcott C 4th (1980) The carotid "string" sign. Differential diagnosis and management. Am J Surg 140:137–143
Morgenstern LB, Fox AJ, Sharpe BL, Eliasziw M, Barnett HJ, Grotta JC (1997) The risks and benefits of carotid endarterectomy in patients with near occlusion of the carotid artery. North American Symptomatic Carotid Endarterectomy Trial (NASCET) Group. Neurology 48:911–915
Nikas DN, Ghany MA, Stabile E, Sorropago G, Saccá S, Favero L, Zakaryan N, Reimers B, Rubino P (2010) Carotid artery stenting with proximal cerebral protection for patients with angiographic appearance of string sign. JACC Cardiovasc Interv 3:298–304
North American Symptomatic Carotid Endarterectomy Trial (1991) Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med 325:445–453
Rothwell PM, Warlow CP (2000) Low risk of ischemic stroke in patients with reduced internal carotid artery lumen diameter distal to severe symptomatic carotid stenosis: cerebral protection due to low poststenotic flow? On behalf of the European Carotid Surgery Trialists' Collaborative Group. Stroke 31:622–630
Rothwell PM, Gutnikov SA, Warlow CP, European Carotid Surgery Trialist's Collaboration (2003) Reanalysis of the final results of the European Carotid Surgery Trial. Stroke 34:514–523
Sekhar LN, Heros RC, Lotz PR, Rosenbaum AE (1980) Atheromatous pseudo-occlusion of the internal carotid artery. J Neurosurg 52:782–789
Terada T, Tsuura M, Matsumoto H, Masuo O, Tsumoto T, Yamaga H, Itakura T (2006) Endovascular treatment for pseudo-occlusion of the internal carotid artery. Neurosurgery 59:301–309
Yadav JS, Wholey MH, Kuntz RE, Fayad P, Katzen BT, Mishkel GJ, Bajwa TK, Whitlow P, Strickman NE, Jaff MR, Popma JJ, Snead DB, Cutlip DE, Firth BG, Ouriel K, Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy Investigators (2004) Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med 351:1493–1501
Author information
Authors and Affiliations
Corresponding author
Additional information
Comments
Gerasimos Baltsavias, Zurich, Switzerland
I think the present report about carotid stenting for near occlusion of the cervical carotid artery is interesting for a couple of reasons. Apart from the technical aspects of the described technique which is eloquently presented, it is very interesting to realize how broad is the spectrum of periprocedural management and techniques which are currently used in various departments from different endovascular groups to treat the same disease: From 9Fr guide catheter, double protection devices and triple antiplatelet agents, as in the current group, to 6Fr guide catheter, without protection devices and double antiplatelet agents practiced by other groups including ours. This variety of approaches reminds us once again how a good result can be achieved through different approaches. On the other hand, it highlights the need for larger studies and guidelines at least for some aspects of our practice as the antithrombotic regime. Personally and despite this interesting aspect of this report, I would be rather reluctant to apply or recommend the presented technique and rational. One significant reason, among others, is its contrariety to the highly appreciated “kisss” principle1 (keep it simple, short and safe). Interestingly enough and despite the double and triple protection, the known and clinically silent DWI lesions were still detected postoperatively in a small percentage of patients.
1. Adopted from: A neurosurgeon’s notebook. One man’s way of trying to avoid trouble. CBT Adams. Oxford Blackwell Science. 1998
William W. Ashley Jr. and Christopher M. Loftus, Maywood, USA.
In “Carotid artery stenting using the proximal or dual protection method for near occlusion of the cervical internal carotid artery”, the authors present a retrospective analysis of data from 10 symptomatic and 4 asymptomatic patients with carotid near occlusion treated at a single institution over an approximately 4.5-year period. All 14 patients were male and the mean age was about 75 years of age. They were treated with carotid artery stenting using proximal protection alone (n = 5) or proximal protection combined with distal protection (n = 9). Four patients received triple antiplatelet regimens and 10 received dual antiplatelet regimens. Symptomatic patients were treated within an average of 29 days from onset of symptoms. MRS data is not presented but the mean follow-up was about 17 months (range 5–29). All near occlusions were successfully dilated and there were no transient or permanent complications. There were only 2 patients who showed new small T2 hyperintensities on follow-up MRI.
We would like to thank the authors for presenting this stimulating paper. As they point out, carotid near occlusion is a controversial topic. Thus we appreciate the effort to add further improve the treatment of these complex patients. As the authors themselves point out, this is a small non-randomized retrospective chart review. It is not clear whether the patients were treated by the same practitioner but there is a great deal of variability (antiplatelet regimen, type of stent, type of protection, symptomatic status in the methods used to treat these patients). As a result, no meaningful comparisons can be made between the very small sub-groups. We want to congratulate the authors on excellent results in this small group of difficult patients. We agree that by using careful technique and patient selection, carotid near occlusion can be safely and effectively treated using CAS in some patients.
This paper brings up some very important issues. First, it is not clear as to the true natural history of this phenomenon. How does near occlusion fall in the continuum between a normal patent vessel and a completely occluded one? Are we seeing a point in the temporal progression toward occlusion or is near occlusion a different pathophysiologic phenomenon? Our understanding of the answers to these questions will inform our understanding of the risk of stroke associated with near occlusion. Some analysis of data from the NASCET and ECST trials suggests that carotid near occlusion is low risk while other suggests that treating near occlusion may be of benefit. Unfortunately, due to the relatively rarity of the problem and heterogeneity of the patient population, we do not have adequate evidence from which to draw conclusions. Thus it is still not clear whether we should aggressive treat carotid near occlusion. We must make decisions based on individual patient data. Patient symptoms, the chronicity of the lesion, the type of prior medical therapy, the type and stability of the collateral network and other factors must be considered. Certainly an acute near occlusion in a symptomatic patient seems like a reasonable candidate for therapy. But what of the asymptomatic patient with a chronic narrowing?
Next even if we should treat, what methodology should we use? Again we do not have any randomized controlled trials comparing revascularization to medical therapy for carotid near occlusion. Similarly we do not have good studies comparing CEA to CAS for this subset of patients. This paper highlights the fact that it can be done but we must organize larger well designed studies or, at least, effective registries with standardized treatment regimens to further understand the issue. In this paper the patient population was relatively old with a mean age of 75 and a range from 60 to 84 years of age. If we are to generalize and believe the CREST data, there are at least some of these patients (35 % of patients in the study were over 80 years old) that would be better treated with CEA. It would be interesting to know more about how the authors chose which patients with near occlusion to treat using CAS versus CEA. And were there any near occlusions that they chose not to treat at all and why? Again, we need more studies.
If we are going to treat using CAS, who do we treat and how do we do it? The CREST study suggested that CAS is not inferior to CEA. In reality CAS is probably better for some and CEA better for others. Improvements in technology may result in further expansion of the group who is best treated using CAS. The authors took advantage of multiple strategies to improve the safety and effectiveness of CAS including multiple protection strategies, dual and triple antiplatelet agents and intravascular ultrasound to check the quality of stent placement. They used these techniques in a heterogeneous fashion and achieved excellent results. We need to know more about when to choose each one of these strategies to optimize results. In terms of protection, there is mounting evidence that proximal protection may provide benefit over distal protection because one can avoid crossing the lesion unprotected. But it may be harmful in patients with poor collaterals or the isolated hemisphere. However, the benefit of a dual strategy of proximal and distal protection has not been proven. The authors used a dual strategy when they thought balloon deployment would be less than optimal. But in these cases would it be better to just use a distal protection device? We need to know more about the collateral networks in this group of patients to help us decide which device to use. In those with robust collaterals, proximal protection with or without a DPD may be all that is necessary. The choice to use an additional antiplatelet agent is also controversial. In coronary stenting and peripheral vascular studies, an additional agent has been shown to improve rates of restenosis and need for re-treatment. But it is postulated that is likely relate to providing a failsafe for those that are resistant to ASA or clopidogrel. Indeed, it may be that a more effective pre-treatment analysis of ASA or clopidogrel sensitivity may be more useful and safer than adding a third agent to all patients. The authors chose to use three agents in 4 patients but do not mention why they made this choice. IVUS has been shown to be effective in quality assurance after carotid stenting. It helps discover cases of poor stent apposition, or positioning or intravascular clot extruded through the stent. In small studies, it has not been shown to yield major improvements in outcomes from carotid stenting but has lead to decreased need for retreatment due to restenosis.
In addition to the three studies mentioned in this paper, there are at least three other papers that deal with this topic and show high procedural success rates with good outcomes. Most recently Ruiz-Salmerón et al. (2013) analyzed 54 patient with carotid near occlusion treated with CAS. They used proximal protection in over 50 % of the patients and, like the current study presented herein, they showed a 96 % success rate. However, they also showed that stenting in cases of near-occlusion caused increased detachment of plaque, as shown by higher percentages of macroscopic plaque captured by protection devices (18.5 % vs. 7 %, P = .01) and of perioperative ischemic brain lesions (47 % vs. 31 %, P = .07). They showed that in 30 days of follow-up, the tendency toward adverse neurological events (death, major and minor stroke) was higher in the near-occlusion group (9.2 % vs. 3.2 %, P = .08). These data are different from the experience of the current authors. These differences emphasize the need for further and better controlled studies.
It is an exciting time. We now have many more tools available. This study and others highlight the breadth and variation of techniques that can be employed to treat carotid near occlusion and and also the need also the need for further stringent investigative to help us optimize our treatment choices.
References
Choi BS, Park JW, Shin JE, Lü PH, Kim JK, Kim SJ, Lee DH, Kim JS, Kim HJ, Suh DC. Outcome evaluation of carotid stenting in high-risk patients with symptomatic carotid near occlusion. Interv Neuroradiol. 2010 Sep;16(3):309–16. Epub 2010 Oct 25.
Ruiz-Salmerón RJ, Gamero MA, Carrascosa C, Pérez S, de Araujo D, Marcos F, Rodríguez de Leiras S, Vizcaíno M, Caparrós C, Izquierdo G. Neurologia. Carotid artery stenting: clinical and procedural implications for near-occlusion stenosis. 2013Mar 1. doi: pii: S0213-4853(13)00002-9. 10.1016
Miyamoto N, Naito I, Takatama S, Shimizu T, Iwai T, Shimaguchi H. Urgent stenting for patients with acute stroke due to atherosclerotic occlusive lesions of the cervical internal carotid artery. Neurol Med Chir (Tokyo). 2008 Feb;48(2):49–55; discussion 55–6.
Rights and permissions
About this article
Cite this article
Sakamoto, S., Kiura, Y., Kajihara, Y. et al. Carotid artery stenting using the proximal or dual protection method for near occlusion of the cervical internal carotid artery. Neurosurg Rev 36, 551–558 (2013). https://doi.org/10.1007/s10143-013-0481-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-013-0481-y