Abstract
Stereotactic biopsies represent a routine neurosurgical procedure for the diagnosis of intracranial lymphomas and selected diffusely infiltrating gliomas. Acquisition of tissue samples that do not allow correct tumor typing and grading is, however, not uncommon. Five-aminolevulinic acid (5-ALA) has been shown to accumulate in malignant tumor tissue. The aim of this study was to prospectively investigate the clinical usability of 5-ALA for intraoperative detection of representative tissue in stereotactic tumor biopsies. Fifty consecutive patients underwent frameless stereotactic biopsy for a suspected brain tumor. 5-ALA was administered 4 h before anesthesia. Serial biopsy samples were obtained and intraoperatively checked for 5-ALA fluorescence (strong, vague, or none) using a modified neurosurgical microscope. All samples were examined for the presence of representative tumor tissue according to neuroimaging (MRI, positron emission tomography, and/or chemical shift imaging) and histopathological parameters. Visible 5-ALA fluorescence was observed in 43/50 patients (strong in 39 and vague fluorescence in four cases). At biopsy target, 52/53 samples of glioblastomas, 9/10 samples of gliomas grade III, and 14/16 samples of lymphomas revealed strong 5-ALA fluorescence. Samples with strong 5-ALA fluorescence were only observed at, but not outside the biopsy target. All tissue samples with strong 5-ALA fluorescence were representative according to our neuroimaging and histopathological criteria (positive predictive value of 100%). Our data indicate that strong 5-ALA fluorescence is a reliable and immediately available intraoperative marker of representative tumor tissue of malignant gliomas and intracranial lymphomas in stereotactic biopsies. Thereby, the application of 5-ALA in stereotactic brain tumor biopsies may in future reduce costs for operating room and neuropathology and may decrease procedure-related morbidity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Although stereotactic biopsies are well-established for the diagnosis of intracranial lymphomas and selected cases of diffusely infiltrating gliomas (DIG), specific shortcomings are inherent to this standard neurosurgical procedure: (1) The acquisition of non-diagnostic samples from outside the viable tumor volume (such as necrotic/gliotic tissue or normal white matter), which has been reported in up to 24% of stereotactic biopsy series [3, 4, 11, 38], with the need for a second neurosurgical intervention. Typical causes for this technical failure are registration inaccuracy or patient movement due to an inadequate holding of the head. (2) The acquisition of non-representative tumor samples, which has been reported in up to 64% of biopsies [1, 16, 24], that do not allow the correct tumor typing and grading of DIG. This is typically caused by inappropriate definition of the target during the planning phase. Histopathological undergrading and non-diagnostic biopsies may subsequently impede allocation of patients to adequate adjuvant treatments.
Therefore, intratumoral serial biopsies [18, 19, 33, 37] and intraoperative neuropathological assessment [4, 11, 14, 33] are commonly applied to improve the diagnostic yield and accuracy of stereotactic biopsies. These techniques, however, are associated with major drawbacks: (1) Intraoperative neuropathological assessment is time consuming, costly, and generally not permanently available; [4, 9, 29] (2) Acquisition of serial biopsies is associated with an increased risk of intracranial hemorrhages [5, 28], which have been reported in 0.3–59.8% of cases [3, 8, 10, 19–21] and contribute considerably to the reported morbidity of 0–16.1% [4, 6, 11, 20, 23, 33] and mortality of up to 3.9% [3, 4, 11, 20, 23, 33] of this minimally invasive procedure.
Therefore, safe alternative techniques to improve the diagnostic yield and accuracy of stereotactic biopsies are warranted.
Five-aminolevulinic acid (5-ALA) leads to intracellular accumulation of fluorescing protoporphyrin IX, especially in malignant glioma tissue which can be visualized by specifically modified neurosurgical microscopes. 5-ALA is primarily applied for fluorescence-guided resection of malignant gliomas [31]. Recently, 5-ALA has been identified also as a promising intraoperative marker, that is unaffected by brain-shift, for visualization of anaplastic foci in DIG with non-significant contrast-enhancement (CE) [35]. Furthermore, 5-ALA fluorescence was reported also in malignant non-glial tumors [7, 13].
Based on the observation by Hefti et al. [13], who first described the successful application of 5-ALA in ten stereotactic biopsies for malignant gliomas, we designed the present study to investigate the clinical usability of 5-ALA for intraoperative detection of representative tissue samples in stereotactic biopsies for suspected brain tumors.
Methods
Study cohort
Our study cohort consists of a consecutive series of 50 patients who underwent 5-ALA controlled stereotactic biopsy for diagnosis of a suspected brain tumor at the Department of Neurosurgery, Medical University Vienna between April 2008 and May 2011. All patients gave informed consent and this clinical prospective study was approved by the ethics committee of the Medical University Vienna (for patients characteristics, see Table 1).
WHO grading and typing
In the 50 patients, DIG was diagnosed in 39 cases: 6 WHO grade II (3 astrocytomas, 2 oligodendrogliomas and 1 oligoastrocytoma), 8 WHO grade III (7 astrocytomas and 1 oligodendroglioma), and 25 WHO grade IV gliomas, respectively. Of the remaining 11 patients, 7 were diagnosed as primary central nervous system lymphoma and 4 patients with brain metastasis.
Preoperative imaging
Preoperative imaging was performed within 2 weeks prior to stereotactic biopsy (Table 1).
Magnetic resonance imaging
All patients were examined on a 3 Tesla scanner (Tim Trio, Siemens, Erlangen, Germany) using our routine magnetic resonance imaging (MRI) protocol for brain tumors with axial fluid-attenuated-inversion-recovery sequences, diffusion-weighted images, axial, coronal T1- and T2-weighted sequences, and contrast-enhanced axial, coronal and sagittal T1-weighted sequences. The pattern of CE of all tumors was classified by two neuroradiologists (D.P.,J.F.) as being significant (nodular or ring-like) or non-significant (none or patchy/faint).
¹¹C-methionine-positron emission tomography
To detect potential anaplastic foci as the optimal biopsy target, ¹¹C-methionine-positron emission tomography (PET) was performed preoperatively in DIG with non-significant CE. The intratumoral area with highest pathological tumor-to-normal-brain ratio, reflecting most tracer uptake, was defined as PETmax and used as biopsy target (for detailed description, see [27, 34]).
Chemical shift imaging
If PET was unable to depict an area of increased intratumoral metabolism, the intratumoral area with highest pathologic chemical shift imaging (CSI) choline/N-acetyl-aspartate ratio, reflecting the most abnormal metabolite ratio, was defined as CSImax and used as biopsy target (for detailed description, see [34]).
5-ALA management
All patients received 5-aminolevulinic acid hydrochloride (Biosynth® AG, Switzerland) at 20 mg/kg body weight 4 h before anesthesia. We use a modified neurosurgical microscope (NC4/Pentero, Carl Zeiss Surgical GmbH, Oberkochen, Germany) with the feasibility to switch from conventional white light to violet-blue excitation light for detection of potential 5-ALA fluorescence [32]. According to Stummer et al. [30], two clearly discernible fluorescence qualities can be distinguished: (1) Strong (=solid) fluorescence as characterized by a vivid red fluorescence impression and (2) vague fluorescence as defined by a less vivid pink fluorescence (Fig. 1). Each biopsy sample was evaluated for the determination of the fluorescence status (strong, vague, none) by two independent observers (G.W., S.W.).
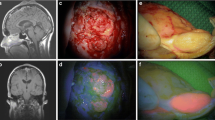
Macroscopic tissue appearance and corresponding 5-ALA fluorescence status and histopathology (hematoxylin/eosin stain, HE, original magnification ×100) of the obtained biopsy cylinders in different locations of a glioblastoma: a Specimen from outside the tumor (gray/white matter interface): Macroscopic appearance reveals no abnormalities, 5-ALA is negative and HE shows only slightly increased cellularity. b Biopsy cylinder from the white matter in the non-contrast-enhancing tumor periphery: The specimen shows slight macroscopic pathological features, is of vague 5-ALA fluorescence and HE shows non-representative infiltrating tumor tissue. c Specimen from the contrast-enhancing tumor part (=biopsy target): The specimen clearly depicts pathologic macroscopic appearance, shows strong 5-ALA fluorescence and corresponds to representative glioblastoma tissue including increased cellularity, high mitotic activity, endothelial proliferation, and focal necrosis. d Specimen from the central necrosis: biopsy cylinder reveals brown/red appearance, 5-ALA is negative and HE depicts necrotic tissue
To avoid potential skin phototoxicity after 5-ALA application, all patients were protected from light sources for 24 h postoperatively.
Stereotactic biopsies
Navigation-guided frameless stereotactic biopsies (Stealth Station Cranial Mach 5; Medtronic, CO, USA) were performed in all patients. The biopsy target was defined as follows:
Lesions with significant CE
Contrast-enhancing area (nodular part or contrast-enhancing ring).
Lesions with non-significant CE
Anatomical MR images were co-registered with PET or CSI on the navigation workstation: PETmax or CSImax were selected as biopsy target.
All procedures were performed under general anesthesia. After fixation of the patient in the head clamp, registration of the navigation system was performed using surface registration or anatomical landmarks [36]. Under sterile conditions, the navigation biopsy arm was fixed to the head clamp, the biopsy needle was registered and aligned with the predefined trajectory. After skin incision, placement of a burr hole, and local opening of the dura, the navigated biopsy needle was advanced to the target by “real time” navigation and serial samples were taken at +5, 0, −5, and −10 mm from the target if appropriate. Between one and four tissue samples were taken from the same level.
For each sample, the needle insert was withdrawn and the 5-ALA fluorescence status of the obtained specimen was checked with violet-blue excitation light of a sterile microscope in the darkened operating room. This check-up for 5-ALA fluorescence took less than 10 s. All samples were labelled according to the 5-ALA fluorescence status as 5-ALA positive (strong or vague) or 5-ALA negative. One target sample was immediately sent for intraoperative histology (frozen section and/or smear preparation), all other samples were formalin-fixed and paraffin-embedded and routinely processed for definitive histopathological workup.
For assessment of accuracy, 0.5 ml of air was injected at the biopsy target, and the position of the small air bubble on postoperative computerized tomography (CT) was later topographically correlated with the target on co-registered preoperative T1-weighted contrast-enhanced MRI [23].
Finally, the needle was removed and the procedure was terminated. All stereotactic procedures were performed by two neurosurgeons (G.W.,S.W.). In eight cases, microsurgical tumor resection was performed after stereotactic biopsy as described previously [34].
A CT of the brain was routinely performed on first postoperative day.
Neuropathology
Histopathological tumor diagnosis was established by the local neuropathology team (J.A.H, A.W.) according to the WHO 2007 criteria [22] on a multi-headed microscope. The neuropathologists were blinded to the 5-ALA fluorescence status.
A tissue sample was considered representative if histopathology allowed—in conjunction with the neuroimaging features (MRI, PET, and/or CSI)—the correct typing and grading of a given tumor:
-
1.
Lymphomas and metastases: presence of malignant lymphatic or epithelial cells (given contrast-enhancing lesion on MRI).
-
2.
Glioblastoma (GBM): presence of a malignant glioma AND necrosis and/or endothelial proliferation, WHO grade IV (given ring-like CE on MRI).
-
3.
High-grade glioma (HGG): diffuse glioma with increased cellularity, nuclear atypia, and marked proliferative activity, WHO grade III (given non-significant or significant nodular CE but no central hypointensity on T1-weighted contrast-enhanced MRI).
-
4.
Low-grade glioma (LGG): well-differentiated glioma tissue (WHO grade II) taken from the PETmax or CSImax within DIG with non-significant CE as described previously [34].
In cases, where the tumor was resected after biopsy, the tumor bulk was systematically analyzed for anaplastic tissue to confirm the initial subtyping and grading of the stereotactic biopsy samples.
Statistical analysis
For statistical analyses, SPSS® version 16.0 software (SPSS Inc., Chicago, Illinois, USA) was used. Sensitivities, specificities, positive and negative predictive values for fluorescence status and histopathological representativity and the 95% confidence interval were calculated.
Results
The stereotactic biopsy using 5-ALA was feasible and accurate in all cases as assessed by the air bubble location at the target on postoperative CT co-registered with preoperative MRI. Altogether, 150 samples were collected from stereotactic biopsies of 50 patients (median 3, range 1–7 samples): 104 tissue samples were taken from the target and 46 specimes from outside the target. According to the independent judgement of the two performing neurosurgeons, strong 5-ALA fluorescence was clearly discernable from vague fluorescence in all fluorescing samples of our study. We never found ambiguous findings in the interpretation of a strong versus vague fluoresceing biopsy tissue sample between the two observers.
5-ALA fluorescence of biopsy target tissue
The biopsy tissue cylinders at the target showed visible 5-ALA fluorescence in 43/50 patients (strong in 39 and vague fluorescence in 4 cases). Strong 5-ALA fluorescence (n = 75 samples) was only observed at the biopsy target, never outside the target (Table 2).
DIG
At target, 52/53 samples of GBM and 9/10 specimens of HGG revealed strong 5-ALA fluorescence, in the remaining samples vague fluorescence was observed. No sample at the target of GBM or HGG was 5-ALA negative. All 19 samples of LGG were 5-ALA negative.
Non-glial tumors
All 16 samples at the target of lymphomas showed 5-ALA fluorescence (strong in 14 and vague fluorescence in two samples). In none of the six samples of brain metastases, strong fluorescence was identified at the target: Three of these samples showed vague and three no 5-ALA fluorescence.
5-ALA fluorescence of tissue outside the target
None of the 46 samples taken from serial biopsies outside the target revealed strong fluorescence (vague in 14 and no fluorescence in 32 samples). In GBM, all 11 samples from the low intensity center on MRI were 5-ALA negative. Eight of 18 samples from the non-contrast-enhancing periphery of GBM showed vague fluorescence. It is of note that three samples from outside the target of metastases revealed vague fluorescence as well.
Correlation of strong 5-ALA fluorescence and CE on MRI, PET, and CSI
All DIG with ring-like or nodular CE on MRI revealed strong 5-ALA fluorescence at biopsy target. Strong 5-ALA fluorescence was never observed in DIG with no CE. All lymphomas showed nodular CE and revealed strong 5-ALA fluorescence. In contrast, none of the patients with metastasis with ring-like or nodular CE showed strong 5-ALA fluorescence. In gliomas with non-significant CE (n = 11), 4/8 tumors showed strong fluorescence in the PETmax (all were WHO grade III). In the remaining three gliomas with negative PET, none of the patients showed strong fluorescence in the CSImax (all were WHO grade II) (Table 3).
Histopathology representative samples
All samples from the target (n = 104) contained diagnostic tumor tissue as evaluated by intraoperative histology and were found representative at definitive postoperative histopathological work-up in conjunction with the neuroimaging parameters. All tissue samples with strong 5-ALA fluorescence were derived from the target and were representative according to our neuroimaging and histopathological criteria. The positive predictive value of a strong fluoresceing sample for representative tumor tissue was therefore 100% (Table 4; Fig. 2).
Fraction of representative versus non-representative biopsy tissue samples in defined areas of diffusely infiltrating gliomas, lymphomas, and metastases in relation to the 5-ALA fluorescence status. a Glioblastomas: at biopsy target, all samples showed strong or vague fluorescence and were representative. All specimens from the low intensity center on MRI were 5-ALA negative and non-representative. Only four of the eight vague fluoresceing samples from the non-contrast-enhancing periphery were representative. b High-grade gliomas: all samples from the target showed strong or vague fluorescence and were representative. Only one of the three vague fluoresceing samples from outside the target was representative. c Low-grade gliomas: all samples from serial biopsies of LGG were representative, none of them revealed visible 5-ALA fluorescence. d Lymphomas: all samples from the target showed strong or vague fluorescence and were representative. The two 5-ALA negative samples from outside the target were non-representative. e Metastases: all samples from the target were representative. None of these samples revealed strong fluorescence. Interestingly, three of eight samples from outside the target showed vague fluorescence and all were non-representative
GBM
All samples from the target were representative and showed strong or vague fluorescence. All samples from the low intensity center on MRI were 5-ALA negative and histopathologically corresponded to necrosis. Of the eight vague fluoresceing samples from the non-contrast-enhancing periphery, four corresponded histologically to representative tumor tissue, the remaining four to infiltrating tumor tissue.
HGG
All samples from the target were representative and showed strong or vague fluorescence. Of the three vague fluoresceing samples from outside the target, one corresponded histologically to representative tumor tissue, the remaining two to LGG tissue.
LGG
All samples from serial biopsies of LGG were representative and none of them revealed visible 5-ALA fluorescence.
Lymphomas
All samples from the target were representative and showed strong or vague fluorescence. The two samples from outside the target revealed non-fluoresceing white matter.
Metastases
At target, all samples were representative and none of them revealed strong fluorescence. Outside the target, 3/8 samples had vague fluorescence and all were non-representative.
Tumor resection after biopsy
In 11/50 patients, tumor resection was performed after initial stereotactic biopsy: In eight cases, stereotactic biopsy was performed immediately before tumor resection and the three remaining patients underwent tumor resection in a separate procedure within 2 weeks after stereotactic biopsy. In all cases, the histopathology of the open surgery confirmed the previous histopathological diagnosis of the biopsy with regard to tumor type and grade.
Complications of stereotactic biopsies
Procedure-related morbidity due to a symptomatic intracerebral hemorrhage was observed in 3/50 patients (6%). One patient required neurosurgical evacuation of the hemorrhage and one patient underwent conservative management; both patients recovered to preoperative condition. The third patient with an extensive brain stem GBM developed a posthemorrhagic hydrocephalus with the need for cerebrospinal fluid shunting. There was no-procedure related mortality.
Adverse events of 5-ALA application
No side effects of 5-ALA were observed in any patient.
Discussion
In the present study, we prospectively investigated the clinical value of 5-ALA for detection of representative tissue samples in stereotactic tumor biopsies. We observed that all tissue samples with strong 5-ALA fluorescence were representative according to neuroimaging and histopathological criteria.
Despite advances in imaging techniques, stereotactic biopsy is still an indispensable technique for establishing a definitive diagnosis and for planning of further treatment. In this respect, the aim of a stereotactic tumor biopsy is to acquire tissue that is representative for the given pathologic process and thereby enables the diagnosis of the correct tumor type and accurate tumor grade to assign patients to the correct treatment.
Currently, most series evaluate the success rate of stereotactic biopsies by “the percentage of cases in which a definitive histopathological diagnosis could be reached” [1, 2]. This so-called “diagnostic yield” has been reported between 76% and 99% [1, 3, 11, 17, 29, 33, 38]. Diagnostic yield, however, rates the correct distinction between tumor and other tissue but does not necessarily include the correct tumor type and grade. For the latter, the term “diagnostic accuracy” was defined [2].
We believe that modern stereotactic biopsy series should be evaluated primarily on the basis of diagnostic accuracy. In cases of DIG, intratumoral tissue heterogeneity with frequently focal malignant progression decreases accuracy of stereotactic biopsies if only standard imaging (CT and/or MRI) for target determination is used. Jackson et al. [16] reported an undergrading of malignant gliomas by stereotactic biopsies in 63% of cases. However, identification of potential anaplastic foci is essential, because only representative histology indicates which postoperative therapy is adequate. Therefore, acquisition of serial biopsies was proposed to improve diagnostic accuracy as one single tissue cylinder may not contain all the relevant histologic features of a malignant glioma [18, 19, 33, 37]. However, multiple biopsies may be associated with an increased risk of intracranial hemorrhage and neurologic deficit [5, 28].
For preoperative visualization of areas of highest malignancy and definition of the biopsy target, the metabolic information of PET [25, 26] or multivoxel MR spectroscopy CSI [15, 34] have been suggested: The high diagnostic accuracy of these advanced imaging methods has previously been confirmed by histopathological comparison of a biopsy specimen from the region of highest metabolism with the tumor bulk from subsequent open surgical removal [34]. An intraoperative confirmation of the correct targeting during stereotactic biopsy, however, is still missing as intraoperative MRI is not widely available [12].
In a randomized controlled multicenter phase 3 trial on malignant gliomas with significant CE, Stummer et al. [31] demonstrated that 5-ALA fluorescence-guided glioma resection leads to a significantly higher rate of complete resections of the contrast-enhancing tumor part and a significantly prolonged 6-month progression-free survival as compared with the conventionally operated control group. Recently, we were able to identify 5-ALA as a promising intraoperative marker for representative histology in DIG with non-significant CE that is unaffected by intraoperative brain shift: Focal 5-ALA fluorescence correlated topographically with maximum PET tracer uptake and high-grade pathology in all patients [35]. Cell proliferation rates were significantly higher in 5-ALA-positive than in non-fluorescent areas within a given tumor. In sum, current literature provides strong evidence that PET, CSI, and 5-ALA are clinically reliable techniques for identification of anaplastic foci in DIG that improve acquisition of representative tumor samples and therefore increase diagnostic accuracy.
So far, few studies have reported the effect of 5-ALA in tumor entities that are relevant in the differential diagnosis of gliomas: Hefti et al. and Eljamel [7, 13] observed 5-ALA fluorescence in lymphomas and metastases as well. Further, Hefti et al. [13] described his first experience with the application of 5-ALA in ten patients who underwent stereotactic biopsy for suspected malignant gliomas: In these tumors, all samples revealed strong 5-ALA fluorescence and histology was conclusive in all cases.
On the basis of this first observation, we systematically analyzed the value of 5-ALA for stereotactic biopsies of suspected brain tumors for the first time in the current study. At the target, we found strong fluorescence in the vast majority of samples of GBM, HGG, and lymphomas (98%, 90%, and 88%, respectively). All samples with strong 5-ALA fluorescence were found to contain representative tumor tissue according to neuroimaging and histopathological criteria corresponding to a positive predictive value of 100%. In contrast, the MRI low-intensity center of GBM was 5-ALA negative in all patients. The MRI non-enhancing periphery of GBM and the tissue outside the target of HGG were of vague fluorescence in approximately half of all samples. Of the specimens with vague fluorescence, representative material was present in only 50% of GBM and 33% of HGG. None of the samples of LGG showed visible 5-ALA fluorescence. Vague fluorescence at the target was also observed in half of the samples of metastases, none of them showing strong fluorescence. It is of note that some of the samples from the non-contrast-enhancing periphery of metastases also exhibited vague fluorescence unrelated to the fluorescence status of the metastasis itself and from histopathological view being non-representative.
From these observations, we conclude that only strong, but not vague 5-ALA fluorescence is a reliable and immediately available intraoperative marker of representative tumor tissue of GBM, HGG, and lymphomas. As we observed that all DIG and lymphomas with ring-like or nodular CE on MRI revealed strong 5-ALA fluorescence at the biopsy target, the use of 5-ALA seems justified in stereotactic biopsies of suspected GBM, HGG, and lymphomas with CE (ring-like, nodular, or patchy/faint).
Intraoperative histopathologic assessment of biopsy samples was reported to improve the diagnostic yield up to 99% [4, 11, 14, 33]. However, intraoperative histopathology is time consuming, costly, and not permanently available [4, 9, 29]. Therefore, Dammers et al. [4] recommended the application of an “on-demand” intraoperative frozen-section-analysis only if macroscopic aspects of the tissue specimens were not clearly pathological. Recently, Shooman et al. [29] even reported that intraoperative neuropathology should no longer be routinely recommended. However, according to our experience, we regard intraoperative histopathology as a crucial point. Our findings demonstrate that the application of 5-ALA in stereotactic tumor biopsies may be used in future to select cases that require intraoperative histopatholgy, namely only if vague or no fluorescence is present. Consequently, time and cost of operating room, operating room personnel, and neuropathology may be reduced in cases that do not require intraoperative histopathology.
Limits of 5-ALA in stereotactic tumor biopsies
5-ALA is currently only approved in the European Union, but not in the United States, for surgical resection of malignant gliomas under the trade name Gliolan® (Medac GmbH, Germany). The data from our current study suggest that this approval should be extended to stereotactic biopsies of suspected malignant gliomas.
As the aim of our study was to investigate the value of 5-ALA in stereotactic biopsies of brain tumors, the fluorescence status of 5-ALA in brain abscesses, infections or demyelinating diseases remains to be clarified.
Finally, despite the use of our neuroimaging and histopathological criteria for the definition of representative tumor tissue, we cannot rule out histopathological undergrading of malignant gliomas by stereotactic biopsy as the whole tumor specimen was not available for histopathological analysis in most patients.
Our proposal
From the observations of our present series, we propose the following strategy for stereotactic 5-ALA-controlled tumor biopsies (Table 5):
-
(1)
Strong 5-ALA fluorescence: Considering that all tissue samples of our series with strong 5-ALA fluorescence did always include representative tumor tissue, we will not routinely require intraoperative histopathology in such a case. Further, serial samples are not required and the procedure can be terminated. Consequently, the operation time might be significantly reduced by this approach in future. Oncoming studies will clarify, if the application of 5-ALA can also reduce the complication rate of stereotactic biopsies, considering that procedure-related morbidity/hemorrhages may directly be related to the number of tissue cylinders bioptically removed [5, 28].
-
(2)
Vague 5-ALA fluorescence: If the sample shows vague 5-ALA fluorescence, we will always demand intraoperative histopathology. If this reveals representative tissue of a metastasis, GBM, HGG, or lymphoma, the procedure can be terminated. On the other hand, if intraoperative histolopathology reveals an infiltration zone of GBM or white matter in a patient with suspected metastasis, the navigation accuracy should be checked and a re-biopsy taken into consideration. If intraoperative histology reveals LGG in a tumor with vague 5-ALA fluorescence, the biopsy was most likely taken from outside the anaplastic target of a HGG. In this case, serial biopsies are recommended, if they still contain only LGG tissue, the accuracy should be checked and re-biopsy performed.
-
(3)
5-ALA negative: A 5-ALA negative sample from the target may indicate a potential technical failure of the stereotactic targeting. In such a case, intraoperative histology is crucial and, if negative for tumor tissue or only infiltration zone of GBM or necrosis is present, the accuracy should be checked and a re-biopsy performed. In case of representative histology of a metastasis the procedure can be terminated. If histopathology reveals LGG, serial sampling will be required.
Conclusion
This prospective study investigates the clinical value of 5-ALA for detection of representative tissue in stereotactic brain tumor biopsies. According to our observations, strong 5-ALA fluorescence is a reliable novel intraoperative marker that indicates the sampling of representative tumor tissue of malignant gliomas and intracranial lymphomas. Thereby, strong 5-ALA fluorescence obviates the need for intraoperative histopathology and serial biopsies.
References
Aker FV, Hakan T, Karadereler S, Erkan M (2005) Accuracy and diagnostic yield of stereotactic biopsy in the diagnosis of brain masses: comparison of results of biopsy and resected surgical specimens. Neuropathology 25(3):207–213
Calisaneller T, Ozdemir O, Ozger O, Ozen O, Kiyici H, Caner H, Altinors N (2008) The accuracy and diagnostic yield of computerized tomography guided stereotactic biopsy in brain lesions. Turk Neurosurg 18(1):17–22
Dammers R, Haitsma IK, Schouten JW, Kros JM, Avezaat CJ, Vincent AJ (2008) Safety and efficacy of frameless and frame-based intracranial biopsy techniques. Acta Neurochir (Wien) 150(1):23–29
Dammers R, Schouten JW, Haitsma IK, Vincent AJ, Kros JM, Dirven CM (2010) Towards improving the safety and diagnostic yield of stereotactic biopsy in a single centre. Acta Neurochir (Wien) 152(11):1915–1921
Dickerman RD, Mittler MA, Morgan JT (2005) Stereotactic brain biopsies and operative complications: technique to further decrease risks. Acta Neurochir (Wien) 147(8):911–912
Dorward NL, Paleologos TS, Alberti O, Thomas DG (2002) The advantages of frameless stereotactic biopsy over frame-based biopsy. Br J Neurosurg 16(2):110–118
Eljamel MS (2009) Which intracranial lesions would be suitable for 5-aminolevulenic acid-induced fluorescence-guided identification, localization, or resection? A prospective study of 114 consecutive intracranial lesions. Clin Neurosurg 56:93–97
Field M, Witham TF, Flickinger JC, Kondziolka D, Lunsford LD (2001) Comprehensive assessment of hemorrhage risks and outcomes after stereotactic brain biopsy. J Neurosurg 94(4):545–551
Gralla J, Nimsky C, Buchfelder M, Fahlbusch R, Ganslandt O (2003) Frameless stereotactic brain biopsy procedures using the Stealth Station: indications, accuracy and results. Zentralbl Neurochir 64(4):166–170
Grossman R, Sadetzki S, Spiegelmann R, Ram Z (2005) Haemorrhagic complications and the incidence of asymptomatic bleeding associated with stereotactic brain biopsies. Acta Neurochir (Wien) 147(6):627–631
Hall WA (1998) The safety and efficacy of stereotactic biopsy for intracranial lesions. Cancer 82(9):1749–1755
Hall WA, Truwit CL (2008) Intraoperative MR-guided neurosurgery. J Magn Reson Imaging 27(2):368–375
Hefti M, von Campe G, Moschopulos M, Siegner A, Looser H, Landolt H (2008) 5-aminolevulinic acid induced protoporphyrin IX fluorescence in high-grade glioma surgery: a one-year experience at a single institution. Swiss Med Wkly 138(11–12):180–185
Heper AO, Erden E, Savas A, Ceyhan K, Erden I, Akyar S, Kanpolat Y (2005) An analysis of stereotactic biopsy of brain tumors and nonneoplastic lesions: a prospective clinicopathologic study. Surg Neurol 64(Suppl 2):S82–88
Hermann EJ, Hattingen E, Krauss JK, Marquardt G, Pilatus U, Franz K, Setzer M, Gasser T, Tews DS, Zanella FE, Seifert V, Lanfermann H (2008) Stereotactic biopsy in gliomas guided by 3-tesla 1H-chemical-shift imaging of choline. Stereotact Funct Neurosurg 86(5):300–307
Jackson RJ, Fuller GN, Abi-Said D, Lang FF, Gokaslan ZL, Shi WM, Wildrick DM, Sawaya R (2001) Limitations of stereotactic biopsy in the initial management of gliomas. Neuro Oncol 3(3):193–200
Jain D, Sharma MC, Sarkar C, Deb P, Gupta D, Mahapatra AK (2006) Correlation of diagnostic yield of stereotactic brain biopsy with number of biopsy bits and site of the lesion. Brain Tumor Pathol 23(2):71–75
Kelly PJ (1991) Tumor stereotaxis. WB Saunders, Philadelphia
Kondziolka D, Firlik AD, Lunsford LD (1998) Complications of stereotactic brain surgery. Neurol Clin 16(1):35–54
Krieger MD, Chandrasoma PT, Zee CS, Apuzzo ML (1998) Role of stereotactic biopsy in the diagnosis and management of brain tumors. Semin Surg Oncol 14(1):13–25
Kulkarni AV, Guha A, Lozano A, Bernstein M (1998) Incidence of silent hemorrhage and delayed deterioration after stereotactic brain biopsy. J Neurosurg 89(1):31–35
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK (2007) WHO classification of the central nervous system. IARC, Lyon
Lunsford LD, Niranjan A, Khan AA, Kondziolka D (2008) Establishing a benchmark for complications using frame-based stereotactic surgery. Stereotact Funct Neurosurg 86(5):278–287
Muragaki Y, Chernov M, Maruyama T, Ochiai T, Taira T, Kubo O, Nakamura R, Iseki H, Hori T, Takakura K (2008) Low-grade glioma on stereotactic biopsy: how often is the diagnosis accurate? Minim Invasive Neurosurg 51(5):275–279
Pirotte B, Goldman S, David P, Wikler D, Damhaut P, Vandesteene A, Salmon L, Brotchi J, Levivier M (1997) Stereotactic brain biopsy guided by positron emission tomography (PET) with [F-18]fluorodeoxyglucose and [C-11]methionine. Acta Neurochir Suppl 68:133–138
Pirotte B, Goldman S, Massager N, David P, Wikler D, Lipszyc M, Salmon I, Brotchi J, Levivier M (2004) Combined use of 18F-fluorodeoxyglucose and 11C-methionine in 45 positron emission tomography-guided stereotactic brain biopsies. J Neurosurg 101(3):476–483
Potzi C, Becherer A, Marosi C, Karanikas G, Szabo M, Dudczak R, Kletter K, Asenbaum S (2007) [11C] methionine and [18F] fluorodeoxyglucose PET in the follow-up of glioblastoma multiforme. J Neurooncol 84(3):305–314
Sawin PD, Hitchon PW, Follett KA, Torner JC (1998) Computed imaging-assisted stereotactic brain biopsy: a risk analysis of 225 consecutive cases. Surg Neurol 49(6):640–649
Shooman D, Belli A, Grundy PL (2010) Image-guided frameless stereotactic biopsy without intraoperative neuropathological examination. J Neurosurg 113(2):170–178
Stummer W, Novotny A, Stepp H, Goetz C, Bise K, Reulen HJ (2000) Fluorescence-guided resection of glioblastoma multiforme by using 5-aminolevulinic acid-induced porphyrins: a prospective study in 52 consecutive patients. J Neurosurg 93(6):1003–1013
Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ (2006) Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol 7(5):392–401
Stummer W, Stepp H, Moller G, Ehrhardt A, Leonhard M, Reulen HJ (1998) Technical principles for protoporphyrin-IX-fluorescence guided microsurgical resection of malignant glioma tissue. Acta Neurochir (Wien) 140(10):995–1000
Tilgner J, Herr M, Ostertag C, Volk B (2005) Validation of intraoperative diagnoses using smear preparations from stereotactic brain biopsies: intraoperative versus final diagnosis—influence of clinical factors. Neurosurgery 56(2):257–265, discussion 257–265
Widhalm G, Krssak M, Minchev G, Wohrer A, Traub-Weidinger T, Czech T, Asenbaum S, Marosi C, Knosp E, Hainfellner JA, Prayer D, Wolfsberger S (2011) Value of 1H-magnetic resonance spectroscopy chemical shift imaging for detection of anaplastic foci in diffusely infiltrating gliomas with non-significant contrast-enhancement. J Neurol Neurosurg Psychiatry 82(5):512–20
Widhalm G, Wolfsberger S, Minchev G, Woehrer A, Krssak M, Czech T, Prayer D, Asenbaum S, Hainfellner JA, Knosp E (2010) 5-Aminolevulinic acid is a promising marker for detection of anaplastic foci in diffusely infiltrating gliomas with nonsignificant contrast enhancement. Cancer 116(6):1545–1552
Wolfsberger S, Rossler K, Regatschnig R, Ungersbock K (2002) Anatomical landmarks for image registration in frameless stereotactic neuronavigation. Neurosurg Rev 25(1–2):68–72
Woodworth G, McGirt MJ, Samdani A, Garonzik I, Olivi A, Weingart JD (2005) Accuracy of frameless and frame-based image-guided stereotactic brain biopsy in the diagnosis of glioma: comparison of biopsy and open resection specimen. Neurol Res 27(4):358–362
Zoeller GK, Benveniste RJ, Landy H, Morcos JJ, Jagid J (2009) Outcomes and management strategies after nondiagnostic stereotactic biopsies of brain lesions. Stereotact Funct Neurosurg 87(3):174–181
Acknowledgments
We thank Dr. Harald Heinzl, Department of Medical Computer Sciences, for statistical advice, Irene Leisser, and Gerda Ricken for technical assistance with preparation of tissue specimens and Ingrid Dobsak for drawing the figure. This study was performed within the PhD thesis project of Clinical Neuroscience (CLINS) at the Medical University Vienna.
Funding, financial disclosures, conflict of interest
None declared
Author information
Authors and Affiliations
Corresponding author
Additional information
Comments
Michel Mittelbronn, Frankfurt, Germany
The suitability of 5-ALA application has been validated in several studies using fluorescence-guided full open resections of malignant brain tumors also showing a beneficial effect in a randomized phase III trial [1]. In constrast, there is only poor data about the suitability of 5-ALA in the stereotactic neurosurgical setting [2]. In their manuscript, Widhalm et al. aim to investigate the routine suitability of 5-aminolevulinic acid (5-ALA) for neurosurgical stereotactic biopsies by intraoperatively checking for 5-ALA fluorescence using a modified neurosurgical microscope with the feasibility to switch from conventional white light to violet-blue excitation light for detection of potential 5-ALA fluorescence. Using this approach, they successfully reached a positive predictive value of 100% according to the final neuropathological diagnoses in conjunction with clinico-radiological data—making an intraoperative neuropathological diagnostic procedure unnecessary in those cases. However, the authors acknowledge the necessity of neuropathologists coming to the operation theater to perform an intraoperative diagnoses in cases of vague or absent 5-ALA fluorescence. If this appraoch is used in a routine base from now one, it would also be of great interest to collect also cases (if there will be any) which show strong 5-ALA fluorescence without making a definite diagnoses possible or in which final neuropathological diagnostics reveal unexpected findings other than high-grade glioma or lymphoma. In summary, the presented study is an important contribution improving the diagnostic neurosurgical–neuropathological setting and thereby also consecutive patient treatment. Especially, Table 5 of the manuscript is a very helpful guideline for both neurosurgeons and neuropathologists which might further considerably reduce working time and costs.
References
1. Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ: Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicenter phase III trial. Lancet Oncol 7:392–401, 2006.
2. Hefti M, von Campe G, Moschopulos M, Siegner A, Looser H, Landolt H: 5-aminolevulinic acid induced protoporphyrin IX fluorescence in high-grade glioma surgery: a one-year experience at a single institutuion. Swiss Med Wkly 138 (11–12):180–185, 2008.
Walter Stummer, Muenster, Germany
The authors present their interesting experience using 5-ALA for intra-operative validation of biopsy material in 50 patients harboring a number of lesions (HGG, LGG, metastasis, lymphoma). They find that all strongly fluorescing biopsies were diagnostic. They conclude that in case strong fluorescence is observed, this would obviate the necessity for serial biopsies or frozen sections.
The authors approach is interesting and a comparably large experience has not been published before. This series provides a basis for 5-ALA to be used in the context of stereotactic biopsies and the method may be of value for reducing the number of biopsies in a given patient. Also, it may help those neurosurgeons performing biopsies that do not have access to intraoperative neuropathology.
There are some weaknesses that need to be addressed. The authors rely on visible “strong” fluorescence. In my experience, the assumption of “strong” versus “weak” fluorescence requires experience gained from open resection, which many neurosurgeons do not have. Furthermore, there is the risk of photo bleaching which might be an issue with small samples. Bacterial (abscess, cerebritis) nor non-infectious inflammation was not part of this study. Abscesses are a standard differential diagnosis for marginally enhancing cerebral lesions. We have seen fluorescence in the adjacent brain in these lesions, which has been weak and we have also seen fluorescence in non-infectious inflammation. Finally, diagnosis of glioblastomas cannot rely on “strong” fluorescence alone. According to the WHO criteria (Louis DN, Ohgaki H, Wiestler OD, et al. WHO classification of tumours of the central nervous system. Lyon: IARC, 2007) the detection of necrosis is still required, which may be missed when relying on fluorescence alone and may result in undergrading and wrong therapeutic decision.
At the end of the day, 5-ALA will not replace the need for careful planning and immaculate neurosurgical technique. However, in the context of stereotactic biopsies using 5-ALA-induced tissue fluorescence carries the potential to reduce the number of samples required for confident diagnosis.
Rights and permissions
About this article
Cite this article
Widhalm, G., Minchev, G., Woehrer, A. et al. Strong 5-aminolevulinic acid-induced fluorescence is a novel intraoperative marker for representative tissue samples in stereotactic brain tumor biopsies. Neurosurg Rev 35, 381–391 (2012). https://doi.org/10.1007/s10143-012-0374-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-012-0374-5