Abstract
Magnesium, one of the essential trace elements, plays important roles in maintaining both normal cellular and body functions. S100 calcium-binding protein B (S100B) has been used as a marker of glial damage in several neurological disorders. Thirty patients with ruptured intracranial aneurysms treated by clipping are included. The patients were randomized within 4 days after the attack of hemorrhage. The patients were divided into two groups with 15 patients in each group. Group I received magnesium infusion within 4 days. Group II is the control group. World Federation of Neurological Surgeons, Fisher, and Glasgow outcome scores are evaluated at an intensive care unit in addition to 3 months clinical follow-up evaluation. Samplings of serum S100B were performed on admission and on postoperative days 1, 3, and 7. There is a statistically significant difference between both groups as regards the second reading of the S100B (day 1 postoperative; P < 0.05). There is no statistically significant difference between both groups as regards outcome at 3 months using clinical status and S100B values. There is a tendency in the magnesium group to have better outcomes. Further studies with more number of patients with subarachnoid hemorrhage are needed to determinate the accuracy of S100B protein as a prognostic marker and of magnesium sulfate as a neuroprotector.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Several studies have demonstrated that traumatic injury to the brain causes a decrease in magnesium concentration correlated with injury severity. Since then, more attention has been paid to magnesium sulfate (MgSO4) for its neuroprotective effects. Magnesium sulfate has been widely used in clinical practice for almost 100 years. It is cheap, widely available, and with an established safety profile [8].
Magnesium is the fourth most abundant cation in the body and the second most abundant cation in the intracellular fluid. Magnesium is essential for cell functions such as preservation of membrane integrity, protein synthesis, energy metabolism, maintenance of ionic gradients, maintenance of smooth muscle tone, regulation of calcium transport, and reduction of calcium accumulation. Since magnesium is important in maintaining many cellular processes, changes in magnesium status before, during, and after a brain insult are likely to have a profound effect on neurological outcome. The restoration of magnesium homeostasis in the brain along with magnesium’s known anti-excitotoxic actions and vascular effects has been the rationale for the administration of magnesium as a neuroprotective treatment following traumatic brain injury, seizure, subarachnoid hemorrhage, and cerebral ischemia [6, 14].
Protein S100 is a calcium-binding dimeric protein composed of two immunologically distinct subunits, α and ß. Its ßß dimeric form, which predominates in the central nervous system (CNS), is referred to as S100 calcium-binding protein B (S100B). It is produced and released predominantly by astrocytes in physiological and pathological conditions. Depending on its extracellular concentration, S100B can play a trophic or toxic role in both neurons and glial cells [13].
In a multivariate model, S100B emerged as an outcome predictor that was independent of age, sex, stroke severity, etiology, lesion side, and risk factors. S100B protein serum concentration has been extensively studied in severe head trauma, acute ischemic stroke, and cardiac arrest. Regarding subarachnoid aneurysmal hemorrhage, S100B has been evaluated in blood, cerebrospinal fluid, and microdialysis perfusate. From studies performed in subarachnoid hemorrhage (SAH) and including large series of patients, it can be stated that S100B correlates with neurological deficit at admission and outcome [11].
Methods
The study recruited patients with acute aneurysmal SAH admitted to Alexandria University Hospital, Egypt between December 2008 and February 2010. All human studies have been approved by the appropriate ethics committee and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki. All persons gave their informed consent prior to their inclusion in the study. Written informed consents were obtained from either the patients or their next-of-kin before enrollment.
Patients were eligible for the study if they were 18 years or older and had an intracranial aneurysm that was considered as the cause of hemorrhage. Patients who were moribund on admission or could not receive study drug treatment within 4 days after the onset of SAH were excluded from the study. Other exclusion criteria were pregnancy, major cardiac disease, significant renal impairment, and contraindication to magnesium infusion like allergy, preexisting neurological disability from stroke, dementia, other neurological diseases; or concurrent participation in another clinical trial [25].
Normotension, defined as systolic arterial pressure between 120 and 160 mmHg, was maintained except during episodes of vasospasm when hypertension, hypervolemia, and hemodilution were induced. Patients of both groups also received nimodipine infusion at a rate of 20 μg/kg/h for 14 days. The maintenance dose was adjusted according to the hemodynamics of the patients and the use of vasopressor or inotropes for hypotension that is defined as systolic blood pressure less than 110 mmHg. The study drugs (nimodipine and magnesium) would be stopped if the systolic blood pressure is less than 110 mmHg and the patient is unresponsive to intravenous administration of fluids and/or vasopressor or inotropes.
After confirmation of intracranial aneurysm by computed tomogram (CT) or digital subtraction angiogram, patients were randomly allocated to receive either MgSO4 or saline infusion. For patients receiving the active treatment, MgSO4 16 mmol was administered over 20 min; this was followed by a continuous infusion of 65 mmol per day for 14 days after occlusion of the aneurysm. Plasma magnesium concentration was measured regularly. Laboratory results were reviewed by a medical staff independent to the neuroanesthesia team. Infusion was adjusted so that the plasma magnesium concentration was raised to approximately twice the baseline value and less than 2.5 mmol/L. Patients in the control group received an equivalent volume of normal saline.
Clinical and CT evaluation
At admission, clinical severity was assessed using the World Federation of Neurological Surgeons (WFNS) [19] score and Glasgow Coma Scale. The initial CT was reviewed by an independent radiologist blinded to clinical history, therapeutics, and S100B values, and these were classified according to the modified Fisher score [9, 24]. Neurological outcome was assessed using the Glasgow Outcome Scale (GOS) [10] score at 3 months by an anesthesiologist and a neurosurgeon unaware of the patient original treatment.
Grading was done by the WFNS. For statistical purpose, it will be divided into two groups—good-grade patients group (I, II, and III) and poor-grade patients group (IV and V).
GOS was done 3 months after surgery. For statistical purpose, the five categories of GOS will be combined into two categories—either unfavorable outcome, including the first two grades, or favorable outcome, including the last three grades.
The modified Fisher score is used and defined as follows: grade 1, no subarachnoid blood; grade 2, broad diffusion of subarachnoid blood; grade 3, with clots or thick layers of blood; grade 4, intraventricular hemorrhage or intracerebral hematoma, no clot; and grade 5, intraventricular hemorrhage or intracerebral hematoma with clot [24].
S100B protein measurement
Venous blood samples to measure S100B protein were immediately centrifuged and serum frozen. The serum concentrations of S100B protein were measured by an enzyme-linked immunosorbent assay. The detection limit of the assay is 20 pg/ml. Values in healthy individuals are considered to be below 175 pg/ml. For measuring S100B, blood samples were taken at the following intervals:
-
A baseline sample was withdrawn just after admission in the ICU.
-
Twenty-four hours after the start of the operation
-
The third postoperative day.
-
The seventh postoperative day.
Statistical analysis
Statistical analysis was performed with the use of Statistical Package for Social Sciences software. Parametric data were analyzed using the t test, and nonparametric data (e.g., Fisher, WFNS, and GOS scores) by the Chi-square and Mann–Whitney rank-sum test. Differences were considered significant at the P < 0.05 level.
Results
During the study period, 37 patients were admitted to our intensive care unit with a diagnosis of SAH. Of these, seven were excluded for the following reasons: delay of more than 4 days between admission and onset of symptoms (n = 3), delay of more than 4 days between admission and date of intervention (n = 2), or no surgical treatment (n = 2). Eventually, 30 patients were included (9 men and 21 women).
The remaining 30 patients received the assigned treatment. Clinical data collected at the time of randomization are summarized in Table 1. Patient characteristics were similar between groups, and there was no statistically significant difference between both groups as regards gender, age, and sex.
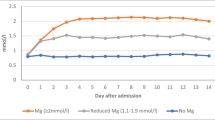
All patients received surgical clipping treatment within 4 days after SAH. The dose regime of 64 mmol/L per day was based on a dose finding study and aimed at maintaining serum magnesium levels within the range of 1.0 to 2.0 mmol/L during magnesium treatment [22]. Other than a flushing sensation, no side effects of magnesium infusion, such as bradycardia, hypotension, or bradypnea, were observed.
Three patients treated with nimodipine in the control group experienced inadvertent systemic hypotension, and the dosage had to be reduced but not stopped. There are three patients in the magnesium group and two patients in the control group who suffered from vasospasm; therefore, inotropes had to be used (norepinephrine 0.1–0.2 μg/kg/min until systolic blood pressure 120–150 mmHg if the aneurysm is not secured and 160–200 mmHg if the aneurysm is secured).
The S100B serum concentrations in the second reading (first postoperative day) showed a statistically significant difference between both groups. The control group readings reach a peak with a median of 392 pg/ml, P = 0.004, but the magnesium group showed a gradual decrease without a peak (median, 225 pg/ml). There was no statistically significant difference between both groups as regards the first, third, or the fourth readings (Fig. 1 and Table 2).
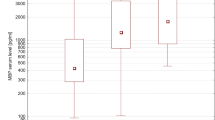
The receiver operating characteristic curve (ROC) showed a cutoff point for S100B initial value of 387.5 pg/ml above, which indicates a bad prognosis. The cutoff point of the S100B average readings is 336.9 pg/ml. The ROC curves showed that the initial and average values of the four readings of S100B significantly predicted a poor outcome (Fig. 2). The area under the curve was significantly higher for the average values of the four readings of S100B than the initial value (1.000 and 0.965, respectively).
As regards WFNS and Fisher score, there were no statistically significant differences between the two studied groups at admission, discharge, and 3 months after SAH (Tables 3 and 4). Glasgow outcome scale 3 months after SAH showed an improvement in the favorable outcome, 73.3% in the magnesium group to 60% in the control group, but it did not reach a statistically significant difference (P = 0.43; Tables 5 and 6).
Discussion
The use of MgSO4 in aneurysmal SAH has a sound theoretical basis, with enough circumstantial evidence in the obstetric and neurological literature to warrant clinical investigation in neurosurgical practice. This study found that magnesium may have a neuroprotective effect. Patients who received magnesium had statistically significant decreases in the S100B protein readings in the first postoperative day. As regards S100B protein readings on postoperative day 7, there was a difference between both groups, but the difference was not statistically significant [2, 17].
As regards outcome measured by the Glasgow outcome score at 3 months after SAH, magnesium sulfate infusion reduced the risk of unfavorable outcome in these patients from 40% in the control group to 26.7% in the magnesium group. This number of patients remains too small for definitive conclusions.
Muroi C et al. found that vasospasm-induced infarctions occurred less often in the magnesium group although without reaching statistical significance. Regarding the outcome, the on treatment analysis showed a statistically significant better outcome after 3 months in patients treated with MgSO4. A trend toward better outcome compared to the placebo group was found after 1 year [15].
Veyna et al. found that administration of high-dose MgSO4 following aneurysmal SAH in 40 patients was safe, and steady magnesium levels in the range of 4 to 5.5 mg/dl were easily maintained. A higher percentage of patients (15%) obtained GOS scores of 4 or 5 in the group treated with MgSO4, but the trend did not reach a statistically significant level [23].
Schmid-Elsaesser et al. conducted a pilot study that compared intravenous magnesium to nimodipine. They concluded that the efficacy of magnesium in preventing delayed ischemic neurological deficit (DIND) in patients with aneurysmal SAH is comparable with that of nimodipine. This study advised a large, multicenter trial to define the role of magnesium in SAH better, and especially studies on the combined administration of nimodipine and magnesium seem to be promising [21].
On the other hand, in Intravenous Magnesium Sulphate for Aneurysmal Subarachnoid Hemorrhage study, a phase III clinical trial for magnesium sulfate infusion after aneurysmal subarachnoid hemorrhage was needed to establish or confirm its potential benefit. In conclusion, the results do not support a significant clinical benefit of intravenous magnesium sulfate infusion over placebo infusion. Possible explanations of the lack of action could be the low cerebrospinal fluid penetration of peripherally infused magnesium sulfate or the inability to provide neuroprotection for early brain injury with the current time window of administration [25].
Nimodipine is a dihydropyridine that blocks calcium influx through the l-type calcium channels. It is the most rigorously studied and the only drug approved by the US Food and Drug Administration for use in the treatment of vasospasm [12]. In the present study, nimodipine was used in both groups by continuous infusion at a rate of 20 μg/kg/h after induction of anesthesia and continued for 2 weeks postoperatively.
Barker and Ogilvie et al., in a well-organized meta-analysis, showed notable improvements in good and good-plus-fair outcomes and reductions in death due to vasospasm and infarcts with nimodipine. Although it has never been tested formally, in a review of several thousand reported cases, the overall incidence of DIND (15.9%) was somewhat lower when intravenous rather than oral nimodipine was used [1].
In contrary to the present study, one meta-analysis failed to show a statistically significant reduction in angiographically detected cerebral vasospasm among patients treated with intravenous nimodipine [7]. This was confirmed by an in vitro study in which nimodipine failed to promote relaxation in the spastic vascular smooth muscle [3].
In the present study, S100B initial values and average values of the four readings correlated with outcomes at 3 months. The cutoff point of the initial value and average of the four readings are 387.5 and 336.9 pg/ml, respectively. S100B initial values more than 387.5 pg/ml (0.387 μg/l) correlated significantly with poor outcome at 3 months.
Weiss et al. showed that a time course evaluation of S100B had a useful and independent prognostic value to assess outcome in SAH patients. Initial and mean daily values above 0.4 μg/l significantly predicted a poor outcome [24]. This threshold is close to the one defined by Raabe et al., at 0.5 μg/l for various neurologic disorders, and by Foerch et al., at 0.35 μg/l in ischemic stroke [18].
Weiss et al. concluded also that measurement of S100B plasma concentration is a good indicator of severity and predictor of 6-month outcome in SAH patients. Similar to troponin and heart disease, S100B may be important for the future improvement of clinical management and outcome in SAH patients [24]. Sanchez-Pena et al. found that mean 15-day S100B performed better than mean 8-day S100B and initial S100B. This indicates that only prolonged assessment of biological markers for brain ischemia is warranted in patients with SAH [20].
Oertel et al. found that S100B may be a reliable predictor of vasospasm and long-term outcome after SAH. Interestingly, patients with high S100B are unlikely to develop vasospasm, but an S100B that is higher than 1 lg/l within the first 3 days after SAH is predictive of unfavorable outcome [16]. On the contrary, Schmid-Elsaesser et al. despite finding higher levels of S100B in WFNS grade 4 and 5 patients compared with WFNS grade 1–3 patients, there was no correlation between the neuronal markers NSE and S100 and patient outcome in the study [21].
There are many biochemical markers demonstrating the CNS damage following coronary artery bypass grafting (CABG). The protein S100B is one of them, and its concentrations depend on the severity of CNS damage. In the present study, infusion of magnesium sulfate reduced serum S100B protein concentrations especially just after surgery in the first postoperative day [5].
Dabrowski et al. concluded that extracorporeal circulation resulted in S100beta elevation and infusion of 10 mg of MgSO4 per minute reduced serum S100beta concentrations. They also concluded that S100beta increased while total Mg decreased during CABG, the highest serum S100beta concentrations were noted just after surgery, the changes in serum S100beta concentrations correlated with those in serum total Mg concentrations, and finally, the decrease in serum magnesium concentrations resulted in an elevation in serum S100beta concentrations [4, 5].
Recommendations
-
Magnesium sulfate may be used in SAH as it may have a neuroprotective effect on the cellular level with minimal side effects.
-
It is necessary to reappraise the usefulness of daily S100B measurements in SAH and to further study its relation with clinical and CT evaluations as well as with treatment strategy and outcome.
-
It is recommended for further study with large number of cases to see the effect of magnesium sulfate on the outcome.
References
Barker FG, Ogilvy CS (1996) Efficacy of prophylactic nimodipine for delayed ischemic deficit after subarachnoid hemorrhage: a metaanalysis. J Neurosurg 84(3):405–414
Boet R, Chan MT, Poon WS, Wong GK, Wong HT, Gin T (2005) Intravenous magnesium sulfate to improve outcome after aneurysmal subarachnoid hemorrhage: interim report from a pilot study. Acta Neurochir Suppl 95:263–264
Clark JF, Pyne GJ, Choutka J, Carrozzella JA, Khoury J, Broderick JP (2001) In vitro therapy with dobutamine, isoprenaline and sodium nitroprusside protects vascular smooth muscle metabolism from subarachnoid haemorrhage induced cerebral vasospasm. Acta Neurochir (Wien) 143(7):721–728
Dabrowski W (2009) Magnesium supplementation significantly reduces serum S100beta concentrations in patients who have undergone coronary artery bypass surgery. Magnes Res 22(1):21–31
Dabrowski W (2007) Do changes in S100beta protein correlate with serum magnesium concentrations in patients undergoing extracorporeal circulation? Magnes Res 20(3):168–176
Dorhout Mees SM, van den Bergh WM, Algra A, Rinkel GJ (2007) Achieved serum magnesium concentrations and occurrence of delayed cerebral ischaemia and poor outcome in aneurysmal subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry 78(7):729–731
Feigin VL, Rinkel GJ, Algra A, Vermeulen M, van Gijn J (1998) Calcium antagonists in patients with aneurysmal subarachnoid hemorrhage: a systematic review. Neurology 50(4):876–883
Feng DF, Zhu ZA, Lu YC (2004) Effects of magnesium sulfate on traumatic brain edema in rats. Chin J Traumatol 7(3):148–152
Fisher CM, Kistler JP, Davis JM (1980) Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning. Neurosurgery 6(1):1–9
Jennett B, Bond M (1975) Assessment of outcome after severe brain damage. Lancet 1(7905):480–484
Kay A, Petzold A, Kerr M, Keir G, Thompson E, Nicoll J (2003) Decreased cerebrospinal fluid apolipoprotein E after subarachnoid hemorrhage: correlation with injury severity and clinical outcome. Stroke 34(3):637–642
Keyrouz SG, Diringer MN (2007) Clinical review: prevention and therapy of vasospasm in subarachnoid hemorrhage. Crit Care 11(4):220
Kleine TO, Benes L, Zofel P (2003) Studies of the brain specificity of S100B and neuron-specific enolase (NSE) in blood serum of acute care patients. Brain Res Bull 6(3):265–279
Meloni BP, Zhu H, Knuckey NW (2006) Is magnesium neuroprotective following global and focal cerebral ischemia? A review of published studies. Magnes Res 19(2):123–137
Muroi C, Terzic A, Fortunati M, Yonekawa Y, Keller E (2008) Magnesium sulfate in the management of patients with aneurysmal subarachnoid hemorrhage: a randomized, placebo-controlled, dose-adapted trial. Surg Neurol 69(1):33–39, Discussion 9
Oertel M, Schumacher U, McArthur DL, Kastner S, Boker DK (2006) S-100B and NSE: markers of initial impact of subarachnoid haemorrhage and their relation to vasospasm and outcome. J Clin Neurosci 13(8):834–840
Prevedello DM, Cordeiro JG, de Morais AL, Saucedo NS Jr, Chen IB, Araújo JC (2006) Magnesium sulfate: role as possible attenuating factor in vasospasm morbidity. Surg Neurol 65:S1:14–S1:21
Raabe AKO, Woszczyk A, Lang J, Gerlach R, Zimmermann M, Seifert V (2004) S-100B protein as a serum marker of secondary neurological complications in neurocritical care patients. Neurol Res 26:440–445
(1988) Report of World Federation of Neurological Surgeons Committee on a Universal Subarachnoid Hemorrhage Grading Scale. J Neurosurg 68(6): 985–986
Sanchez-Peña P, Pereira AR, Sourour NA, Biondi A, Lejean L, Colonne C, Boch AL, Al Hawari M, Abdennour L, Puybasset L (2008) S100B as an additional prognostic marker in subarachnoid aneurysmal hemorrhage. Crit Care Med 36:2267–2273
Schmid-Elsaesser R, Kunz M, Zausinger S, Prueckner S, Briegel J, Steiger HJ (2006) Intravenous magnesium versus nimodipine in the treatment of patients with aneurysmal subarachnoid hemorrhage: a randomized study. Neurosurgery 58(6):1054–1065
Van den Bergh WM, Albrecht KW, Berkelbach van der Sprenkel JW, Rinkel GJ (2003) Magnesium therapy after aneurysmal subarachnoid haemorrhage a dose-finding study for long term treatment. Acta Neurochir (Wien) 145(3):195–199
Veyna RS, Seyfried D, Burke DG, Zimmerman C, Mlynarek M, Nichols V, Marrocco A, Thomas AJ, Mitsias PD, Malik GM (2002) Magnesium sulfate therapy after aneurysmal subarachnoid hemorrhage. J Neurosurg 96(3):510–514
Weiss N, Sanchez-Pena P, Roche S, Beaudeux JL, Colonne C, Coriat P, Puybasset L (2006) Prognosis value of plasma S100B protein levels after subarachnoid aneurysmal hemorrhage. Anesthesiology 104(4):658–666
Wong GK, Poon WS, Chan MT, Boet R, Gin T, Stephanie CP, Zee B (2010) Intravenous Magnesium Sulphate for Aneurysmal Subarachnoid Hemorrhage (IMASH): a randomized, double-blinded, placebo-controlled, multicenter phase III trial. Stroke 41:921–926
Author information
Authors and Affiliations
Corresponding author
Additional information
Comments
Claudius Thomé, Innsbruck, Austria
Hassan et al. have performed a randomized study on the effect of intravenous magnesium sulfate on outcome and S100B levels after aneurysmal subarachnoid hemorrhage (SAH) in a series of 30 patients. S100B was chosen as a surrogate parameter for neurological damage. Patients were included, treated by surgical clipping, and started on an established dose of magnesium or an equal volume of saline within 4 days after SAH. Unfortunately, S100B levels were analyzed in relation to the time point of surgery rather than the time point of SAH, so that it is unclear whether the significantly lower S100B value on the first postoperative day in the magnesium group is a therapeutic effect or merely due to intergroup differences in timing. Vasospasm was present in 3 of 15 treated versus 2 of 15 control patients, but a definition of vasospasm—be it clinically, angiographically, or per TCD—is lacking. There are some methodological flaws like the absence of a power calculation as the small patient numbers would have never allowed meaningful conclusions. Most of these drawbacks can be somewhat overcome by the dedication to perform a randomized study, which is considered the most appropriate means to establish scientific knowledge. One criticism, however, cannot be stressed enough: Favorable outcome of 73% in the magnesium group versus 60% in the control group reaching a P value of 0.43 simply states that there is no difference in outcome! This cannot be interpreted as “a tendency in the magnesium group to have better outcomes” as concluded in the abstract!
Overall, I like the idea of the manuscript to combine a surrogate biomarker with a randomized study design for a potentially neuroprotective agent. Nevertheless, there already is a large amount of data on magnesium sulfate in SAH from six phase II and one phase III randomized-controlled trials, which has been recently reviewed by Suarez et al. (1) The phase III trial with 327 patients revealed no difference in outcome whatsoever (2), which is in line with the results of the present study.
References
1. Suarez JI (2011) Participants in the International Multidisciplinary Consensus Conference on the Critical Care Management of Subarachnoid Hemorrhage. Magnesium sulfate administration in subarachnoid hemorrhage. Neurocrit Care 15:302–307.
2. Wong GK, Poon WS, Chan MT, Boet R, Gin T, Ng SC, Zee BC (2010) Plasma magnesium concentrations and clinical outcomes in aneurysmal subarachnoid hemorrhage patients: post hoc analysis of intravenous magnesium sulfate for aneurysmal subarachnoid hemorrhage trial. Stroke 41:1841–1844.
Rights and permissions
About this article
Cite this article
Hassan, T., Nassar, M., Elhadi, S.M. et al. Effect of magnesium sulfate therapy on patients with aneurysmal subarachnoid hemorrhage using serum S100B protein as a prognostic marker. Neurosurg Rev 35, 421–427 (2012). https://doi.org/10.1007/s10143-011-0368-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-011-0368-8