Abstract
Direct revascularization has been used successfully to prevent strokes by improving regional cerebral blood flow (rCBF) to the affected hemisphere faster in patients with moyamoya disease (MMD). Since most literatures have focused on the rCBF changes of operative hemisphere, we evaluated the hemodynamics of nonoperative side by xenon-enhanced computed tomography (Xe-CT) and acetazolamide challenge test in patients with MMD during a short time follow-up. Fifteen MMD patients with unilateral ischemic presentations who received direct revascularization on the symptomatic hemispheres with complete hemodynamic evaluations by Xe-CT and acetazolamide challenge test were enrolled. Hemodynamic evaluations were performed 1, 3, and 6 months, postoperatively. The postoperative rCBF and cerebral vascular reserve (CVR) were recorded and correlated with clinical outcome. Angiography was performed if the patient had neurological deterioration or deficits. The average follow-up time was 8.5 ± 3.5 months. Three months after the ipsilateral direct revascularization, the CVR of nonoperative hemispheres (25.8 ± 8.1%) began to decrease significantly (P = 0.003). Six months later, the rCBF showed a downward trend in nonoperative hemispheres (47.4 ± 8.0 ml·100 g−1 min−1) than the preoperative status, but the difference was not significant (P = 0.053). Three patients presented with decreased rCBF and impaired CVR in the nonoperative hemispheres. Among them, two patients were symptomatic. Unilateral direct revascularization in symptomatic hemisphere for MMD patient could induce CVR impaired in primary asymptomatic hemisphere during the short term after the surgery. Therefore, critical follow-up, especially the hemodynamic follow-up in the asymptomatic hemispheres should be performed in patients with MMD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Moyamoya disease (MMD) is a progressive occlusive disease in bilateral distal internal carotid arteries (ICA) associated with a secondary fine vascular network serving as collateral channels at the basal brain which give rise to the characteristic reticulate (“puff of smoke”) on angiography. The natural history of MMD is variable. However, regardless of its slow or rapid progression with neurological decline, MMD will progress in the majority of patients inevitably. The medical therapy alone cannot interrupt its progression. However, direct revascularization has been used successfully to prevent strokes by improving regional cerebral blood flow to the affected hemisphere faster and more reliable in the last three decades [6, 9, 15]. But most published literatures have focused on the regional cerebral blood flow (rCBF) changes in the operative hemisphere. There are few researches focusing on the contralateral hemodynamic changes after unilateral direct revascularization is performed on the hemisphere demonstrated with ischemic events initially [2, 13]. Therefore, we evaluated the cerebral hemodynamics of nonoperative hemispheres during a short-time follow-up by xenon-enhanced computed tomography (Xe-CT) and acetazolamide challenge test in 15 patients with MMD.
Methods
Patient population and revascularization
Fifteen cases of MMD treated at our center from June 2008 to July 2009 were enrolled. All the patients presented with unilateral ischemic events. The diagnosis of MMD was made based on the typical angiographic findings of bilateral terminal internal carotid artery occlusion with development of abnormal collateral vessels at the base of brain. The bilateral steno-occlusive changes in the ICA were classified into six stages according to the Suzuki and Takaku Grading system [17]. Patients with intracranial hemorrhage or unilateral moyamoya phenomenon were excluded. Other exclusive criteria included the presence of any other diseases that might be responsible for the vasculopathy, including atherosclerosis, neurofibromatosis, sickle cell anemia, meningitis, and skull-base radiotherapy.
Unilateral superficial temporal artery (STA)–middle cerebral artery (MCA) bypass surgeries were performed on the hemispheres with ischemic symptoms, decreased rCBF, and compromised cerebral vascular reserve (CVR).
Hemodynamic and angiographic evaluations
Cerebral hemodynamic evaluations were performed by xenon–CT scans (Diversified Diagnostic Products Inc., Houston, USA). During xenon–CT scans, patients inhaled through a face mask a 28% concentration of medical-grade Xe gas in 40% oxygen for 4.3 min. At the same time, rapid sequential CT scanning (Siemens, GER) of four preselected levels of the brain was performed. This protocol was then repeated at the identical location 15 min after intravenous injection of 1 g acetazolamide (Bedford Laboratories, USA). Patients were continuously monitored clinically and via end-tidal carbon dioxide, and xenon saturation curves were obtained by the software (XeCT System, Diversified Diagnostic Products Inc., Houston, USA). Regions of interest (ROI) of bilateral rCBF are positioned, and each ROI should contain a minimum of 300 pixels in order to reliably determine rCBF [8]. rCBF was adjusted by current hematocrit. CVR was calculated as (rCBF after acetazolamide − rCBF before acetazolamide)/rCBF before acetazolamide [18]. Follow-up cerebral hemodynamic evaluations were performed 1, 3, and 6 months, postoperatively.
All the patients in our consecutive case series received early postoperative (7–10 days) digital subtraction angiography to assess the patency of anastomoses. Clinical presentations and cerebral hemodynamic evaluations were closely followed up. Angiography will be performed to assess the collateral angioarchitecture if the patient presents with neurological deterioration or deficits.
Clinical evaluation after direct revascularization
All the patients were followed routinely at the outpatient department before they were performed the regular hemodynamic evaluations. Their neurological status was assessed according to postoperative symptoms and signs comparing with preoperative ones. The short-term neurological outcomes were analyzed separately for each hemisphere.
Data analysis and statistics
CVR less than 30% was considered impaired and rCBF less than 40 ml/100 g/min was considered decreased [18]. The most severely compromised hemodynamics demonstrated the “steal flow” phenomenon (CVR was negative value) [19]. Continuous variables were expressed as percentage or as mean ± SD. Statistical analysis was performed using paired t test as appropriate. The statistical level of significance was set at P < 0.05. Statistical analysis was completed by SPSS 11.5.
Results
Patient demography and revascularization
The patients consisted of seven men and eight women with a mean age of 36.2 ± 9.8 years. According to Suzuki and Takaku [17], three hemispheres were grade II, 19 hemispheres were grade III, and eight hemispheres were grade IV. All the patients suffered from symptoms due to ipsilateral cerebral ischemia including 12 (80%) transient ischemic attacks (TIAs) and three (20%) complete strokes and were performed the STA–MCA bypass surgery on the hemispheres with ischemic symptoms matching the decreased hemodynamics. The interval between stroke and direct revascularization is not less than 3 weeks. Twenty-nine anastomoses were performed in all 15 cases. The patency of all the anastomoses was verified by external carotid artery angiography 7–10 days after direct revascularization. The demographic, clinical, and angiographic data were summarized in Table 1.
Hemodynamic evaluations
Before direct revascularization, among the 15 symptomatic (operative) hemispheres, 14 hemispheres demonstrated with rCBF decreased, all CVR of these hemispheres was impaired including three cases with “steal flow” phenomenon after acetazolamide challenge test. However, the rCBF of all asymptomatic (nonoperative) hemispheres was normal and CVR of six cases was impaired. The rCBF of ROI in operative hemispheres (32.8 ± 5.5 ml·100 g−1 min−1) was much lower than that in contralateral sides (50.4 ± 8.4 ml·100 g−1·min−1; P < 0.001) and the CVR (6.1 ± 13.2%) in operative hemispheres was also less than that in the other side (31.7 ± 9.0%; P < 0.001). The preoperative rCBF and CVR data of bilateral hemispheres were also summarized in Table 1.
The average follow-up time was 8.5 ± 3.5 months (ranging from 5 to 17 months). One month after the ipsilateral direct revascularization, the rCBF (43.4 ± 6.4 ml·100 g−1·min−1) and CVR (17.2 ± 12.0%) of the operative hemisphere showed obvious improvement (P < 0.001 and P = 0.012, respectively). While 3 months later, the CVR of nonoperative hemispheres (25.8 ± 8.1%) began to decrease significantly (P = 0.003; Fig. 1). Six months after the revascularization, the rCBF in nonoperative hemispheres showed a downward trend (47.4 ± 8.0 ml·100 g−1·min−1), but the difference was not significant (P = 0.053; Fig. 2). Till 6 months followed-up postoperatively, three patients presented with decreased rCBF and impaired CVR in the nonoperative hemispheres and their clinical data were summarized in Table 2.
Clinical evaluation after direct revascularization
Clinical progression due to the ischemia in the operative hemisphere was not observed in our study after revascularization. Among the three patients with severe compromised hemodynamics in the nonoperative hemispheres, two patients were symptomatic with TIA due to the poor perfusion in the nonoperative sides and the other one was followed up routinely without any discomfortable complains.
Case illustration: clinical, hemodynamic, and angiographic manifestations of contralateral hemisphere after unilateral direct revascularization
Case 4
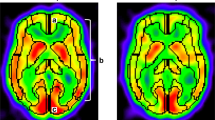
A 16-year-old girl presented with intermittent left upper limb weakness. After the diagnosis of MMD, hemodynamic evaluation by Xe-CT and acetazolamide challenge test demonstrated hypoperfusion and decreased reserve in the right parietal cortex (Fig. 3a, b). She had an uneventful recovery with no ischemic attacks until 6 months after right STA–MCA bypass, when right limbs numbness after laughter was noted. Xe-CT showed poor perfusion in the left temporal and parietal cortex and the rCBF was decreased obviously (Fig. 3c, d). Angiography at that time disclosed a patent bypass which confirmed successful revascularization (Fig. 4). However, bilateral leptomeningeal collaterals from the posterior circulation decreased (Fig. 5). Then she was performed the left STA–MCA bypass and bilateral rCBF improved without any ischemic attacks during follow-up.
Preoperative and postoperative (6 months after right STA–MCA bypass) hemodynamic evaluations by Xe-CT and acetazolamide challenge test. a Preoperative Xe-CT image in resting state demonstrated hypoperfusion in the right parietal cortex. b Preoperative acetazolamide challenge test showed decreased CVR in the right parietal cortex. c Postoperative Xe-CT image in resting state demonstrated poor perfusion in the left temporal and parietal cortex. d Postoperative acetazolamide challenge test showed decreased CVR in corresponding cortex
Discussion
MMD is a chronic cerebrovascular disorder with the characters of progressive occlusion of the arteries in Willis’ circle and the formation of collateral capillaries network. Since 1969, Suzuki and Takaku published their landmark article to describe and designate the “moyamoya disease” [17], our understanding of natural history and optimal treatment of this disease has grown. In Asian, MMD presents a bimodal age distribution. One peak occurs in early childhood with a predilection for ischemic cerebrovascular events and the other peak affects adults in the fourth decade of life with a preference for hemorrhagic strokes [7, 12, 14]. While in North American and European [1, 3], MMD demonstrates several differences with the Far East. In Western countries, it most commonly involves women in their third and fourth decades of life and the most frequent clinical presentation is ischemic stroke and TIA. What is more, it remains a disease with unknown origin and uncertain natural history. So the selection of treatment is still controversial. In recent years, some investigators recommended surgical revascularization in most symptomatic patients to reduce ischemic symptoms and improve hemodynamic status [6, 9, 15]. In our study, nearly all the symptomatic hemispheres suffered from severe impairment of cerebral hemodynamics preoperatively. After the direct revascularization, the hemodynamics and clinical symptoms were relieved significantly. This result is not much different from previous studies with short-term follow-up.
However, in our study, 3–6 months after ipsilateral direct revascularization for symptomatic hemispheres, the CVR of nonoperative hemispheres decreased obviously and rCBF also showed a downward trend although the difference was not significant. Among them, two asymptomatic hemispheres (13.3%) demonstrated with ischemic events during the short-term follow-up. Maybe this phenomenon is related with the progression of MMD. However, the progression of MMD in adult patients was believed to need relative long time. A recent study disclosed that MMD progression occurred in nearly 20% of patients during a mean follow-up period of 6 years [11]. Obviously, the progression of MMD could not elucidate our findings.
There were few articles about the asymptomatic MMD or moyamoya hemisphere. Ikeda et al. used a standard “brain check-up” protocol to assess the clinical feature of adult asymptomatic MMD [5]. They found five patients suffering from decreased perfusion in the eight total asymptomatic MMD patients. During a follow-up period of 2 years, two of them (40%) developed TIA and required surgical STA–MCA anastomosis. Kuroda et al. published the first multicenter, nationwide (Japan) survey focused on asymptomatic patients with MMD [10]. They found the annual risk for any stroke was 3.2% and the symptomatic changes to ischemic episodes were significantly linked to disturbed hemodynamics in asymptomatic MMD. In our series, the preoperative hemodynamic evaluations disclosed that six of 15 (40%) nonoperative hemispheres had a moderate reduction of CVR, despite the fact that the hemispheres remained asymptomatic. But these were probably related with the subsequent ischemic strokes and still could not explain the progressive decrease of the CVR in nonoperative hemispheres after unilateral direct revascularization.
The precise mechanism for compromised hemodynamic in nonrevascularized hemisphere after unilateral revascularization is unknown. This phenomenon occurs in the early stage after the successful unilateral direct revascularization, so the first possible mechanism is related to hemodynamic stress changes. After unilateral direct revascularization, increased blood flow through the anastomosis causes hemodynamic stress then decreases the compensation from other collateral circulation. Posterior circulation including the leptomeningeal collaterals from bilateral posterior cerebral arteries serves as an important role to supply the ischemic brain in MMD [4]. After successful bypass surgery, reduced demand from posterior circulation compensation could induce collateral blood supply decreasing which maybe contribute to the compromised hemodynamics of contralateral side. In our study, decreased bilateral leptomeningeal collaterals from the posterior circulation could be observed in the above case 6 months after unilateral direct revascularization.
The other possible mechanism is related to the changes of some cytokines which modulate the cerebral vasomotor tone after revascularization. Suzuki et al. [16] reported successful direct bypass surgery can suppress the metabolism of nitric oxide (NO) released from endothelium which may participate in the increasing collateral blood flow by dilating these vessels in MMD patients. They found NO metabolites concentrations were suppressed obviously after the first bypass surgery performed on one hemisphere. The interval between pre- and post-bypass to measure the NO metabolites concentrations was approximately 6 months. This interval does correspond with our result of impaired hemodynamics of contralateral hemisphere after the first unilateral bypass surgery.
Before making the conclusion of this research, the limitations of our study must be pointed out. Our research is a case series to illustrate a neglected phenomenon after direct revascularization. The selection of patients was not randomized and the sample size of patients was limited. Our study focused mainly on the changes of hemodynamic after unilateral direct revascularization and the correlation with clinical manifestations. Only a few of the patients had complete DSA documents during the short-time follow-up postoperatively. This makes it difficult to analyze the relationship between the hemodynamic changes and collateral vascular reconstruction after unilateral direct revascularization.
Conclusion
In summary, unilateral direct revascularization in symptomatic hemispheres for MMD patients could induce CVR impaired in primary asymptomatic hemispheres during the short term after the surgery. Therefore, critical follow-up especial the hemodynamic evaluations should be essential for the asymptomatic hemispheres in patients with MMD.
References
Chiu D, Shedden P, Bratina P, Grotta JC (1998) Clinical features of moyamoya disease in the United States. Stroke 29:1347–1351
Fujiwara F, Yamada H, Hayashi S, Tamaki N (1997) A case of adult moyamoya disease showing fulminant clinical course associated with progression from unilateral to bilateral involvement. No Shinkei Geka 25:79–84
Hallemeier CL, Rich KM, Grubb RL, Chicoine MR, Moran CJ, Cross DT (2006) Clinical features and outcome in North American adults with moyamoya phenomenon. Stroke 37:1490–1496
Huang APH, Liu HM, Lai DM, Yang CC, Tsai YH, Wang KC, Yang SH, Kuo MF, Tu YK (2009) Clinical significance of posterior circulation changes after revascularization in patients with moyamoya disease. Cerebrovasc Dis 28:247–257
Ikeda K, Iwasaki Y, Kashihara H, Hosozawa K, Anan K, Tamura M, Satoyoshi E, Ikeda H (2006) Adult moyamoya disease in the asymptomatic Japanese population. J Clin Neurosci 13:334–338
Ishikawa T, Houkin K, Kamiyama H, Abe H (1997) Effects of surgical revascularization on outcome of patients with pediatric moyamoya disease. Stroke 28:1170–1173
Iwama T, Hashimoto N, Yonekawa Y (1996) The relevance of hemodynamic factors to perioperative ischemic complications in childhood moyamoya disease. Neurosurgery 38:1120–1126
Johnson DW, Stringer WA, Marks MP, Yonas H, Good WF, Gur D (1991) Stable xenon CT cerebral blood flow imaging: rationale for and role in clinical decision making. Am J Neuroradiol 12:201–213
Karasawa J, Touho H, Ohnishi H, Miyamoto S, Kikuchi H (1992) Long-term follow-up study after extracranial–intracranial bypass surgery for anterior circulation ischemia in childhood moyamoya disease. J Neurosurg 77:84–89
Kuroda S, Hashimoto N, Yoshimoto T, Iwasaki Y (2007) Radiological findings, clinical course, and outcome in asymptomatic moyamoya disease: results of multicenter survey in Japan. Stroke 38:1430–1435
Kuroda S, Ishikawa T, Houkin K, Nanba R, Hokari M, Iwasaki Y (2005) Incidence and clinical features of disease progression in adult moyamoya disease. Stroke 36:2148–2153
Nishimoto A (1979) Moyamoya disease (author’s transl). Neurol Med Chir (Tokyo) 19:221–228
Oka Y, Kusunoki K, Nochide I, Igase K, Sadamoto K, Kohno K, Kumon Y, Sakaki S (2000) A case of adult moyamoya disease progressed after vascular reconstructive surgery. No Shinkei Geka 28(4):373–378
Saeki N, Yamaura A, Hoshi S, Sunami K, Ishige N, Hosoi Y (1991) Hemorrhagic type of moyamoya disease. No Shinkei Geka 19:705–712
Starke RM, Komotar RJ, Hickman ZL, Paz YE, Pugliese AG, Otten ML, Garrett MC, Elkind MSV, Marshall RS, Festa JR, Meyers PM, Connolly ES (2009) Clinical features, surgical treatment, and long-term outcome of adult moyamoya patients. J Neurosurg 111:936–942
Suzuki Y, Fujita M, Mizutani N, Seki Y, Kimura M, Kajita Y, Takayasu M (2000) Role of nitric oxide in the control of cerebral microcirculation under physiological and pathological condition. Clin Hemorheol and Micro 23:307–312
Suzuki J, Takaku A (1969) Cerebrovascular ‘Moyamoya’ disease: disease showing abnormal net-like vessels in base of brain. Arch Neurol 20:288–299
Touho H, Karasawa J, Shishido H, Morisako T, Yamada K, Shibamoto K (1990) Hemodynamic evaluation in patients with superficial temporal artery-middle cerebral artery anastomosis. Neurol Med Chir (Tokyo) 30:1003–1010
Yonas H, Smith HA, Durham SR, Pentheny SL, Johnson DW (1993) Increased stroke risk predicted by compromised cerebral blood flow reactivity. J Neurosurg 79:483–489
Author information
Authors and Affiliations
Corresponding author
Additional information
Comments
Liu-Guan Bian, Shanghai, China
Moyamoya disease (MMD) is characterized by progressive stenosis of the terminal portion of the bilateral internal carotid arteries and is associated with an abnormal vascular network, called moyamoya vessels. Cerebral revascularization surgery is believed to reduce the incidence and improve the long-term prognosis in patients with MMD. Direct revascularization has been used successfully to prevent strokes by improving regional cerebral blood flow (rCBF) to the affected hemisphere faster in patients with MMD. Since most literatures have focused on the rCBF changes of operative hemisphere, authors evaluated the hemodynamics of nonoperative side by xenon-enhanced computed tomography (Xe-CT) and acetazolamide challenge test in patients with MMD during a short-time follow-up. They found that the CVR of nonoperative hemispheres began to decrease significantly, 3 months after the ipsilateral direct revascularization. Six months later, the rCBF showed a trend toward a decrease in nonoperative hemispheres than the preoperative status. Interestingly, among the three patients presented with decreased rCBF and impaired CVR in the nonoperative hemispheres, two patients were symptomatic. They conclude that unilateral direct revascularization in symptomatic hemisphere for MMD patient could induce CVR impaired in primary asymptomatic hemisphere during the short term after the surgery. It is an interesting paper.
Louis J. Kim, Seattle, Washington, USA
Ma et al. report their experience with 15 moyamoya patients that were followed for cerebral hemodynamic changes on xenon CT with acetazolamide challenge following unilateral revascularization. A trend toward decreased relative cerebral blood flow (rCBF) was noted in the contralateral, untreated hemisphere during postoperative follow-up. In three patients, a profound decrease in rCBF and cerebrovascular reserve was noted. The authors conclude that close hemodynamic monitoring of bilateral hemispheres should be performed following revascularization.
The phenomenon that the authors report here has not been well described in the literature previously. In my practice, most cases of moyamoya present with bilateral hemispheric symptoms. These patients undergo staged bilateral revascularization surgery. As a result, the intermediate-term effects of unilateral treatment on moyamoya can be problematic to assess during follow-up. Nonetheless, as Ma et al. have implied, the physiological consequences of direct revascularization can be quixotic. A small but not insignificant percentage of moyamoya patients develop TIA’s or delayed ischemic deficits even after a technically successful bypass operation. Delayed postoperative ischemic changes may occur in both the ipsi- and contralateral hemisphere. While the authors offer reasonable putative explanations for their findings, these remain speculative, and underscore our rudimentary understanding of cerebral hemodynamic following revascularization. These data shed new light on moyamoya that deserves further inquiry and scrutiny.
Yasuhiro Yonekawa, Zurich, Switzerland
Ma et al. is to be congratulated for a thoughtful analysis and consideration on the cerebral hemodynamic change on the contralateral hemisphere after unilateral revascularization on Moyamoya disease patients.
Ischemic complication on the side of revascularization or on the contralateral side, in the perioperative stage or chronic stage have been not infrequently reported and discussed on their etiology and their prevention since the application of EC-IC bypass surgery for this disease at the beginning of 1970s. Ma et al. focused their attention on the contralateral hemisphere by the use of Xe-CT. Their detection in findings significant decrease in CVR and decrease of CBF (although not statistically significant) in the chronic stage are interesting and give some clue for treatment of ischemic complication. They presumed two reasons: decrease of natural collaterals including from the posterior circulation and some changes in cytokine nitric oxide level.
By the way, I missed in this paper some points to be discussed: changes of the blood pressure over time and their analysis, mention on any abnormalities (stenosis, hypoplasia) of the posterior cerebral artery which contribute to the natural collateral circulation significantly, and one-staged construction of EC-IC bypasses on both hemisphere. Furthermore, I missed the reason why children with Moyamoya disease and Moyamoya syndrome on the whole are excluded from this study as they also can have the same complication.
Rights and permissions
About this article
Cite this article
Ma, Y., Li, M., Jiao, L.Q. et al. Contralateral cerebral hemodynamic changes after unilateral direct revascularization in patients with moyamoya disease. Neurosurg Rev 34, 347–354 (2011). https://doi.org/10.1007/s10143-011-0312-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-011-0312-y