Abstract
Skull base chordoma are still challenging. Between May 1993 and June 2005, 106 consecutive patients with skull base chordoma underwent surgical removal at Skull Base Division of Neurosurgery, Beijing Tiantan Hospital, China. Retrospective analysis included medical charts and images. The age of the patients ranged from 7 to 65 years old, with an average age of 35.6 years. Sixty patients were male; the other 46 were female (1.3:1). Follow up data were available in 79 cases ranging from 10 to 158 months (average 63.9 months) after operation. The prognostic factors for recurrence and survival were analyzed with Kaplan‐Meier, Cox regression and t‐test. Overall, 1, 3, 5 and 10 years survival rates were 87.2%, 79.4%, 67.6%and 59.5% respectively. One, 3, 5 and 10 year recurrent rates were 19.1%, 34.7%, 52.9% and 88.3%, respectively. The long term outcome of the skull base chordomas is poor. The previous radiotherapy or surgery, dedifferentiated pathology, and less tumor resection are risk factors for longterm survival and recurrence (p < 0.05). Although there is no statistic significant role of tumor adherent to vital structure for outcome (p = 0.051), it can not exclude its importance for favorable outcome. Gender, age, tumor size and staging are not independent risk factors for outcome. Surgical technique leading to radical tumor resection with less morbidity is advocatory and beneficial for patients with skull base chordoma with long term outcome, if the tumor could be exposed and resected completely, the recurrence rate was very low for most benign chordomas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Skull base chordoma accounts for 25–30% of all chordomas. It is a rare disease for which treatment is a challenge due to deep location, pattern of extension, high rate of recurrence, and the proximity of important structures such as the brain stem [8, 15, 16]. As they are not sensitive to radiotherapy (RT) or chemotherapy, gross resection remains the preferred method of treatment. Some factors have been reported to associate with outcome including resection degree, tumor pathology, prior surgical history, and RT [1, 4–6, 9, 10, 14, 21, 23]. In the present study, we review prognostic factors for long-term outcome among patients who have undergone surgical resection of skull base chordomas.
Patients and methods
Between May 1993 and June 2005, 106 consecutive patients with chordoma underwent surgical removal at Beijing Tiantan Hospital, China. Retrospective analysis included medical charts and images. The age of the patients ranged from 7 to 65 years old, with an average age of 35.6 years. Sixty patients were male; the other 46 patients were female (1.3:1). All exhibited solitary lesions; none had family history of the disease.
Statistical analysis
The data were collected by database software (Epidata 3.02). Kaplan–Meier survival analysis and the Cox regression method for multivariate regression were done with SPSS 12.0.
Results
The length of the medical history ranged from 1 to 134 months; the average was about 18.8 months. The most frequent complaints included headache and neck pain in 46 cases (41.1%), diplopia in 32 (28.6%), hoarseness and difficulty swallowing in 18, facial palsy and numbness in 12, tinnitus and deafness in seven, and paralysis of the limbs in ten.
Surgical approaches
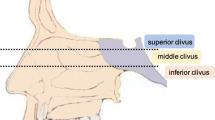
According to tumor location and growth manner, different approaches were chosen. Because dura had not been intact in most cases, subdural approaches were used in most cases. The presigmoidal approach was used in 39 cases for tumor occupying upper and middle clivus accompanying parasellar invading, far later approach in 16 for tumor occupying lower clivus, middle fossa approach in 19 for those unilateral parasellar invading, pterional approach in 11 for parasellar and sphenoid sinus invading bilateral cavernous sinus, subtemporal approach in five for upper clivus invading, bilateral transfrontal in five for giant tumor invading bilateral cavernous sinus, sphenoid and ethmoid sinuses, transoral approach in four for those in lower clivus with intact dura, suboccipital midline in three in foramen magnum, retrosigmoidal approach in three for tumor in cerebellopontine angle, transsphenoidal approach in two for sphenoid and/or ethmoid invading with intact dura, and supraorbital subfrontal approach in four with tumor in tuberculum sellae.
Tumor size, stage, surgical resection degree, and types
Tumor size was evaluated as (D1 × D2 × D3)/2. Tumors ranged in volume from 5 to 180 ml, with an average of 46.0 ml. Tumor consistency were classified into three types: hard, referring to unsuckable during removal; soft, referring to suckable during removal; and moderate, referring to containing both hard and soft. The adherence to adjacent vital structure were classified into three types: loose, referring to the tumor that can be peeled off without sharp dissection; tight, referring to the tumor that is difficult to be peeled off even with sharp dissection in most times; and moderate, referring to part of the tumor that needs sharp dissection to be peeled off in some times. Stage was classified into four types: stage I, primary tumor localizes epidurally without intracranial invasion, with minor neurological deficits; stage II, more than 50% of the primary tumor occupies epidurally with minor or moderate neurological deficits; stage III, primary tumor with extensive growth, more than half of the tumor occupies subdurally, compressing or adhering to brainstem with moderate or severe neurological deficits; and stage IV, tumor metastasis. According to Carstens’ standard [7], pathology findings revealed conventional chordoma in 95 cases, chondroid chordoma in three, and dedifferentiated chordoma in eight. The tumors that invaded or infiltrated the bone were removed as much as possible with drill or rongeur. Tumor resection degree was assessed by postoperative CT and MRI. Bone window of high-resolution CT scan was sensitive to demonstrate the extent of resection of chordoma which invaded the bone. Tumor type, location, and resection degree are listed in Tables 1 and 2. Among the cases studied, 46 patients underwent the transparent electronic microscopic test. Cytokeratin and epithelial membrane antigen (EMA) detection were used for immunohistochemistry diagnosis. Some other tumor markers such as E-cadherin [17] were not used.
Radiotherapy
Within the 79 patients with full follow-up data information, 40 patients underwent RT (10 to 42 months after surgery) as adjuvant therapy for their rejection of re-operation for surgical removal of tumor residual or definitive tumor recurrence. Because of the unavailability of proton beam RT in China, patients with tumor residual smaller than 3 cm in diameter underwent gamma knife radiotherapy (GKR), and the rest underwent linear accelerator radiotherapy (LINAC).
Outcome
Follow-up information was available for 79 patients, ranging from 10 to 158 months after the operation (average 63.9 months).The recurrence rates (5.3 months to 133.3 months after operation) at 1, 3, 5, and 10 years are 19.1%, 34.7%, 52.9%, and 88.3%, respectively. Six recurrent cases repeated RT, 13 repeated surgical removal procedure, and two repeated RT and surgical operation. The survival rates at 1, 3, 5, and 10 years were 87.2%, 79.4%, 67.6%, and 59.5%, respetively. Forty patients received RT after surgical resection. Two patients died within 2 months due to severe operation complications; 15 patients died of brainstem failure due to tumor recurrence. Two deaths were unrelated to chordoma. Among the 60 currently surviving patients, 15 (25%) presented neurological deficits including 10 that presented with impaired eye abduction due to palsy. All three cases of chondroid chordoma lived.
Survival analysis
Despite higher average volume (85.7 ml), longer illness history (615.6 days) and older age (37.8 years) in the non-survival group as compared to the survival group (68.4 ml, 506.7 days, and 35.2 years), these differences were not significant (p = 0.181, 0.165, 0.675, respectively) (Table 1).
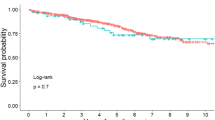
Kaplan–Meier univariate survival analysis demonstrated that prior history of surgical removal or RT represents risk factors for long-term survival (p < 0.01) (Table 2). In terms of pathology type, the chondroid chordoma displayed the most favorable outcome, while the dedifferentiated chordoma displayed the poorest (Fig. 2). Patients benefited significantly more from gross total resection (GTR) than from subtotal resection and partial removal (Fig. 1) (p < 0.01). Although patients with stage I or II skull base chordoma exhibited better outcomes than those with stage III, this difference was not significant (p = 0.48).
Multivariate Cox regression analysis showed significant correlations between long-term survival and pathology type (Fig. 2), prior surgical removal or RT, and degree of surgical removal (Fig. 1). The degree of tumor adherence to the brainstem had no significant correlation with survival outcome (Table 3).
Analysis of recurrence
The recurrence group showed older age, larger tumor size, and shorter illness history than the group free of recurrence. However, these differences were not significant (Table 1).
Kaplan–Meier univariate analysis revealed that the degree of surgical resection (Fig. 3), pathology type, prior surgical history, and RT were significantly correlated with recurrence. The dedifferentiated chordoma was more likely to recur than the other two types; no instances of recurrence were documented for chondroid chordoma. GTR displayed maximum efficacy in achieving tumor control when compared to subtotal and partial resection. Tumor location did not represent a risk factor for recurrence. Similar trends of statistically significant difference were obtained with multivariate Cox regression analysis.
Patients were evaluated with the Karnofsky Performance Scale (KPS) before the operation, 2 weeks after the operation, and at subsequent follow-up visits. Two weeks postoperatively, patients’ KPS scores did not differ significantly from values obtained preoperatively. KPS scores obtained during follow-up increased significantly from preoperative values.
Discussion
Our study supports the finding that chordoma is more common among males [1, 4, 9, 10, 19]; however, this gender bias is not associated with a particular outcome. Dorfman [11] reported that chordoma in females is associated with worse outcomes than in males as revealed by both univariate and multivariate analyses.
It is still debatable whether age is a risk factor for outcome. The present study did not find any association of age with outcome, consistent with reports by Benk [1, 4, 19]. However, others have reported age younger than 40 years [12], older than 60 years [20], or older than 50 years [10] to be risk factors for outcome.
Although the incidence of skull base chordoma is very low, it represents a substantial challenge for the neurosurgeon due to its deep location and the intimacy of vital vessels and neural construction [15, 16]. Fortunately, most cases develop slow tumor growth. Furthermore, the long-term outcomes for patients with skull base chordoma have improved greatly since the advancement of microneurosurgical technique and multidisciplinary collaborations.
Although Sekhar [21], Crockard [10], Al-Mefty, and Borba [6] recommend aggressive surgical removal, opinions regarding postoperative RT are varied. The former two authors recommend RT for definitive recurrence, while the latter two recommend routine RT for those who have undergone partial resection. Because chordoma grows in a slow manner, it is insensitive to conventional RT. However, proton beam RT is unavailable in China, so the present study utilized GKR and LINAC. We subsequently found that prior RT is a risk factor for long-term outcome, possibly due to RT-induced arachnoid membrane proliferation that interfered with tumor exposure and dissection. The same factors would explain why prior operations were demonstrated to be risk factors for outcome.
The present study found that the degree of resection correlated with outcome. Gross total resection led to better long-term survival and lower recurrence than subtotal and partial resection, consistent with the findings of Forsyth [12] and Colli [9]. This is the reason why many patients included in the present study underwent the presigmoidal approach, which led to greater tumor exposure and resection despite its complexity. Advancements in microneurosurgery have allowed surgeons to resect as much tissue as possible during one operation; this postpones recurrence and prolongs survival [18]. In particular, the navigation technique and image-guided techniques greatly increase exposure and the degree of resection [22].Although Akmal [1] and Mark [19] reported no correlation between resection degree and outcome, their reports implied that the extensive resection did not result in poorer outcomes. Gay [13] found no correlation of resection degree with postoperative mortality.
The present study showed that tumor pathology is strongly correlated with survival and recurrence; specifically, dedifferentiated chordoma leads to high levels of recurrence and poor survival. The malignant nature of dedifferentiated chordoma necessitates RT and surgical operation, which are risk factors for outcome; the 5-year survival rate for dedifferentiated chordoma is only 25%, while that for chondroid chordoma is 100%. This is consistent with Al-Mefty’s finding [2, 6]. However, several reports found no difference between chondroid and conventional chordomas in terms of overall survival and tumor-free survival [1, 4, 9, 12].
Different classifications have been provided for various anatomical points [2, 24]. The present study showed only extensive chordoma results in poorer outcomes than other types. Extensive chordoma is correlated with more difficult, challenging total or subtotal resections. The lack of a difference in resection degree among stages I–III might explain the lack of improvement as related to outcome, which would support the importance of tumor resection for outcome. Because this study did not include any cases of metastasis referring at stage IV, we cannot rule out the possibility that metastasis will lead to poorer outcomes [3].
Crockard proposed large tumor size as a risk factor for outcome; such a trend was present in our study but lacked statistical significance. A lack of correlation between volume and outcome was reported by Benk [4] and Akmal [1]. Dorfman found size larger than 70 ml to be a risk factor for outcome through Cox analysis [11].
Currently, there are no objective criteria for the evaluation of tumor consistency and adherence to adjacent structures. We classified tumors into three groups as determined by consistency: hard, intermediate, and soft. There was no correlation between consistency and outcome. Tight adherence to a vital structure was a risk factor for survival as determined by univariate analysis, but this factor exerted only a minor effect as determined by multivariate analysis.
The present study found a 10-year recurrence rate of 90%, demonstrating the malignant nature of skull base chordoma. The average time before recurrence was 48 months, longer than previously reported by Al-Mefty [2] and Crockard [10]. Previous surgery or RT history [5, 9, 12], partial tumor resection [12], and female gender [12] have been reported to correlate with recurrence, while tumor size has not. The degree of resection was inversely correlated with recurrence rate. If the tumor could be exposed and resected completely, the recurrence rate was very low for most benign chordomas. Because of the difficulty of exposure resulting from a deep location, residual tumor leads to recurrence. Therefore, the endoscopy assistant technique is helpful for resection. This staged operation might also benefit patients because of the increased extent of resection. Another difficulty for complete resection is the bony involvement by tumor. Sometimes, it is impossible to remove the bone for no applicable tool and reconstruction technique or avoidance of cerebrospinal fluid leakage. Other factors such as age, gender, tumor volume, location, or staging are not independent risk factors for recurrence.
In addition to surgical management for chordoma, recent studies showed that proton beam RT and chemotherapy are promising treatments for chordoma [8]. Because patients in the present study had not received proton beam RT and chemotherapy, we cannot conclude regarding the influence of these two modalities on surgical management of skull base chordoma.
References
Akmal S, Ole SN, Anne GJ et al (1997) A retrospective clinicopathological study of 37 patients with chordoma: a Danish national series. Sarcoma 1:161–165
Al-Mefty O, Borba LA (1997) Skull base chordoma: a management challenge. J Neurosurg 86:182–189
Arnautovic KI, Al-Mefty O (2001) Surgical seeding of chordomas. J Neurosurg 95(5):798–803
Benk V, Liebsch NJ, Munzenrider JE et al (1995) Base of skull and cervical spine chordomas in children treated by high-dose irradiation. Int J Radiat Oncol Biol Phys 31:577–581
Berven S, David Z, Henry JM et al (2002) Clinical outcome in chordoma: utility of flow cytometry in DNA determination. Spine 27:374–379
Borba LA, Colli B, Al-Mefty O (2001) Skull base chordomas. Neurosurg Q 11:124–139
Carstens PH (1995) Chordoid tumor a light, electron microscopic and immunohistochemical study. Ultrastruct Pathol 19:291–295
Chugh R, Tawbi H, Lucas DR, Biermann JS, Schuetze SM, Baker LH (2007) Chordoma: the nonsarcoma primary bone tumor. Oncologist 12:1344–1350
Colli B, Al-Mefty O (2001) Chordomas of the craniocervical junction: follow-up review and prognostic factors. J Neurosurg 95:933–943
Crockard HA, Steel T, Plowman N et al (2001) A multidisciplinary approach to skull base chordomas. J Neurosurg 95:175–183
Dorfman HD, Czerniak B (1995) Bone cancers. Cancer 75(1Suppl):203–210
Forsyth PA, Cascino TL, Shaw EG et al (1993) Intracranial chordomas: a clinicopathological and prognostic study of 51 cases. J Neurosurg 78:741–747
Gay E, SekharLN RE et al (1995) Chordomas and chondrosarcomas of the cranialbase: results and follow-up of 60 patients. Neurosurgery 36:87–896
Kaneko Y, Sato Y, Iwaki T et al (1991) Chordoma in early childhood: a clinicopathological study. Neurosurgery 29:442
Krayenbuhl H, Yasargil MG (1975) Cranial chordomas. Prog Neurol Surg 6:380–434
McMaster ML, Goldstein AM, Bromley CM et al (2001) Chordoma: incidence and survival patterns in the United States, 1973–1995. Cancer Causes Control 12:1–11
Mori K, Chano T, Kushima R (2002) Expression of E-cadherin in chordomas diagnostic marker and possible role of tumor cell affinity. Virchows Arch 440:123–127
Mortini P, Mandelli C, Franzin A et al (2001) Surgical excision of clival tumors via the enlarged transcochlear approach. Indications and results. J Neurosurg Sci 45:127–139
Soo MY (2001) Chordoma: review of clinicoradiological features and factors affecting survival. Australas Radiol 45:427–434
Thieblemont C, Biron P, Rocher F et al (1995) Prognostic factors in chordoma: role of postoperative radiotherapy. Eur J Cancer 31:2255–2259
Tzortzidis F, Elahi F, Wright D et al (2006) Patient outcome at long-term follow-up after aggressive microsurgical resection of cranial base chordomas. Neurosurgery 59:230–237
Vougioukas VI, Hubbe U, Schipper J et al (2003) Navigated transoral approach to the cranial base and the craniocervical junction: technical note. Neurosurgery 52:247–250
Zhang JT, Wu Z, Jia GJ et al (2006) Microsurgical treatment of skull base chordoma. Chin J Neurosurg 22:29–31
Zhou DB, Yu XG, Xu BN et al (2005) Skull base chordoma. Chin J Neurosurg 21:156–159
Author information
Authors and Affiliations
Corresponding author
Additional information
Comments
Luis A. B. Borba, Curitiba, Brazil
We read with great interest the paper entitled “Prognostic factors for long-term outcome of patients with surgical resection of skull base chordomas—106 cases review in one institution” by Wu Zhen et al.
In this article, the authors reported their large experience in the treatment of this challenging tumor. They follow 79 patients with a mean follow-up of 63.9 months. The recurrence rates at 1, 3, 5, and 10 years were 19.1%, 34.7%, 52.9%, and 88.3%, respectively. The degree of surgical resection, pathology type, prior surgical removal, and RT were directly related with tumor control.
We disagree with the authors in a few topics.
Chordoma is an extradural tumor and should be attacked from the extradural compartment to the intradural. In our series, the incidence of intradural invasion is around 45% of the cases, and it is not the main concern because usually the tumor has a good plan with the CNS except in cases of recurrence or prior RT. A rare situation of pial invasion can be found, however it is not a place for recurrent tumor.
For us, chordomas should be understood and approached from these perspectives:
(a) The tumor comes from the bone.
(b) The free-time recurrence is directly related with the extension of surgical removal.
(c) Sometimes more than one surgical procedures need to get a radical removal.
(d) The tumor is extradural with or without intradural extension; therefore, approach it from an extradural fashion.
(e) Chordoma is different than chondrosarcoma; the cytokeratin and EMA staining are essential to the diagnosis. The old reported “chondroid chordoma” may be a low-grade chondrosarcoma which has a better prognosis.
(f) The tumor will recur (it’s a matter of time). Islands of residual tumor occur away from the main tumor mass, inside the bone of skull base.
(g) A radical bony removal is mandatory; however, the time to stop to do it is the most challenging part in the surgical procedure.
(h) A radical removal does not mean a high surgical morbidity. Tumor extension to the cavernous sinus, petroclival region, occipital condyles, etc., can be easily removed without adding surgical morbidity or mortality.
(i) The role of high dosage of radiation treatment with proton beam is the best additional therapy. The capacity to deliver a high dose protecting the adjacent tissue (Braga peak effect) is the main point to indicate it.
We would like to congratulate the authors for the excellent work in the management of these truly challenging tumors.
Anil Nanda, Shreveport, USA
Zhen et al. reviewed retrospectively one of the largest series reported to date on skull base chordomas. The authors report the outcomes of 106 consecutive cases of chordomas operated between 1993 and 2005. Follow-up data obtained on 79 patients showed overall survival rates and recurrence rates over 1, 3, 5, and 10 years. They report a 60% survival over 10 years and recurrence rate of 88%. Conventional radiation therapy was administered as an adjuvant. Pathology and extent of surgical excision (and previous treatment) were significant risk factors for both survival and recurrence. Tumor size and grading were not independent risk factors for outcome. The authors need to be congratulated for their superb management of these difficult tumors.
Rights and permissions
About this article
Cite this article
Wu, Z., Zhang, J., Zhang, L. et al. Prognostic factors for long-term outcome of patients with surgical resection of skull base chordomas—106 cases review in one institution. Neurosurg Rev 33, 451–456 (2010). https://doi.org/10.1007/s10143-010-0273-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-010-0273-6