Abstract
The significance of surgery for choroid plexus tumors is well established, but surgical resection of those in the fourth ventricle has not been evaluated. This study reviewed five consecutive patients with choroid plexus tumors in the fourth ventricle treated in our institute between 1996 and 2005, focusing on the factors that hindered total extirpation. Two cases were choroid plexus papillomas, and three cases were choroid plexus carcinomas. Preoperative T2-weighted magnetic resonance imaging showed a diffuse high-intensity lesion in the brain stem in four patients. Infiltration into the fourth ventricle floor was apparent in all five patients during surgery, which hindered total resection of the tumors without neurological deterioration. Intraoperative bleeding was well controlled in all five patients by cauterizing the feeding arteries at the early stage of surgery through the telovelar approach. Performance status was improved in all patients postoperatively. All patients with choroid plexus carcinomas underwent radiation therapy after the surgical removal. No patient suffered tumor progression within the follow-up of 24–129 months (mean 64 months). Total resection of choroid plexus tumors in the fourth ventricle is difficult because of invasion into the fourth ventricle floor. Adjuvant therapy for choroid plexus tumors with brain stem infiltration must be established.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Choroid plexus tumors (CPTs) are rare intraventricular tumors accounting for about 0.4% of brain tumors according to the Brain Tumor Registry of Japan [19]. Choroid plexus papilloma (CPP) was as rare as 0.3% (147 cases), and malignant plexus papilloma (choroid plexus carcinoma; CPC) was even rarer with only 0.1% (29 cases) among 52,196 cases of brain tumor [19]. Only a few reports have described the treatment for this entity [1–3, 6, 8–12, 14–17, 20].
The extent of the surgical resection is widely accepted as the most important prognostic factor in patients with CPTs and particularly in patients with CPCs [3, 8, 9, 16, 17, 20]. However, total removal of CPC is achieved in as few as 36–64% of cases because of problems including intraoperative bleeding and infiltration of the normal brain structure [3, 8, 14–16]. More than 70% of patients with CPTs were under the age of 2 years [4, 6]. CPT is more common in the lateral ventricle in children but is more common in the fourth ventricle among adult patients [5, 19]. Surgical treatment for supratentorial CPTs has been discussed elsewhere [8, 14, 17], but that for fourth-ventricle CPTs has not been established.
The present study reviewed our surgical experience with CPTs in the fourth ventricle, especially focusing on the problems regarding surgical resection.
Materials and methods
The present study included five consecutive patients, three male and two female aged 3 to 37 years, treated for CPTs in the fourth ventricle in our institute between 1996 and 2005. Two cases were CPPs, and three cases were CPCs. All patients with CPCs underwent radiation therapy after the surgical removal. A retrospective review of the case notes that pre- and postoperative magnetic resonance (MR) imaging, operation reports, and pathology was performed.
Surgical technique
All patients were operated on in the prone position. After suboccipital osteoplastic craniotomy and C-1 laminectomy, the dura was opened with a Y-shaped incision. The telovelar approach (transcerebellomedullary fissure approach) described previously [5, 7, 13] was applied to all cases. Any tumor protruding through the obex and/or the foramina of Luschka was initially removed using an Ultrasonic Surgical Aspirator (Sonopet; Miwatec, Tokyo, Japan) to expose and preserve the medulla oblongata, lower cranial nerves, and vascular structures including the vertebral arteries and posterior inferior cerebellar arteries (PICAs). The tela choroidea and inferior medullary velum were involved with the tumor in most of our cases. The concept of the surgery was as follows. The tela choroidea was incised along the taeniae choroidea with coagulation of the feeding arteries from the PICA to the tumor, to avoid damage to the branches to the brain stem. The inferior medullary velum was also incised. These procedures could reduce almost all of the blood supply to the tumor. Tumor debulking was then performed using the Ultrasonic Surgical Aspirator to expose and trace the normal fourth ventricle floor. Considerable care was taken to avoid injury to the fourth ventricle floor.
Results
Table 1 summarizes the clinical history, findings of MR imaging, and treatment of our five patients. Pre- and postoperative MR imaging and intraoperative findings are shown in Figs. 1, 2, 3, 4, and 5.
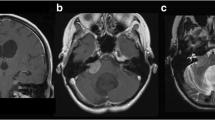
Case 1, a 28-year-old man with choroid plexus papilloma. Axial T2-weighted magnetic resonance (MR) image showing the lesion associated with a high-intensity lesion in the adjacent neural tissue, including the brain stem (a). Sagittal T1-weighted MR image with gadolinium showing an enhanced mass with a cyst in the fourth ventricle (b). The tumor was removed subtotally, and the residual tumor was observed as an enhanced spot (c, arrow). Schematic drawing indicating invasion of the tumor into the fourth ventricle floor as the shaded area (d)
Case 2, a 3-year-old boy with choroid plexus carcinoma. Axial T2-weighted magnetic resonance (MR) image disclosing the lesion associated with a high-intensity lesion in the adjacent neural tissue, including the brain stem (a). Sagittal T1-weighted MR image with gadolinium showing a dorsally exophytic enhanced mass within the medulla oblongata (b). The tumor was removed totally (c). Schematic drawing indicating invasion of the tumor into the hypoglossal and vagal trigone (d, shaded area)
Case 3, a 37-year-old man with choroid plexus papilloma. Axial T2-weighted magnetic resonance (MR) image showing the lesion but no associated high-intensity lesion in the adjacent neural tissue (a). Sagittal T1-weighted MR image with gadolinium showing an enhanced mass in the fourth ventricle (b). The tumor was removed almost totally, and no residual tumor was observed (c). However, intraoperative findings disclosed residual tumor on the fourth ventricle floor. Schematic drawing indicating tumor invasion into the fourth ventricle floor as the shaded area (d)
Case 4, a 25-year-old woman with choroid plexus carcinoma. Axial T2-weighted magnetic resonance (MR) image disclosing the lesion associated with a high-intensity lesion in the medulla oblongata (a). Sagittal T1-weighted MR image with gadolinium showing an enhanced mass filling the fourth ventricle with extension into the cerebellomedullary fissure (b). The tumor was removed subtotally, and the residual tumor was observed as an enhanced lesion (c, arrow). Schematic drawing indicating tumor invasion into the fourth ventricle floor as the shaded area (d)
Case 5, a 34-year-old woman with choroid plexus carcinoma. Axial (a) and sagittal (c) T1-weighted magnetic resonance (MR) images with gadolinium showing an enhanced mass with cystic formations filling the fourth ventricle. Axial T2-weighted MR image disclosing the lesion associated with a high-intensity lesion in the adjacent neural tissue, including the brain stem (b). The tumor was removed partially, and residual tumor was observed (d). Sagittal T1-weighted MR image with gadolinium obtained 1 year after extended local radiation and gamma knife stereotactic radiosurgery demonstrating shrinkage of the tumor (e). Intraoperative photograph after debulking of the tumor demonstrating the residual tumor invading the fourth ventricle floor (f). Schematic drawing indicating tumor invasion into the fourth ventricle floor as the shaded area (g)
All patients except for case 3 (Fig. 3) had preoperative neurological deficits. Case 2 suffered severe dysphagia and respiratory disturbance because of infiltration of the tumor into the brain stem (Fig. 2). Three patients showed signs of high intracranial pressure because of obstructive hydrocephalus (Figs. 1, 4, and 5).
Preoperative T1-weighted MR imaging of all five patients demonstrated relatively well-demarcated lesions in the fourth ventricle enhanced by contrast material, whereas T2-weighted MR imaging showed a diffuse high-intensity lesion in the brain stem in four of the five cases (Figs. 1, 2, 4, and 5). Marked infiltration into the fourth ventricle floor was identified during surgery in these four cases, which hindered total resection of the tumors at the site of invasion. Brain stem invasion was also evident, to a lesser extent, in case 3 without high-intensity lesion in the brain stem on preoperative T2-weighted MR imaging (Fig. 3). The tumor had not always infiltrated into the brain stem at the site of taeniae choroidea, as brain stem infiltration occurred at the midline portion of the fourth ventricle floor in cases 1, 3, and 5 (Figs. 1, 3, and 5).
Total removal of the tumor was performed only in case 2 with CPC, who had already developed severe dysphagia and respiratory disturbance. Total resection of the tumor was planned preoperatively in this patient. Cases 1, 3, and 4 underwent subtotal removal, and case 5 underwent partial removal with maximum preservation of the normal brain stem structure. Postoperative MR imaging detected residual tumor in case 5 (Fig. 5) and small spot-enhanced nodules on the fourth ventricle floor in cases 1 and 4 (Figs. 1 and 4). Postoperative MR imaging showed no residual tumor in case 3, although the small amount of invasive tumor was left on the fourth ventricle floor (Fig. 3).
Intraoperative bleeding was well controlled in all five patients by cauterizing the tumor-feeding arteries at the early stage of surgery through the telovelar approach.
Performance status was improved in all patients postoperatively, and no patient had suffered tumor progression by May 2007, with a follow-up of 24 to 129 months (mean 64 months).
Illustrative case (case 5)
A 34-year-old woman in the 25th week of pregnancy was restricted to bed rest because of vertigo and nausea on admission. Preoperative T1-weighted MR imaging with contrast material demonstrated a well-delineated tumor with cystic formation in the fourth ventricle causing obstructive hydrocephalus and distortion of the brain stem (Fig. 5a and c). T2-weighted MR imaging disclosed the high-intensity lesion in the adjacent neural tissue, including the dorsal aspect of the brain stem (Fig. 5b). The patient initially underwent endoscopic third ventriculostomy for obstructive hydrocephalus, followed by successful cesarean section at the 30th week of pregnancy. Then, she underwent tumor removal by craniotomy. Surgical removal was extremely difficult because of the wide infiltration of the tumor into the fourth ventricle floor (Figs. 5f and g). The upper part of the tumor could not be resected because the normal fourth ventricle floor could not be traced above the striae medullaris (Fig. 5d). Adjuvant therapy using extended local irradiation and gamma knife stereotactic radiosurgery resulted in marked regression of the residual tumor (Fig. 5e). She was independent at the last follow-up, 2 years after the surgery. Her child is also doing well.
Discussion
The telovelar approach (transcerebellomedullary fissure approach) is one of the optimal surgical approaches for fourth-ventricle tumor [5, 7, 13]. This approach without splitting of the inferior vermis can avoid the development of cerebellar mutism syndrome. Because the blood supply to the choroid plexus in the fourth ventricle derives from the branch of PICA, the telovelar approach allows us to cauterize the feeding artery to the CPTs at the early stage of surgery. The advantage of this approach and the smaller size of CPTs in the fourth ventricle may together contribute to less intraoperative bleeding compared to CPTs in the lateral ventricle or the third ventricle. In the present series, intraoperative bleeding was well controlled in all five cases using this approach.
Infiltration into the fourth ventricle floor was apparent in all five cases during surgery. Anatomically, the attachment of the choroid plexus in the fourth ventricle is located at the side of ventricular wall and runs longitudinally along the midline, then passes laterally toward the bilateral lateral recesses [18], forming an inverted T-shape. The lateral parts of the choroid plexus extend to the bilateral cerebellopontine angles through the foramina of Luschka. Based on this anatomy, CPTs would not infiltrate the brain stem at the very beginning of the tumorigenesis. CPTs in the fourth ventricle may later infiltrate to the brain stem via the taeniae choroidea laterally or infiltrate directly through the fourth ventricle floor after a period of tumor adherence. CPCs are considered to be highly infiltrative, and this characteristic is a diagnostic indicator of CPCs. The present series demonstrated that both CPCs and CPPs may present with apparent infiltration into the fourth ventricle floor, which hinders total extirpation of the tumor during surgery. Tumor adherence to the floor of the fourth ventricle was encountered in one child with fourth ventricular papilloma, which necessitated subtotal removal [17]. Evidence of stromal invasion and/or focal brain invasion without other cellular changes may be clinically consistent with benign CPPs [12].
Therefore, we recommend preoperative evaluation of possible brain stem invasion by MR imaging in patients with suspected CPTs. Careful evaluation of the anatomical relationship between the normal brain structure and the tumor, as well as the detection of the high-intensity lesion in the brain stem by T2-weighted MR imaging, may indicate the extent of brain stem invasion preoperatively.
The extent of surgical resection is generally accepted to be the most significant prognostic factor for CPCs [3, 8, 9, 16, 17, 20], but accurate histological diagnosis and evaluation of malignancy during surgery is not always possible to obtain. Such difficulties do not allow us to perform complete removal of the tumor, which may result in significant postoperative morbidity caused by destruction of the fourth ventricle floor. Therefore, we recommend removal of CPTs in the fourth ventricle as far as possible without affecting the normal brain stem structure. Complete surgical resection may be necessary for survival, and “second-look” procedures should be considered for patients who undergo subtotal resection [3, 8, 14]. If the lesion is localized and the patient’s condition permits, most primary tumors of the choroid plexus should be removed completely via as many procedures as required, regardless of the size, site, and histological features [8]. However, total resection of CPTs in the fourth ventricle may be hindered by invasion into the fourth ventricle floor, not by the excessive blood loss during surgery. Second-look surgery has the same risk of damage to the brain stem functions as the first surgery. Therefore, after the definitive histological diagnosis was established, we performed adjuvant radiochemotherapy for CPCs after discussion with a multidisciplinary team and also with the patients and family. We currently await the results of long-term follow-up of the patients with CPPs. The optimal postoperative treatment for the residual tumor in the fourth ventricle floor, including adjuvant radiochemotherapy or radiological follow-up without adjuvant therapy, is undetermined because of the limited number of patients with CPTs. The establishment of a worldwide registry for CPTs [9] would allow the development of a growing database of treatment strategies to address this critical issue.
Conclusion
The telovelar approach (transcerebellomedullary fissure approach) for CPTs in the fourth ventricle can control intraoperative bleeding by cauterizing the feeding arteries at the early stage of surgery. However, total resection is difficult because of infiltration into the brain stem. Future establishment of adjuvant therapy for CPTs with the brain stem infiltration is necessary to improve the prognosis.
References
Araki K, Aori T, Takahashi JA, Nozaki K, Nagata I, Kikuchi H, Yokoyama M, Hattori H, Akiyama Y, Kubota Y, Yokomizo H (1997) A case of choroid plexus carcinoma. No Shinkei Geka 25:853–857
Asai A, Hoffman HJ, Matsutani M, Takakura K (1991) Choroid plexus tumors in infancy. No Shinkei Geka 19:21–26
Berger C, Thiesse P, Lellouch-Tubiana A, Kalifa C, Pierre-Kahn A, Bouffet E (1998) Choroid plexus carcinomas in childhood: clinical features and prognostic factors. Neurosurgery 42:470–475
Boyd MC, Steinbok P (1987) Choroid plexus tumors: problems in diagnosis and management. J Neurosurg 66:800–805
Deshmukh VR, Figueiredo EG, Deshmukh P, Crawford NR, Preul MC, Spetzler RF (2006) Quantification and comparison of telovelar and transvermian approaches to the fourth ventricle. Neurosurgery 58:ONS–202-ONS-207
Dohrmann GJ, Collias JC (1975) Choroid plexus carcinoma. Case report. J Neurosurg 43:225–232
El-Bahy K (2005) Telovelar approach to the fourth ventricle: operative findings and results in 16 cases. Acta Neurochir (Wien) 147:137–142
Ellenbogen RG, Winston KR, Kupsky WJ (1989) Tumors of the choroid plexus in children. Neurosurgery 25:327–335
Fitzpatrick LK, Aronson LJ, Cohen KJ (2002) Is there a requirement for adjuvant therapy for choroid plexus carcinoma that has been completely resected? J Neurooncol 57:123–126
Kimura M, Takayasu M, Suzuki Y, Negoro M, Nagasaka T, Nakashima N, Sugita K (1992) Primary choroid plexus papilloma located in the suprasellar region: case report. Neurosurgery 31:563–566
Kumabe T, Tominaga T, Kondo T, Yoshimoto T, Kayama T (1996) Intraoperative radiation therapy and chemotherapy for huge choroid plexus carcinoma in an infant. Neurol Med Chir (Tokyo) 36:179–184
Levy ML, Goldfarb A, Hyder DJ, Gonzales-Gomez I, Nelson M, Gilles FH, McComb JG (2001) Choroid plexus tumors in children: significance of stromal invasion. Neurosurgery 48:303–309
Matsushima T, Inoue T, Inamura T, Natori Y, Fukui M (2001) Transcerebellomedullary fissure approach with special reference to methods of dissecting the fissure. J Neurosurg 94:257–264
McEvoy AW, Harding BN, Phipps KP, Ellison DW, Elsmore AJ, Thompson D, Harkness W, Hayward RD (2000) Management of choroid plexus tumors in children: 20 years experience at a single neurosurgical centre. Pediatr Neurosurg 32:192–199
Noshita N, Kumabe T, Kayama T, Tominaga T (2006) Choroid plexus tumors: report of 7 cases in a single institution. No Shinkei Geka 34:73–81
Packer RJ, Perilongo G, Johnson D, Sutton LN, Vezina G, Zimmermann RA, Ryan J, Reaman G, Schut L (1992) Choroid plexus carcinoma of childhood. Cancer 69:580–585
Pencalet P, Sainte-Rose C, Lellouch-Tubiana A, Kalifa C, Brunelle F, Sgouros S, Meyer P, Cinalli G, Zerah M, Pierre-Kahn A, Renier D (1998) Papillomas and carcinomas of the choroid plexus in children. J Neurosurg 88:521–528
Rhoton AL Jr (2000) Cerebellum and fourth ventricle. Neurosurgery 47:S7–S27
The Committee of Brain Tumor Registry of Japan (2003) Report of brain tumor registry of Japan (1969–1996). Neurol Med Chir (Tokyo) 43(Suppl):36–43
Wolff JE, Sajedi M, Brant R, Coppes MJ, Egeler RM (2002) Choroid plexus tumours. Br J Cancer 87:1086–1091
Author information
Authors and Affiliations
Corresponding author
Additional information
Comments
Veit Rohde, Göttingen, Germany
The authors report five cases of CPT in the fourth ventricle. Only few larger series have been published so far, including patients with CPTs in the lateral and the third ventricles. The authors confirm again that papillomas as well as carcinomas invade brain tissue, here the floor of the fourth ventricle. Therefore, the authors did not try to remove the tumor completely to avoid neurological deficits. There was a progression-free survival after 64 months (mean), which supports the author’s concept of a less aggressive surgical strategy. Using the telovelar approach, brisk bleeding could be controlled effectively. Even before this report, many neurosurgeons were reluctant to proceed to complete CPT removal in cases of infiltration of the fourth ventricular floor. Now, the neurosurgeons have the scientific justification for this strategy.
Rights and permissions
About this article
Cite this article
Kumabe, T., Fujimura, M., Jokura, H. et al. Surgical treatment for choroid plexus tumors in the fourth ventricle: brain stem infiltration hinders total extirpation. Neurosurg Rev 31, 165–172 (2008). https://doi.org/10.1007/s10143-007-0103-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-007-0103-7