Abstract
Secretory meningiomas constitute a relatively rare subtype of meningiomas, accounting for only 1.1% at our institution, with a 6:1 predominance of female patients. This study aimed to obtain more information about the immunohistochemical characteristics of this histological entity, and to analyse the effects of histological factors such as the presence of mast cells on the radiological evidence of surrounding tumour oedema that frequently occurred in this subtype of meningioma. Fourteen cases of secretory meningioma were examined. Relevant clinical information was obtained from the patient files. Peritumoural oedema was determined either by CT or MRI scans and graded as small, moderate and severe. In order to perform the quantitative evaluation of mast cells in secretory meningiomas in a comparison with other meningiomas, 14 non-secretory meningiomas were randomly selected and used as a control group. The immunohistochemical staining of carcinoembryonic antigen was positive within the secretory droplets and the cells surrounding them in all cases. Ki 67 (MIB 1) proliferative index mean values were 2.4%, indicating low expression in all secretory meningiomas. Moreover, from our statistical analysis, there is no clear-cut pattern of various types of cytokeratins emerging in secretory meningiomas. The secretory meningiomas were characterized by a significantly increased number of mast cells as compared with non-secretory meningiomas of different grades. As the present clinical findings and laboratory results could not confirm a correlation between mast cell density and radiological evidence of oedema, further studies of mediators are warranted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Secretory meningioma is a subtype of meningioma characterized by unique epithelial differentiation with glandular lumina containing secretory globules (hyaline bodies). This histological subtype was first described by Cushing and Eisenhardt [15]. Subsequently, Kepes and other authors described these hyaline bodies as pseudopsammoma bodies [8, 19, 24, 25, 27, 28]. In 1986, Alguacil-Garcia investigated the secretory features of these meningiomas and proposed the term “secretory meningioma”[1].
The secretory subtype of meningiomas is relatively rare, with only a limited number of cases reported up to 2003 [14]. Although immunohistochemical and electron microscopic studies demonstrated the epithelial differentiation features of secretory meningioma [1, 9, 18, 19, 25, 32, 40, 46, 48], the types of cytokeratin and mast cell numbers have never been investigated. According to our group’s previous studies, the other common feature of secretory meningioma is a high proportion of mast cells, immunohistochemically demonstrable in and around the pseudopsammoma bodies [20]. Therefore, we examined 14 cases of secretory meningiomas diagnosed and/or treated in our institution from 1980 to 2004 in order to provide more information on the histological characters of this tumour. This study also aimed to analyse the effects of histological factors (the presence of mast cells as well as pericytic proliferation) on radiological evidence of surrounding tumour oedema, which occurred more frequently in this subtype of meningioma.
Materials and methods
Between January 1980 and February 2004, 1310 cases of meningioma were diagnosed and/or operated on at the Philipps University Hospital, Marburg. Fourteen cases (1.1%) were diagnosed as secretory meningioma. Relevant clinical information was obtained from the patient files. The peritumoural oedema was determined either by computerized tomography (CT) or magnetic resonance imaging (MRI) scans, and graded as small, moderate and severe [9]. The surgical specimens were processed after fixation in 4% buffered formalin. Sections (4 μm) were cut from paraffin blocks. Sections were routinely stained with haematoxylin and eosin, periodic acid-Schiff without diastase, toluidine blue and other immunohistochemical antibodies.
Immunohistochemistry was performed using the avidin-biotin complex method according to the manufacturer’s instructions with primary antibodies to epithelial membrane antigen (EMA) (monoclonal; DAKO, Hamburg, Germany), carcinoembryonic antigen (CEA) (polyclonal; DAKO), Ki-67 (MIB-1, monoclonal; Dianova, Hamburg, Germany), pancytokeratins (CK MNF 116), cytokeratin 5/6, cytokeratin 7, cytokeratin 8 and cytokeratin 20. Additionally, pericytic proliferation was demonstrated using SM-actin staining. For mast cell analysis, anti-human CD 117 was used to label the transmembrane tyrosine kinase receptor CD117/c-kit, located in the mast cells (see applied primary antibodies in Table 1).
In order to perform quantitative evaluation of mast cells in secretory meningiomas in a comparison with other meningiomas, 14 non-secretory meningiomas were randomly selected and used as a control group. The control group of meningiomas comprised mostly endotheliomatous types, but included one malignant and one angiomatous meningioma. CD 117 marker was evaluated in a simple counting procedure. Positively stained mast cells located in five regions of secretory meningioma slice and control tumour slice were randomly counted with a single square microscopic counting ocular.
Statistics
Descriptive and explorative statistics was performed. Data are presented as mean±SD or median and range. Both the number of the whole cell content in the field and the number of mast cells were evaluated and calculated. For statistical evaluation, the non-parametric Mann-Whitney U-test was used. A P-value <0.05 was considered significant.
Results
Clinical feature
Tumour location, grade of oedema and the results of mast cell density are summarized in Table 2. Twelve patients were female and two were male. The common feature of the secretory meningiomas was the tendency to evoke brain oedema (Fig. 1). Five convexity and four middle fossa meningiomas revealed perifocal oedema. Six cases (43%) presented with a grade 3 oedema, one case (7%) with a grade 2 oedema and two cases (14%) with a grade 1 oedema, whereas oedema was absent in five cases (36%).
Histology
All secretory meningiomas had light microscopic appearance in accordance with the criteria of revised WHO classification of tumours of the central nervous system [31]. Each meningioma revealed an endotheliomatous growth pattern with the presence of moderate to abundant secretory globules of different sizes, staining intensely red with PAS (Fig. 2a) and blue with toluidine (Fig. 2b).
a Secretory meningioma with secretory products that stain vividly with the periodic acid-Schiff (PAS, original magnification ×500). Mast cells locating in the vicinity of these secretory products are observed (black arrows). Note: typical meningioma cells demonstrate the nuclear indentations (white arrows). b Toluidine stain-positive hyaline inclusions are shown. Mast cells also reveal deep blue reactions in their granules (original magnification ×1000)
In all tumours of this type, obviously more slightly basophilic mast cells could be detected. Regressive changes and transition into other subgroups of meningioma were noticed at random. Throughout all specimens, a prominent spongy state was observed. These frequent vacuoles are considered as oedema formation within the tumours.
Immunohistochemistry of secretory meningioma
Cytokeratin
Pancytokeratins were strongly expressed in the surrounding of the secretory droplets in almost all tumour specimens (11/13) (Fig. 3a). One patient’s specimen was excluded from this analysis because the tissue was unavailable. However, expression varied in strength from one specimen to the others. The immunoreactivity of different cytokeratins is shown in Table 3. The expression of CK 5/6, which represents squamous differentiation, was found in six cases (Fig. 3b), whereas markers of mucosal epithelial cells (CK 7, CK 8) were found in seven and four tumours (Fig. 3c,d), respectively; some expressed both CK7 and CK8. Cytokeratin 20 was absent in all specimens.
a Pancytokeratin expression is demonstrated in the cells surrounding the secretory droplets (original magnification ×500). b In some tumours, the epithelial marker CK5 or CK6 are expressed in these secreting cells (original magnification ×500). c CK7 is present in the same manner surrounding those pseudopsammoma bodies (original magnification ×250). d CK8 expresses in few secreting cells (original magnification ×500). CK cytokeratin
CEA, vimentin, epithelial membrane antigen, MIB-1
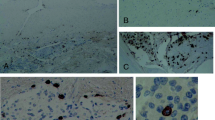
CEA was expressed within the secretory droplets and the cells surrounding them in all cases (Fig. 4a). Generally, the membrane and the cytoplasm of tumour cells were positive for EMA and vimentin. Positive vimentin staining was seen in clusters of focally arranged cells, whereas EMA was found both in the focally distributed tumour cells and the tumour cells surrounding secretory globules. The EMA expression was almost identical to the expression of pancytokeratin in cells those lay around the secretory product. Ki 67 (MIB 1) proliferative index mean values were 2.4%, indicating low expression in all secretory meningiomas.
a Vimentin expression is found in tumour cells with rather clonal expression. Both diffusely scattered tumour cells and those surrounding the secretory product are stained (original magnification ×500). b With CD 117, mast cells are detected. Expression is clearer near the cell membrane (original magnification ×500). c SM-actin demonstrates vascular proliferation (original magnification ×250). d Only the center of pericytic proliferation reacts with SM-actin. Cells that form the periphery of the proliferation zone (dotted line) obviously lose smooth muscle differentiation. Thin arrows point to the surrounding oedema. The thick arrow points to a secretory droplet (original magnification ×250)
Mast cell immunohistochemistry
The presence of mast cells was assessed by immunohistochemistry using polyclonal rabbit anti-human CD 117 (Fig. 4b). The quantitative evaluation of mast cells in secretory meningioma in comparison with other non-selected meningiomas of different cell types is demonstrated in Fig. 5.
The quantitative evaluation of mast cells in secretory meningioma in comparison with non-selected meningiomas of different cell type is revealed. In the control group, the general cellular density was significantly higher than corresponding values in the secretory meningioma group. However, secretory meningiomas have significantly more numbers of mast cells than control meningiomas
Although the general cellular densities of control meningiomas were significantly higher than corresponding values of secretory meningioma (mean 310 (range 186 –496) versus 207 (range 132–340) cells per 0.0575 mm2; P=0.001, Mann-Whitney U-test), the secretory meningioma had significantly more mast cells (mean 2.43 (range 0.020–5.60) versus 0.27 (range 0,0–2.0) cells per 0.0575 mm2; P=0.0001, Mann-Whitney U-test).
Sm-actin marker for pericytic proliferation
A remarkable pericytic proliferation within the vessel walls was observed in 12/13 cases (92%) (Fig. 4c). Sm-actin immunoreactivity was found in perivascular pericytes located adjacent to the capillary endothelial cells. Sm-actin content gradually decreased with increasing distance from the lumen of vessel. This expression pattern was confirmed by electron microscopic findings (data not shown). The vascular endothelial proliferation rim is regularly surrounded by a zone of spongy holes representing perivascular oedema (Fig. 4d).
Discussion
Incidence and clinical aspects
Secretory meningiomas are rare tumours with a reported incidence of 1.2–4.4% [19, 37, 40]; however, other early reports quote higher incidences of 8.1–9.3% [1, 42]. The frequency of the secretory type among the meningiomas diagnosed in our institution is 1.1%. A previous study revealed a female-to-male ratio of 10:1 among patients with secretory meningioma [40]. In our study, the female predominance was noticeable with the value of 6:1 (12 females: 2 males), surpassing the 3:2 ratio among patients with intracranial meningioma [10]. Although a variable presence of both progesterone and oestrogen receptors has been reported by several authors [12, 17, 34, 40], the pathophysiological function and molecular role of sex hormones with regard to pathogenesis of meningioma remains unknown.
Many authors conclude that the higher incidence or the trend toward a high incidence of peritumoral oedema is associated with tumour volume [3, 4, 6, 7, 21, 30, 33, 49]. Although all secretory meningiomas except one (4 cm) of our series were smaller than 4 cm, peritumoral oedema was observed in about two-thirds of cases and two-thirds of cases with this peritumoral oedema were classified as severe oedema. This is in agreement with early reports of a higher incidence of oedema in secretory meningiomas [13, 23, 35, 40, 46].
Immunohistochemistry
It is well recognized that meningioma can develop multiple mesenchymal differentiation (fibroblastic, transitional, meningoendothelial, etc.) and meningioma with hyaline inclusions is thought to represent the focal epithelial differentiation of meningothelial cells [1, 36, 47]. Additionally, epithelial differentiation can be easily demonstrated via staining of cytokeratin [40]. Although positive staining with cytokeratin or pancytokeratin in the inclusions and in the cytoplasm of cells surrounding secretory meningiomas has been reported [1, 18, 40, 46], we have not found data focusing on the types of cytokeratin. From our study of cytokeratins, the secretory meningiomas may express markers of squamous or columnar epithelia, or even both. This could indicate a progressive undirected process of differentiation. Moreover, from our statistical analysis there is no clear-cut pattern of various types of cytokeratins emerging in secretory meningioma (Table 3).
Histological similarity of secretory meningioma and other carcinoma
Intracellular and extracellular lumina with prominent microvilli surrounding hyaline inclusion in both meningiomas [19, 25] and other carcinomas [2, 44] was shown using ultrastructural studies. These findings indicate the association between epithelial and secretory differentiation. Light microscopic findings of similar cytoplasmic hyaline inclusions have been also reported in carcinomas of the breast, lung, colon and liver [2, 16]. Our immunohistochemical study demonstrated CEA and EMA expressed in epithelial cells and inclusions of secretory meningiomas. The strong positivity for CEA and EMA in cells forming intracellular lumina and duct-like structures support the epithelial secretory nature of these benign meningiomas and malignant tumour cells [1, 11, 26, 43].
Although these malignant tumours and secretory meningiomas are very different in terms of progression, some morphological aspects and immunohistochemical features of intracellular hyaline bodies are similar suggesting a similar pathogenesis of these structures in both benign and malignant tumours. Additionally, both secretory meningioma and intracerebral metastatic carcinoma are associated with severe brain oedema.
Mast cells and pericytic proliferation
According to the report of Bo et al., mononuclear cell infiltrates in meningiomas are mainly composed of T-cells and macrophages, indicating an immune system surveillance and response to the tumour cells, with mast cells found only in nine out of 32 tumours [5].
In contrast to the above finding, we previously demonstrated that high mast cell numbers are a common feature in secretory meningiomas and these were found in and around pseudopsammoma bodies [20]. After investigating the mast cell density in secretory meningiomas and the control group (non-secretory meningiomas), the number of mast cells in secretory meningiomas was significantly increased compared with those cells in tissues of the control group (Fig. 5). Mast cell derived mediators, e.g. histamine, may play an important role in creating pathological brain oedema. Further studies of mediators are required to clarify the correlation between the presence of mast cells and the development of oedema, although our preliminary data of clinical findings and results from the laboratory did not clarify the relation between mast cell density and radiological evidence of oedema.
Pericytic proliferation is another component that aids diagnosis of secretory meningioma [1, 8, 13, 18, 40]. Pericytic proliferation was observed in 83% of cases in a recent study [14]. In our study, a high ratio (92%) of pericytic proliferation was also observed using sm-actin staining. We agree that this histological feature is a helpful finding in the diagnosis of secretory meningiomas, especially with cases where secretory inclusions are not prominent [14]. However, an unusual vascular pericytic proliferation as a cause of peritumoral cerebral oedema is still a matter of debate [13, 37, 40].
Peritumoral oedema and possible pathogenesis
A noteworthy finding of secretory meningioma is that they are accompanied more often by massive peritumoral oedema than other meningiomas of similar location or size [23]. However, the exact pathogenesis of meningioma-associated brain oedema is not completely understood. Controversies in the literature concerning the correlation between the degree of peritumoral oedema in intracranial meningiomas and tumor size, location, or histological subtype have been reported [9, 40]. According to the venous compression theory, the incidence of oedema would be higher in meningioma located at the frontal or sphenoidal region. In our study, 13 secretory meningiomas are less than 4 cm and ten of them are located at either the frontal convexity or the sphenoid ridge. Thus, venous compression might have some influence on peritumoral oedematous formation. The other possible mechanisms seem related to the secretion of vascular mediating substances such as vascular endothelial growth factor (VEGF) [29, 38, 39] or other modulating factors regulating its secretion [45]. Particularly in secretory meningioma, which demonstrate a higher number of mast cells than other subtype of meningioma, other mast cell mediators (e.g. histamine, serotonin) or VEGF might play a significant role in oedemous formation. Further research should be conducted in order to demonstrate the possible relation of secretion of mast cell mediators or VEGF and oedematous process. Additionally, we agree that oedematous formation in the intracranial meningioma is probably multifactorial [9, 22].
Conclusion
Secretory meningiomas are often associated with severe peritumoral oedema, causing signs and symptoms of increased intracranial pressure. So far, the initial step inducing peritumoral oedema has not been demonstrated. Many histological findings such as increased numbers of mast cells, pseudopsammoma bodies, CEA, and pericytic proliferation might be involved in the pathophysiological process of this vasogenic brain oedema. Demonstration of mediators in the tumour tissue might define the role of mast cells and provide more information about the nature of this particular type of meningioma.
References
Alguacil-Garcia A, Pettigrew NM, Sima AA (1986) Secretory meningioma. A distinct subtype of meningioma. Am J Surg Pathol 10:102–111
An T, Ghatak N, Kastner R, Kay S, Lee HM (1983) Hyaline globules and intracellular lumina in a hepatocellular carcinoma. Am J Clin Pathol 79:392–396
Bitzer M, Topka H, Morgalla M, Friese S, Wockel L, Voigt K (1998) Tumor-related venous obstruction and development of peritumoral brain edema in meningiomas. Neurosurgery 42:730–737
Bitzer M, Wockel L, Morgalla M, Keller C, Friese S, Heiss E, Meyermann R, Grote E, Voigt K (1997) Peritumoural brain oedema in intracranial meningiomas: influence of tumour size, location and histology. Acta Neurochir (Wien) 139:1136–1142
Bo L, Mork SJ, Nyland H (1992) An immunohistochemical study of mononuclear cells in meningiomas. Neuropathol Appl Neurobiol 18:548–558
Bradac GB, Ferszt R, Bender A, Schorner W (1986) Peritumoral edema in meningiomas. A radiological and histological study. Neuroradiology 28:304–312
Brandis A, Mirzai S, Tatagiba M, Walter GF, Samii M, Ostertag H (1993) Immunohistochemical detection of female sex hormone receptors in meningiomas: correlation with clinical and histological features. Neurosurgery 33:212–217
Budka H (1982) Hyaline inclusions (Pseudopsammoma bodies) in meningiomas: immunocytochemical demonstration of epithel-like secretion of secretory component and immunoglobulins A and M. Acta Neuropathol (Berl) 56:294–298
Buhl R, Hugo HH, Mihajlovic Z, Mehdorn HM (2001) Secretory meningiomas: clinical and immunohistochemical observations. Neurosurgery 48:297–301
Burger PC, Scheithauer BW, Vogel FS (1991) Surgical pathology of the nervous system and its coverings, 3rd edn. Churchill Livingstone, New York, pp 67–91
Burtin P, Escribano MJ (1983) The carcinoembryonic antigen and its cross-reacting antigens. In: Fishman WH (ed) Oncodevelopmental markers. Academic, New York
Carroll RS, Zhang J, Black PM (1999) Expression of estrogen receptors alpha and beta in human meningiomas. J Neuro-oncol 42:109–116
Challa VR, Moody DM, Marshall RB, Kelly DL Jr (1980) The vascular component in meningiomas associated with severe cerebral edema. Neurosurgery 7:363–368
Colakoglu N, Demirtas E, Oktar N, Yuntem N, Islekel S, Ozdamar N (2003) Secretory meningiomas. J Neuro-oncol 62:233–241
Cushing H, Eisenhardt L (1938) Meningiomas. Their classification, regional behaviour, life history and surgical end results. Thomas, Springfield Baltimore. Quoted in: Kepes JJ (1961) Observation on the formation of psammoma bodies and pseudopsammoma bodies in meningiomas. J Neuropathol Exp Neurol 34:255–262
Dekker A, Krause JR (1973) Hyaline globules in human neoplasms. A report of three autopsy cases. Arch Pathol 95:178–181
Donnell MS, Meyer GA, Donegan WL (1979) Estrogen-receptor protein in intracranial meningiomas. J Neurosurg 50:499–502
Ejeckam GC, Azadeh B, Hamad A (1992) Secretory meningioma. Histopathology 21:475–477
Font RL, Croxatto JO (1980) Intracellular inclusions in meningothelial meningioma. A histochemical and ultrastructural study. J Neuropathol Exp Neurol 39:575–583
Hallier-Neelsen M, Mennel HD (2002) CD-117 in astrocytic and meningeal tumors. Acta Neuropathol 104:543–581
Inamura T, Nishio S, Takeshita I, Fujiwara S, Fukui M (1992) Peritumoral brain edema in meningiomas-influence of vascular supply on its development. Neurosurgery 31:179–185
Kalkanis SN, Carroll RS, Zhang J, Zamani AA, Black PM (1996) Correlation of vascular growth factor messenger RNA expression with peritumoral vasogenic cerebral edema in meningiomas. J Neurosurg 85:1095–1101
Kepes JJ (1989) History and diagnosis of meningiomas. In: Fields WC (ed) Primary brain tumors: a review of histologic classification. Springer, Berlin Heidelberg New York, pp 217–230
Kepes JJ (1961) Observation on the formation of psammoma bodies and pseudopsammoma bodies in meningiomas. J Neuropathol Exp Neurol 34:282–294
Kepes JJ (1975) The fine structure of hyaline inclusions (pseudopsammoma bodies) in meningiomas. J Neuropathol Exp Neurol 34:282–294
Klavins JV (1983) Advances in biological markers for cancer. Ann Clin Lab Sci 13:275–280
Kock KF, Teglbjaerg PS (1981) Meningiomas with a non-meningotheliomatous component. A new type of tumour? Acta Neuropathol (Berl) 55:199–203
Kubota T, Hirano A, Yamamoto S (1982) The fine structure of hyaline inclusions in meningioma. J Neuropathol Exp Neurol 41:81–86
Lamszus K, Lengler U, Schmidt NO, Stavrou D, Ergun S, Westphal M (2000) Vascular endothelial growth factor, hepatocyte growth factor/scatter factor, basic fibroblast growth factor, and placenta growth factor in human meningiomas and their relation to angiogenesis and malignancy. Neurosurgery 46:938–947
Lobato RD, Alday R, Gomez PA, Rivas JJ, Dominguez J, Cabrera A, Madero S, Ayerbe J (1996) Brain oedema in patients with intracranial meningioma. Correlation between clinical, radiological, and histological factors and the presence and intensity of oedema. Acta Neurochir (Wien) 138:485–493
Louis DN, Scheithauer BW, Budka H, von Deimling A, Kepes JJ (2000) Meningiomas. In: Kleihues P, Cavenee WK (eds) Pathology and genetics of tumors of the nervous system. IARC Press, Lyon, pp 176–180
Louis DN, Hamilton AJ, Sobel RA, Ojemann RG (1991) Pseudopsammomatous meningioma with elevated serum carcinoembryonic antigen: a true secretory meningioma. Case report. J Neurosurg 74:129–132
Maiuri F, Gangemi M, Cirillo S, Delehaye L, Gallicchio B, Carandente M, Giamundo A (1987) Cerebral edema associated with meningiomas. Surg Neurol 27:64–68
Maxwell M, Galanopoulos T, Neville-Golden J, Antoniades HN (1993) Expression of androgen and progesterone receptors in primary human meningiomas. J Neurosurg 78:456–462
Mirra SS, Miles ML (1982) Unusual pericytic proliferation in a meningotheliomatous meningioma: an ultrastructural study. Am J Surg Pathol 6:573–580
Ng HK, Tse CC, Lo ST (1987) Meningiomas and arachnoid cells: an immunohistochemical study of epithelial markers. Pathology 19:253–257
Nishio S, Morioka T, Suzuki S, Hirano K, Fukui M (2001) Secretory meningioma: clinicopathologic features of eight cases. J Clin Neurosci 8:335–339
Paek SH, Kim CY, Kim YY, Park IA, Kim MS, Kim DG, Jung HW (2002) Correlation of clinical and biological parameters with peritumoral edema in meningioma. J Neuro-oncol 60:235–245
Pistolesi S, Fontanini G, Camacci T, De Ieso K, Boldrini L, Lupi G, Padolecchia R, Pingitore R, Parenti G (2002) Meningioma-associated brain oedema: the role of angiogenic factors and pial blood suppy. J Neuro-oncol 60:159–164
Probst-Cousin S, Villagran-Lillo R, Lahl R, Bergmann M, Schmid KW, Gullotta F (1997) Secretory meningioma: clinical, histologic, and immunohistochemical findings in 31 cases. Cancer 79:2003–2015
Rosai J (1995) Ackerman’s surgical pathology. Mosby, St Louis, Mo, pp 37–38
Schelper RL, Beck DW, Boarini DJ, Hart MN, Baumbach GL (1984) Proteins of hyaline inclusions in meningioma. J Neuropathol Exp Neurol 43:297
Sloane JP, Ormerod MG (1981) Distribution of epithelial membrane antigen in normal and neoplastic tissues and it value in diagnostic tumor pathology. Cancer 47:1786–1795
Syre G, Sehm M (2004) Intracellular storage of IgA and secretory component in carcinomas of the female breast. Virchows Arch (Pathol Anat) 393:315–320
Tsai JC, Hsiao YY, Teng LJ, Shun CT, Goldman CK, Kao MC (1999) Regulation of vascular endothelial growth factor secretion in human meningioma cells. J Formos Med Assoc 98:111–117
Tsunoda S, Takeshima T, Sakaki T, Morimoto T, Hoshida T, Watabe Y, Goda K (1992) Secretory meningioma with elevated serum carcinoembryonic antigen level. Surg Neurol 37:415–418
Vakili ST, Muller J (1988) Intracytoplasmic lumina in meningioma: an ultrastructural and immunohistological study. Neurosurgery 23:180–184
Winek RR, Scheithauer BW, Wick MR (1989) Meningioma, meningeal hemangiopericytoma (angioblastic meningioma), peripheral hemangiopericytoma, and acoustic schwannoma. A comparative immunohistochemical study. Am J Surg Pathol 13:251–261
Yoshioka H, Hama S, Taniguchi E, Sugiyama K, Arita K, Kurisu K (1999) Peritumoral brain edema associated with meningioma: influence of vascular endothelial growth factor expression and vascular blood supply. Cancer 85:936–944
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tirakotai, W., Mennel, HD., Celik, I. et al. Secretory meningioma: immunohistochemical findings and evaluation of mast cell infiltration. Neurosurg Rev 29, 41–48 (2006). https://doi.org/10.1007/s10143-005-0402-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-005-0402-9