Abstract
Long non-coding RNA (lncRNA) small nucleolar RNA host gene 1 (SNHG1) has been found to be highly expressed in gastric cancer (GC). However, the study for exploring the effects of SNHG1 and microRNA (miR)-195-5p on GC is limited. This research commits to unravel the regulatory effects of SNHG1, miRNA-195-5p, and Yes-associated protein 1 (YAP1) on GC. SNHG1, miR-195-5p and YAP1 levels in GC tissues and GC cells were detected. The GC cells were treated with various constructs altering SNHG1 or miR-195-5p expression to determine the biological activities of GC cell in vitro. The effect of SNHG1 inhibition on subcutaneous tumorigenesis of GC cells in a nude mouse model in vivo was detected. The binding relation among SNHG1, miR-195-5p, and YAP1 was validated. SNHG1 and YAP1 levels were elevated and miR-195-5p level was reduced in GC. Reduction of SNHG1 or elevation of miR-195-5p retarded GC cell biological activity in vitro. Downregulated SNHG1 suppressed tumor growth in vivo. SNHG1 bound to miR-195-5p, and miR-195-5p directly targeted YAP1. The downregulated SNHG1 hinders the biological behaviors of GC cells via the modulation of the miR-195-5p/YAP1 axis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer (GC), as one of the most prevailing cancer globally, ranks the top three inducers for cancer-associated death globally (Smyth et al. 2020). The risk factors that contributing to the high incidence of GC include infectious agent, host factors, environmental factors, and virus factors. GC patients usually suffer from abdominal pain, frequent vomit, and weight loss (Correa 2013). Chemotherapy, radiotherapy, molecular-targeted therapies, and immunotherapy are some newly developed treatments for GC patients in different phases (Song et al. 2017), even so, silent symptom still causes the low rate of early diagnosis of GC (Tan 2019), and the median survival for patients with advanced GC is 1 year or less (Zhang and Zhang 2017). Thus, it is necessary and significant to make further research on the effective treatment of GC from more different aspects.
Long non-coding RNA (lncRNA) exerts a vital role in tumor genesis and development by acting as tumor modulators (Wang et al. 2018a). LncRNA small nucleolar RNA host gene 1 (SNHG1) is found to become a crucial regulatory RNA for different cancers types, including glioma, GC, and many other diseases (Huang et al. 2018). SNHG1 is expressed in GC and acts as a promoter for cell growth (Hu et al. 2017). Hu et. al. has reported that SNHG1 is robustly expressed in GC cells, and high expression of SNHG1 has a connection with early tumor stage, tumor size, and reduced overall survival (Thin et al. 2019). In addition, it is verified that lncRNA, as the endogenous RNA, can bind with microRNA (miRNA) (Zhang et al. 2016). According to some articles (Zou et al. 2019; Zhao and Wu 2019), miR-195-5p is lowly expressed in GC patients, and miR-195-5p expression can be used as an independent prognostic factor for poor prognosis of GC patients. In addition, miR-195-5p can restrain the cell activities and the epithelial-mesenchymal transition of hepatocellular carcinoma (HCC) by suppressing the expression of Yes-associated protein (YAP) (Zhao and Wu 2019). YAP is regulated by mechanical cues through the connection of Hippo pathway with cytoskeleton (Qiao et al. 2017). As reported, YAP expression is elevated in GC cells, and its high expression enhances the cell function and survival (Yan et al. 2018). In HCC, the interaction of silenced miR-195-5p and enhanced YAP has already been verified (Zhao and Wu 2019). However, the regulatory mechanism of miR-195-5p and YAP1 in GC needs further clear exploration. So, this research committed to unravel the detailed roles of the SNHG1/miR-195-5p/YAP1 axis in GC, which may help to furnish the research direction for GC treatment strategies.
Materials and methods
Ethics statement
The study got approval from the ethics committee of the Second Xiangya Hospital of Central South University. All participants signed the informed consent form and enjoyed the right to know. The animal protocol for this study was issued by Institutional Animal Care and Use Committee of the Second Xiangya Hospital of Central South University.
Study subjects
The specimens of GC tissues and adjacent normal tissues were selected from 160 participants who underwent the GC resection in the Second Xiangya Hospital of Central South University. The samples were frozen in liquid nitrogen after surgery.
Cell culture
The human normal gastric epithelial cell line GES-1 and GC cell lines (MNK-45 and HGC-27) were purchased from Tong Pai Technology Co., Ltd. (Shanghai, China). After resuscitation, the cells were incubated in Roswell Park Memorial Institute (RPMI-1640) medium (Hyclone, Thermo scientific, USA) supplemented with 10% fetal bovine serum (FBS, Hyclone) at 37 °C with 5% CO2.
Cell transfection and grouping
MNK-45 cells and HGC-27 cells were divided into 8 groups: sh-negative control (NC) (transfected with non-targeting shRNA containing vector), short hairpin RNA (sh)-SNHG1 (transfected with sh-SNHG1 vector), mimic NC (transfected with mimic NC), miR-195-5p mimic (transfected with miR-195-5p mimic), sh-SNHG1 + inhibitor NC (transfected with sh-SNHG1 vector and inhibitor NC), sh-SNHG1 + miR-195-5p inhibitor group (transfected with sh-SNHG1 vector and miR-195-5p inhibitor), sh-SNHG1 + overexpressed (oe)-NC group (transfected with sh-SNHG1 vector and oe-YAP1 vector mock control), and sh-SNHG1 + oe-YAP1 group (transfected with sh-SNHG1 vector and overexpressed YAP1 vector). The Lipofectamine™ 2000 (Invitrogen, Carlsbad, California, USA) was adopted for various constructs transfection (the above various constructs were obtained from Shanghai GenePharma Co, Ltd., Shanghai, China).
Cell counting kit (CCK)-8 assay
After transfection, the 96-well plates were used for cell seeding (approximately 2000 cells per well) and followed by 5-day incubation. The sterile CCK-8 solution (10 μL, Solarbio, Beijing, China) was added every 24 h and followed by 3-h incubation. The microplate reader (Thermo Fisher Scientific, MA, USA) was adopted to examine the optical density value at 450 nm (Sun, et al. 2020).
Colony formation assay
Cells were placed in a 6-well plate (500 cells/well). Then, colonies were observed on the plate and and the medium was discarded 2–3 weeks later to finish cell culture. Then, the cells were fixed by methanol, added with 1 mL Giemsa working fluid, dyed for 30 min, and washed. Ultimately, the plate was dried and photographed (Cai, et al. 2020).
Annexin V-PI staining
The transfected cells were harvested and resuspended in 4 °C precooled phosphate-buffered saline. The cells were resuscitated by 1 × binding buffer, mixed with 5 μL Annexin V-fluorescein isothiocyanate, and incubated for 15 min. Thereafter, 5 μL propidium iodide dye and 200 μL 1 × binding buffered solution were added 5 min before the assay. Finally, the samples were examined by a flow cytometer (Beckman Coulter, Brea, CA, USA). The Cell Quest (BD Bioscience, San Diego, CA) was used for result evaluation. The cells were classified into dead cells (Q1, Annexin V negative, PI positive), late apoptotic cells (Q2, Annexin V positive, PI positive), early apoptotic cells (Q3, Annexin V positive, PI negative), and viable cells (Q4, Annexin V negative, PI negative) (Cai, et al. 2020; Temiz, et al. 2021).
Scratch test
Forty-eight hours after transfection, GC cells were isolated to until the concentration finally reached to 2 × 10 5 mL−1, and then cells were coated in a 12-well plate (2 × 10 5 cells /well) for 24-h deposition. When cell confluence reached to 100%, the wound was evenly scraped with a sterile pipette tip. Cell migration ability was examined by detecting the cell movement after scraping. After 24 h, the speed of wound closure was assessed by calculating the distance of the wound from 0 h (Yang et al. 2020).
Transwell assay
The ability for cell biological behaviors were examined by a 24-well Transwell chamber. During the migration test, 3 × 104 cells in serum-free medium were put in the top chamber, and the bottom chamber was added with 600 μL RPMI-1640 medium with 10% FBS for 24-h incubation. The cells were subjected to fixation with 4% paraformaldehyde, staining with 1% crystal violet and analysis with an inverted microscope. During the invasion test, the Matrigel (Becton, Dickinson and Company, NJ, USA) diluted with serum-free medium was enveloped with the Transwell chamber before the cells were added and followed by 48-h incubation in a humid environment (at 37 °C with 5% CO2). The remaining steps were elucidated in the assay of cell migration.
Reverse transcription quantitative polymerase chain reaction (RT-qPCR)
The Trizol kit (Invitrogen) was adopted for RNA extraction. The RNA concentration and purity were measured with an ultraviolet spectrophotometer. Complementary DNA (cDNA) was generated using PrimeScript RT Reagent Kit (TaKaRa, Dalian, China) for lncRNAs or mRNAs and Mir-X™ miRNA First-Strand Synthesis Kit (TaKaRa) for miRNAs. qPCR amplification was carried out in ABI7500 real-time rapid PCR system (Applied Biosystems, MA, USA). SNHG1, miR-195-5p, and YAP1 primer sequences were shown (Supplementary Table 1). U6 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were used as endogenous references. The gene expression was determined by the 2−ΔΔCt method. The experiment was repeated three times.
Western blot assay
Radioimmunoprecipitation buffer was used to extract proteins. Protein samples (15–30 μg) were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis, followed by electroblotting onto the polyvinylidene fluoride membrane (Millipore, Billerica, MA, USA). Afterwards, the protein sample was subjected to 1-h blocking with 5% bovine serum albumin, and then added with primary antibodies, and incubated at 4 °C overnight. The corresponding secondary antibody was added for 1-h incubation. The samples were imaged by chemiluminescence reagent. The ImageJ (National Institutes of Health, Bethesda, MA, USA) was adopted for determining the gray value of protein bands. YAP1 and GAPDH (both 1:1000; Abcam, Cambridge, MA, USA) were the primary antibodies.
Dual luciferase reporter gene assay
The binding sites of miR-195-5p and SNHG1or YAP1 were predicted by Starbase website (Li, et al. 2014) and validated by dual luciferase reporter gene assay. SNHG1-wild type (wt), SNHG1-mutant (mut), or YAP1-wt, YAP1-mut targeted fragments were amplified by PCR and then cloned into pmirGLO vector (Promega, WI, USA). The constructed vectors were co-transfected of miR-195-5p mimic and mimic NC into MNK-45 and HGC-27 cells, respectively. The cells were collected and lysed 48 h after transfection, and the luciferase activity was examined by the dual luciferase assay system (Promega).
In order to evaluate the variation of the Hippo signaling pathway, the luciferase activity (Promega) was determined. The cells were transfected with transcriptional enhanced associate domains (TEAD) luciferase reporter plasmid (YouBio, Changsha, China). Two days after transfection, the luciferase activity was analyzed (Gao et al. 2018).
RNA immunoprecipitation (RIP) experiment
Magna RIP RNA Binding Protein Immunoprecipitation Kit (Millipore) was used for RIP assay. After MNK-45 and HGC-27 cells were lysed with RIP lysis buffer, we incubated the RIP lysate with protein A/G magnetic beads, which were pre-conjugated with antibodies targeting Argonaute-2 (anti-Ago2) (Abcam) or NC anti-immunoglobulin G (IgG) (Millipore) antibody in RIP immunoprecipitation buffer. After removing the protein through the interaction with protease K buffer (Invitrogen), the immunoprecipitated RNAs were eluted. The purified RNA was analyzed using RT-qPCR assay (Lin et al. 2019).
Tumorigenesis in nude mice
Four-week-old BALB/c nude mice were selected to produce a subcutaneous tumor model in nude mice. MNK-45 and HGC-27 cells (5 × 106) were injected into the left armpit of nude mice. Four weeks later, the mice were euthanized, and the weight and volume of the subcutaneous tumors were measured (Yu et al. 2019).
Statistical analysis
Data were statistically analyzed with SPSS 21.0 (IBM, SPSS, Chicago, IL, USA) and GraphPad Prism 6.0 (GraphPad Software Inc., CA, USA). Measurement data were represented as mean ± standard deviation. The t-test was used for comparison between two groups, while analysis of variance (ANOVA) and Tukey’s test were adopted for comparisons among multiple groups. Correlation analysis was conducted with the Pearson test. P < 0.05 was an indicator of statistically significance.
Results
SNHG1 and YAP1 expression levels are elevated and miR-195-5p expression level is reduced in GC
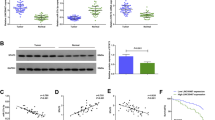
We conducted RT-qPCR and Western blot assay to determine the different expression of SNHG1, miR-195-5p, and YAP1 in GC and normal gastric tissues. It indicated that SNHG1 and YAP1 expression levels were elevated and miR-195-5p expression level was reduced in GC tissues (Fig. 1A–D).
SNHG and YAP1 are highly expressed, and miR-195-5p is lowly expressed in GC tissues. A SNHG1 expression in GC tissue and normal gastric tissues (GADPH was used as an endogenous reference). B miR-195-5p expression in GC tissue and normal gastric tissues (U6 was used as an endogenous reference). C mRNA expression of YAP1 in GC tissue and normal gastric tissues (GADPH was used as an endogenous reference). D Protein expression of YAP1 in GC tissue and and normal gastric tissues. E Correlation between SNHG1 and miR-195-5p. F Correlation between SNHG1 and YAP1. G Correlation between miR-195-5p and YAP1. H Survival time curve between SNHG1 expression and GC patients. *P < 0.05 vs. the normal gastric tissues. The data were represented by mean ± standard deviation (n = 160)
The correlations among SNHG1, miR-195-5p, and YAP1 levels of GC patients were determined by Pearson test, which revealed a negative relation between SNHG1 and miR-195-5p expression, a positive relation between SNHG1 and YAP1 mRNA, and a negative relation between miR-195-5p and YAP1 mRNA (Fig. 1E–G).
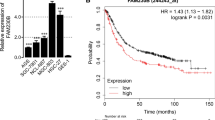
Relationships between SNHG1 expression and clinicopathological characteristics of GC were studied (Supplementary Table 2). The results indicated that SNHG1 level was not correlated to age (P = 0.748), gender (P = 0.518), Lauren tissue type (P = 0.266), and helicobacter pylori infection (P = 0.424). However, the expression of SNHG1 had an association with tumor size (P = 0.001), lymph node metastasis (P < 0.001), and TNM stage (P = 0.011). Furthermore, Kaplan–Meier analysis showed that elevated SNHG1 expression in GC patients was responsible for the poor survival in GC patients (Fig. 1H).
Downregulation of SNHG1 inhibits cell biological activities in vitro, as well as suppresses tumor growth in vivo
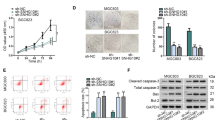
SNHG1 is a valuable biomarker for early examination and prognostic prediction of a range of cancers, such as GC. It is reported that SNHG1 level in GC cell line is significantly elevated (Guo, et al. 2019). SNHG1 expression in GES-1 and GC cell lines (MNK-45 and HGC-27) was assessed by RT-qPCR, which suggested that SNHG1 expressed highly in GC cell lines (Fig. 2A).
Down-regulation of SNHG1 inhibits GC cell activities, as well as suppresses the tumor growth in vivo. A, B. RT-qPCR detected the expression level of SNHG1 in MNK-45 and HGC-27 cells after down-regulation of SNHG1 (GADPH was used as an endogenous reference). C CCK-8 assay detected the growth curve of MNK-45 and HGC-27 cells after down-regulation of SNHG1. D Colony formation assay detected the colony formation ability of MNK-45 and HGC-27 cells after down-regulation of SNHG1. E Flow cytometry detected the apoptosis of MNK-45 and HGC-27 cells after down-regulation of SNHG1: dead cells (Q1, Annexin V negative, PI positive), late apoptotic cells (Q2, Annexin V positive, PI positive), early apoptotic cells (Q3, Annexin V positive, PI negative), and viable cells (Q4, Annexin V negative, PI negative). F Scratch test detected the cell migration ability of MNK-45 and HGC-27 cells after down-regulation of SNHG1. G Transwell assay detected the cell migration and invasion ability of MNK-45 and HGC-27 cells after down-regulation of SNHG1. H Tumor formation assay in vivo detected the volume and weight of tumors after down-regulation of SNHG1. *P < 0.05 vs. the sh-NC group; #P < 0.05 vs. GES-1 cells. The data were represented by mean ± standard deviation. The cell experiment was repeated 3 times with 3 technical repetitions, and the animal experiment was performed with 3 animals in each group with 3 technical repetitions
To detect the biological activities of SNHG1 in GC cells, we transfected the sh-SNHG1 vector into MNK-45 and HGC-27 cells, and the transfection was validated successfully by RT-qPCR (Fig. 2B). The proliferative capacity of MNK-45 and HGC-27 cells was tested by CCK-8 and colony formation assays. The outcomes reflected that the treatment with sh-SNHG1 in MNK-45 and HGC-27 cells inhibited the proliferative and colony formation capacities of MNK-45 and HGC-27 cells (Fig. 2C, D).
The flow cytometry was adopted to assess the apoptotic ability, which of MNK-45 and HGC-27 cells was significantly enhanced by down-regulation of SNHG1 (Fig. 2E).
The migratory capacity of MNK-45 cells and HGC-27 cells was detected by cell scratch test. It was found that the migratory capacity of MNK-45 cells and HGC-27 cells was diminished after transfected with sh-SNHG1 (Fig. 2F).
Transwell assay unraveled that the number of MNK-45 and HGC-27 cells invading and migrating to the lower Transwell chamber was decreased after transfected with sh-SNHG1 (Fig. 2G).
To further unveil the effects of SNHG1 expression on the occurrence of GC in vivo, sh-NC- and sh-SNHG1-transfected cells were injected into nude mice to establish the xenograft tumor model. The weight and volume of tumor transplanted into nude mice were measured, and the findings suggested that depleted SNHG1 reduced the weight and volume of subcutaneous tumor (Fig. 2H).
The results above showed that the silencing of SNHG1 blocked the biological activities of GC cells in vivo.
SNHG1 binds to miR-195-5p
As previously described, miR-195-5p is decreased in tissue and serum of GC patients (Zhao and Wu 2019). The miR-195-5p levels in GES-1 and GC cell lines (MNK-45 and HGC-27) were analyzed by RT-qPCR, which implied that miR-195-5p expressed lowly in GC cells (Fig. 3A). Moreover, when SNHG1 was silenced, miR-195-5p level in MNK-45 and HGC-27 cells was up-regulated (Fig. 3B).
SNHG1 binds to miR-195-5p. A, B RT-qPCR detected the expression of miR-195-5p in MNK-45 and HGC-27 cells (U6 was used as an endogenous reference). C Starbase predicted the binding site of miR-195-5p to SNHG1. D Dual luciferase reporter gene assay verified the targeting relationship between miR-195-5p and SNHG1; E RIP experiment detected the binding of SNHG1 to miR-195-5p. *P < 0.05 vs. the sh-NC group; #P < 0.05 vs. GES-1 cell; ^P < 0.05 vs. the mimic NC group. The data were represented by mean ± standard deviation. The cell experiment was repeated 3 times with 3 technical repetitions
To investigate the regulatory mechanism of SNHG1 and miR-195-5p, it was predicted that SNHG1 and miR-195-5p had specific binding sites through Starbase website (Fig. 3C). The relation between SNHG1 and miR-195-5p was determined by dual luciferase reporter gene reporter assay. It was validated that miR-195-5p enhancement inhibited the luciferase activity of MNK-45 and HGC-27 cells transfected with SNHG1-wt (Fig. 3D). RIP experiment indicated that there was a higher level of SNHG1 enrichment in Ago2 immunoprecipitation and a higher level of SNHG1 enrichment after miR-195-5p mimic treatment (Fig. 3E). These discoveries suggested that SNHG1 could specifically bound to miR-195-5p and modulated miR-195-5p expression.
Up-regulation of miR-195-5p inhibits GC cell activities in vitro
We successfully transfected MNK-45 and HGC-27 cells with mimic NC and miR-195-5p mimic, respectively (Fig. 4A). To further investigate the impacts of miR-195-5p on GC cell biological activities, we up-regulated miR-195-5p, and there exhibition reduced the capacities of proliferation, migration, and invasion, as well as increased apoptosis in MNK-45 and HGC-27 cells (Fig. 4B–G). These findings above uncovered that up-regulation of miR-195-5p impeded the malignant behaviors of GC cells.
Up-regulation of miR-195-5p inhibits GC cell activities. A RT-qPCR detected the expression of miR-195-5p in MNK-45 and HGC-27 cells after up-regulation of miR-195-5p (U6 was used as an endogenous references). B, C CCK-8 assay detected the growth curve of MNK-45 and HGC-27 cells after up-regulation of miR-195-5p. D Colony formation assay detected the colony formation ability of MNK-45 and HGC-27 cells after up-regulation of miR-195-5p. E Flow cytometry detected the apoptosis of MNK-45 and HGC-27 cells after up-regulation of miR-195-5p: dead cells (Q1, Annexin V negative, PI positive), late apoptotic cells (Q2, Annexin V positive, PI positive), early apoptotic cells (Q3, Annexin V positive, PI negative), and viable cells (Q4, Annexin V negative, PI negative). F Scratch test detected the cell migration ability of MNK-45 and HGC-27 cells after up-regulation of miR-195-5p. G Transwell assay detected cell migration and invasion ability of MNK-45 and HGC-27 cells after up-regulation of miR-195-5p. *P < 0.05 vs. the mimic NC group. The data were represented by mean ± standard deviation. The cell experiment was repeated 3 times with 3 technical repetitions
MiR-195-5p targets YAP1
YAP1 expression in GC cells was examined by RT-qPCR and Western blot assay. It was demonstrated that YAP1 expressed highly in MNK-45 and HGC-27 cells (Fig. 5A, B). As predicted by Starbase, miR-195-5p had binding sites with YAP1 (Fig. 5C). As reflected in the luciferase activity experiment, the relative luciferase activity of MNK-45 and HGC-27 cells was impaired after the co-transfection of YAP1-wt and miR-195-5p mimic (Fig. 5D, E). RIP experiments implied that miR-195-5p and YAP1 were enriched in Ago2 immunoprecipitation (Fig. 5F).
MiR-195-5p targets YAP1. A RT-qPCR detected mRNA expression in YAP1 in GES-1, MNK-45 and HGC-27 cells (GADPH was used as an endogenous reference). B Western blot assay detected the protein expression level of YAP1 in GES-1, MNK-45, and HGC-27 cells. C Starbase predicted the target sites between miR-195-5p and YAP1. D, E Luciferase reporter gene experiment verified the targeting relationship between miR-195-5p and YAP1. F RIP experiment detected that the binding of miR-195-5p and YAP1. G RT-qPCR detected the mRNA expression of YAP1 after transfection of miR-195-5p mimic in MNK-45 and HGC-27 cells (GADPH was used as an endogenous reference). H Western blot assay detected the expression of YAP1 protein after transfection with miR-195-5p mimic in MNK-45 and HGC-27 cells. #P < 0.05 vs. GES-1 cells; *P < 0.05 vs. the mimic NC group. The data were represented by mean ± standard deviation. The cell experiment was repeated 3 times with 3 technical repetitions
Next, we detected the expression level of YAP1 by RT-qPCR and Western blot assay. We found that YAP1 expression was reduced in MNK-45 and HGC-27 after up-regulation of miR-195-5p (Fig. 5G, H), which demonstrated that miR-195-5p targeted YAP1.
SNHG1 sponges miR-195-5p to target YAP1, thus promoting GC cell proliferation and metastasis
Committed to verify the impacts of SNHG1, miR-195-5p, and YAP1 on proliferation and metastasis of GC, we transfected sh-SNHG1 + inhibitor NC, sh-SNHG1 + miR-195-5p inhibitor, sh-SNHG1 + oe-NC or sh-SNHG1 + oe-YAP1 to MNK-45 and HGC-27 cells, respectively. The transfection was confirmed successfully by RT-qPCR and Western blot assay detection (Fig. 6A, B). The treatment with miR-195-5p inhibitor or oe-YAP1 was found to rescue the inhibitory effect of downregulated SNHG1 on the proliferation and metastasis of GC cells (Fig. 6C–G). Shortly, SNHG1 bound to miR-195-5p to target YAP1, thus promoting GC proliferation and metastasis.
SNHG1 binds to miR-195-5p to target YAP1, thus promoting GC cell proliferation and metastasis. A RT-qPCR detected the expression level of YAP1 in MNK-45 and HGC-27 cells in the rescue experiments (GADPH was used as an endogenous reference). B Western blot assay detected YAP1 protein expression level of MNK-45 and HGC-27 cells in the rescue experiments. C CCK-8 assay detected the growth curve of MNK-45 and HGC-27 cells. D Colony formation assay detected the clone formation ability of MNK-45 and HGC-27 cell. E Flow cytometry detected the apoptosis of MNK-45 and HGC-27 cells: dead cells (Q1, Annexin V negative, PI positive), late apoptotic cells (Q2, Annexin V positive, PI positive), early apoptotic cells (Q3, Annexin V positive, PI negative), and viable cells (Q4, Annexin V negative, PI negative). F Scratch test detected the cell migration ability of MNK-45 and HGC-27 cells in the rescue experiments. G Transwell assay detected the migration and invasion ability of MNK-45 and HGC-27 cells in the rescue experiments. *P < 0.05 vs. the sh-SNHG1 + inhibitor NC group; #P < 0.05 vs. the sh-SNHG1 + oe-NC group. The data were represented by mean ± standard deviation. The cell experiment was repeated 3 times with 3 technical repetitions
SNHG1/miR-195-5p/YAP1 axis affects the Hippo signaling pathway
Hippo signaling pathway is expressed abnormally in multiple human cancers and exerts crucial influences on tumorigenesis and development. Evidence has shown that non-receptor tyrosine phosphatase 14 can enhance the proliferation and migration capabilities of GC cells by stimulating YAP phosphorylation in the Hippo signaling pathway (Han et al. 2019). To probe the mechanism of the SNHG1/miR-195-5p/YAP1 axis on the Hippo signaling pathway, we verified the transcriptional activity of YAP1 and the activity of Hippo pathway by TEAD luciferase. The results implied that the co-transfection of SNHG1-wt and miR-195-5p mimic increased the activity of TEAD luciferase in MNK-45 and HGC-27 cells, and the co-transfection of SNHG1-mut and miR-195-5p mimic exerted no change in the activity of TEAD luciferase in MNK-45 and HGC-27 cells (Fig. 7A, B). This indicated that SNHG1 could alter YAP1 expression and Hippo signaling activity through modulating miR-195-5p expression.
SNHG1/miR-195-5p/YAP1 axis affects the Hippo signaling pathway. A The TEAD dual luciferase reporter gene assay detected TEAD luciferase in HCC cells. B The TEAD dual luciferase reporter gene assay verified that the SNHG1/miR-195-5p/YAP1 axis affected Hippo signal pathway in MNK-45 cells. *P < 0.05 vs. the SNHG1-wt + mimic NC group. The data were represented by mean ± standard deviation. The cell experiment was repeated 3 times with 3 technical repetitions
Discussion
GC has a low detection rate because of insufficient signs in early stage (Smyth et al. 2020). Based on our study, SNHG1 and YAP1 were high-expressed, and miR-195-5p was low-expressed in GC. After the down-regulation of SNHG1, the GC cell biological activities were impeded, and the tumor growth was suppressed. Through Starbase, it was predicted that SNHG1 had specific binding sites with miR-195-5p, and it was unveiled that elevated miR-195-5p retarded GC cell growth. Later, the target relationship between miR-195-5p and YAP1 was detected, and overexpression of YAP1 was found to deteriorate the proliferation and metastasis of GC cells. Collectively, SNHG1 facilitated the development of GC cells via the modulation of the miR-195-5p/YAP1 axis and regulating the Hippo signaling pathway (Supplementary Fig. 1).
There are some articles highlighting that SNHG1 acts as an oncogene in diverse human diseases. For instance, it has been verified that SNHG1 level is extremely high in GC (Hu et al. 2017), and the median survival of GC patients with up-regulated SNHG1 is shorter than those with downregulated SNHG1 (Huang et al. 2018). Dong et al. have reported that the elevation of SNHG1 is a predictor for poor outcome of digestive system (Dong et al. 2019). Our research validated that SNHG1 level was amplified in GC cells, and the up-regulation of SNHG1 could accelerate the biological functions of GC cells, suggesting the promotive role of SNHG1 in GC. Similarly, in another study, the up-regulation of SNHG1 is demonstrated in colorectal cancer, and the SNHG1 enrichment contributes to accelerate colorectal cancer cells growth, implying that SNHG1 may be a potent biomarker for diagnosis and treatment of colorectal cancer (Xu et al. 2018). Pei et al. have elucidated that SNHG1 expression is elevated in breast cancer patients, which will repress the Treg cell activities, thereby impeding the immune escape of breast cancer (Pei et al. 2018). In another study about laryngeal cancer (LC), it is found that SNHG1 is highly expressed, and the SNHG1 knockdown suppresses LC cell development (Liu et al. 2019). These findings furnished theory references for this study.
The targeted relationship of SNHG1 to miR-195 has been verified, and the augment of SNHG1 and knockdown of miR-195 enhance the cervical cancer cell activities (Ji et al. 2020). In our study, we also found that SNHG1 could bind to miR-195-5p. As previously reported, miR-195-5p could serve as a tumor suppressor gene in many types of human diseases. It is verified that miR-195 is depleted in lung cancer, and thus the overexpression of miR-195 can effectively suppress the tumor cell survival and proliferation (O'Donnell et al. 2020). It is found miR-195-5p is depleted in circulating tumor cells (CRC), and the enrichment of miR-195-5p can effectively suppress CRC biological function (Lin et al. 2019). Furthermore, it has also found that miR-195 displays a low level in GC cells, and overexpression of miR-195 effectively blocks GC migration and invasion (Wang et al. 2018b; Liang et al. 2019). All these aforementioned evidence indicate that suppressive role of miR-195-5p in human disease. Though the preceding studies for probing the mechanism of miR-195-5p in GC were limited, these existed findings still afforded valuable references. In line with these findings, this study demonstrated that miR-195-5p was silenced in GC cells, while the induction of miR-195-5p effectively repressed the growth of GC cells, implying the inhibitory effect of miR-195-5p in GC.
Furthermore, some miRNAs may act as either oncogenes or tumor suppressors via regulating gene expression (Zhang et al. 2007). Another article has indicated that one of the inferior targets of miR-195-5p is YAP1, whose expression levels are suppressed by miR-195-5p (Wang et al. 2020). A previous study has verified the negative relation between miR-195-5p and YAP1, and the rescue experiments further uncovered that YAP1 is involved in the negative effects of miR-195-5p on cell biological function (Liu, et al. 2020). In line with this finding, our research also validated that YAP1 was a downstream target gene of miR-195-5p. Additionally, YAP1 acting as an oncogene in diverse human diseases has been widely in explored. For example, Zhou et al. have mentioned that YAP1 acts an indicator for malignant prognosis and disease recurrence for pancreatic cancer patients (Zhou et al. 2020). Yu et al. have reported that the YAP1 is up-regulated in GC, and high expression of YAP1 will cause deep invasion of GC cells (Yu et al. 2017). In accordance with these article, our study also demonstrated the promotive role of YAP1 in GC. As a cancer signaling network, Hippo pathway has a correlation with tumorgenesis and cancer-related biological function (Harvey et al. 2013). It has been verified the Hippo signaling activity can be enhanced by competitively sponging with miR-375 (Gao et al. 2018). YAP is function as a effector of Hippo pathway (Qiao, et al. 2018), and the Hippo signaling pathway is promoted for the predictor of miR-195-5p (Sun et al. 2017), which is conformed with our study.
To sum up, our study deciphers that SNHG1 and YAP1 express highly while miR-195-5p expresses lowly in GC cells. The depletion of SNHG1 could impede the GC cell biological functions via regulating the miR-195-5p/YAP1 axis. Our study may afford therapeutic targets for the clinical treatment of GC by highlighting the importance of the SNHG1/miR-195-5p/YAP1 axis. However, further investigation should be conducted to enrich the data in the experimental results.
References
Cai J et al (2020) miR-124–3p regulates FGF2-EGFR pathway to overcome pemetrexed resistance in lung adenocarcinoma cells by targeting MGAT5. Cancer Manag Res 12 11597–11609
Correa P (2013) Gastric cancer: overview. Gastroenterol Clin North Am 42(2):211–217
Dong B et al (2019) The prognostic value of lncRNA SNHG1 in cancer patients: a meta-analysis. BMC Cancer 19(1):780
Gao L, Cao H, Cheng X (2018) A positive feedback regulation between long noncoding RNA SNHG1 and YAP1 modulates growth and metastasis in laryngeal squamous cell carcinoma. Am J Cancer Res 8(9):1712–1724
Guo W et al (2019) LncRNA SNHG1 promoted HGC-27 cell growth and migration via the miR-140/ADAM10 axis. Int J Biol Macromol 122:817–823
Han X et al (2019) Nonreceptor tyrosine phosphatase 14 promotes proliferation and migration through regulating phosphorylation of YAP of Hippo signaling pathway in gastric cancer cells. J Cell Biochem 120(10):17723–17730
Harvey KF, Zhang X, Thomas DM (2013) The Hippo pathway and human cancer. Nat Rev Cancer 13(4):246–257
Hu Y et al (2017) LncRNA-SNHG1 contributes to gastric cancer cell proliferation by regulating DNMT1. Biochem Biophys Res Commun 491(4):926–931
Huang L et al (2018) Small nucleolar RNA host gene 1: a new biomarker and therapeutic target for cancers. Pathol Res Pract 214(9):1247–1252
Ji YY, Meng m and Miao Y (2020) lncRNA SNHG1 promotes progression of cervical cancer through miR-195/NEK2 Axis. Cancer Manag Res 12:11423–11433
Li JH et al (2014) starBase v2.0: decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA interaction networks from large-scale CLIP-Seq data. Nucleic Acids Res 42(Database issue):D92–7
Liang M et al (2019) Elevated levels of hsa_circ_006100 in gastric cancer promote cell growth and metastasis via miR-195/GPRC5A signalling. Cell Prolif 52(5):e12661
Lin X et al (2019) miR-195-5p/NOTCH2-mediated EMT modulates IL-4 secretion in colorectal cancer to affect M2-like TAM polarization. J Hematol Oncol 12(1):20
Liu T et al (2019) LncRNA SNHG1 promotes cell proliferation in laryngeal cancer via Notch1 signaling pathway. Eur Rev Med Pharmacol Sci 23(15):6562–6569
Liu X et al (2020) MiR-195–5p inhibits malignant progression of cervical cancer by targeting YAP1. Onco Targets Ther 13:931–944
O’Donnell PH et al (2020) Patient-reported outcomes and inflammatory biomarkers in patients with locally advanced/metastatic urothelial carcinoma treated with durvalumab in phase 1/2 dose-escalation study 1108. Cancer 126(2):432–443
Pei X, Wang X, Li H (2018) LncRNA SNHG1 regulates the differentiation of Treg cells and affects the immune escape of breast cancer via regulating miR-448/IDO. Int J Biol Macromol 118(Pt A):24–30
Qiao Y et al (2017) YAP regulates actin dynamics through ARHGAP29 and promotes metastasis. Cell Rep 19(8):1495–1502
Qiao Y et al (2018) The Hippo pathway as a drug target in gastric cancer. Cancer Lett 420 14–25
Smyth EC et al (2020) Gastric cancer. Lancet 396(10251):635–648
Song Z et al (2017) Progress in the treatment of advanced gastric cancer. Tumour Biol 39(7):1010428317714626
Sun M et al (2017) Integrated analysis identifies microRNA-195 as a suppressor of Hippo-YAP pathway in colorectal cancer. J Hematol Oncol 10(1):79
Sun D et al (2020) The novel long non-coding RNA LATS2-AS1–001 inhibits gastric cancer progression by regulating the LATS2/YAP1 signaling pathway via binding to EZH2. Cancer Cell Int 20:204
Tan Z (2019) Recent advances in the surgical treatment of advanced gastric cancer: a review. Med Sci Monit 25:3537–3541
Temiz E et al (2021) Inhibition of carbonic anhydrase IX promotes apoptosis through intracellular pH level alterations in cervical cancer cells. Int J Mol Sci 22(11)
Thin KZ, Tu JC, Raveendran S (2019) Long non-coding SNHG1 in cancer. Clin Chim Acta 494:38–47
Wang J et al (2018a) LncRNA HOXA-AS2 and its molecular mechanisms in human cancer. Clin Chim Acta 485:229–233
Wang F et al (2018b) TRIM14 promotes the migration and invasion of gastric cancer by regulating epithelial to mesenchymal transition via activation of AKT signaling regulated by miR1955p. Oncol Rep 40(6):3273–3284
Wang X et al (2020) Long noncoding RNA LINC00473/miR1955p promotes glioma progression via YAP1TEAD1Hippo signaling. Int J Oncol 56(2):508–521
Xu M et al (2018) The long noncoding RNA SNHG1 regulates colorectal cancer cell growth through interactions with EZH2 and miR-154-5p. Mol Cancer 17(1):141
Yan H et al (2018) Yap regulates gastric cancer survival and migration via SIRT1/Mfn2/mitophagy. Oncol Rep 39(4):1671–1681
Yang M et al (2020) Bone marrow mesenchymal stem cell-derived exosomal miR-144-5p improves rat ovarian function after chemotherapy-induced ovarian failure by targeting PTEN. Lab Invest 100(3):342–352
Yu L et al (2017) Distinct prognostic values of YAP1 in gastric cancer. Tumour Biol 39(4):1010428317695926
Yu S et al (2019) CLDN6 promotes tumor progression through the YAP1-snail1 axis in gastric cancer. Cell Death Dis 10(12):949
Zhang B et al (2007) microRNAs as oncogenes and tumor suppressors. Dev Biol 302(1):1–12
Zhang Y et al (2016) Comprehensive characterization of lncRNA-mRNA related ceRNA network across 12 major cancers. Oncotarget 7(39):64148–64167
Zhang XY, Zhang PY (2017) Gastric cancer: somatic genetics as a guide to therapy. J Med Genet 54(5):305–312
Zhao DL, Wu QL (2019) Effect of inhibition to Yes-related proteins-mediated Wnt/beta-catenin signaling pathway through miR-195-5p on apoptosis of gastric cancer cells. Eur Rev Med Pharmacol Sci 23(15):6486–6496
Zhou Q et al (2020) YAP1 is an independent prognostic marker in pancreatic cancer and associated with extracellular matrix remodeling. J Transl Med 18(1):77
Zou J et al (2019) Dysregulation of miR-195-5p/-218-5p/BIRC5 axis predicts a poor prognosis in patients with gastric cancer. J Biol Regul Homeost Agents 33(5):1377–1385
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Fahui Cheng and Li Wang are co-first author.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cheng, F., Wang, L., Yi, S. et al. Long non-coding RNA SNHG1/microRNA-195-5p/Yes-associated protein axis affects the proliferation and metastasis of gastric cancer via the Hippo signaling pathway. Funct Integr Genomics 22, 1043–1055 (2022). https://doi.org/10.1007/s10142-022-00876-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10142-022-00876-2