Abstract
Purpose
Evaluate the potential effects of X-ray tube voltage (kV) changes on Hounsfield unit (HU) measurements of hemoperitoneum in patients with blunt splenic injuries.
Methods
Eight different tissue equivalent electron density plugs in the Electron Density Phantom were scanned (muscle, adipose, breast, liver, lung (exhale), lung (inhale), trabecular bone, and dense bone). The phantom was scanned at different kV values (70, 80, 100, 120, and 140 kV). In the clinical study, the local trauma registry database was queried for splenic injuries between January 2015 and December 2016 with a final cohort of 110 patients. The average HU numbers of hemoperitoneum found in three different anatomic locations (pelvic, perisplenic, and perihepatic) were compared at different kV values (100 kV, 120 kV, and 140 kV). ANOVA and pairwise t tests were performed for statistical analysis.
Results
In both studies, HU measurements generally decreased as kV increased, and vice versa. One hundred ten patients were reviewed: 29 for 100 kV, 66 for 120 kV, and 15 for 140 kV. For the perihepatic group, significant differences were observed in average HU in the following pairwise comparisons: 100/140 (13.7 (5.3), p < 0.05) and 120/140 (10.3 (4.5), p < 0.05). For the perisplenic group, significant differences were observed in 100/120 (7.0 (3.5), p < 0.05) and 100/140 (13.2 (4.9), p < 0.05). No significant difference was observed in the pelvic location (p = 0.5594).
Conclusions
HU measurements of hemoperitoneum in patients with blunt splenic injuries significantly varied with the use of different kV values. Radiologists should be aware of the possible effects of altering kV on HU.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Multiple novel computed tomography (CT) imaging techniques have been developed and employed to reduce radiation dose in response to the concern of potential association of radiation with certain malignancies [1]. These include tube current modulation [2], iterative reconstruction algorithms [3], and most recently automatic X-ray tube voltage (kV) selection [4].
Besides well-established tube current modulation, automatic kV adjustment based on body habitus, tissue type of interest, and scan type allows for further dose reductions while maintaining a good contrast-to-noise ratio. Hu et. al. reported that the utilization of the automated kV selection reduces radiation dose by 14–17% when compared with the standard 120 kV protocol in abdominal CT scans [5].
Of interest in the present study is the potential effect of kV on Hounsfield unit (HU) measurements in hemoperitoneum. HU is a CT quantity that corresponds to X-ray attenuation. HU is normalized to water being a value of 0. Measurement of HU has many clinical applications, including presence or absence of lipid in an adrenal nodule [6], characterization of fat-containing lesions [7], identification of fluid (simple or complex) [8], and identification of air [9]. HU cutoff values often aid radiologists in making clinical diagnoses. Although hemoperitoneum is not typically a diagnostic dilemma, in patients with trauma, occasionally HU measurements are used to troubleshoot whether intraperitoneal fluid is hemorrhagic or not. In patients with indeterminate intraperitoneal fluid, a HU cutoff value of > 30 is often utilized, and typically determined to be hemorrhagic [10].
Higher kV increases X-ray photon penetration through a patient’s body, translating to decreased image contrast. For clinical scenarios where HU cutoff values aid radiologists in decision-making, there is potential for missed diagnoses if kV alters HU measurements to a significant degree. The purpose of the study is to evaluate the effect of changing tube voltages (kV) on average HU measurements in patients with a known source of hemoperitoneum.
Methods
This was an IRB-approved, HIPAA-compliant single-institution retrospective study.
Phantom study
A phantom study was performed using the Electron Density Phantom (Computerized Imaging Reference Systems, Inc.). The phantom was scanned using a SOMATOM Force CT scanner (Siemens Healthineers). Eight different tissue equivalent electron density plugs in the Electron Density Phantom were scanned (muscle, adipose, breast, liver, lung (exhale), lung (inhale), trabecular bone, and dense bone). The Electron Density Phantom was also scanned at different kV values (70, 80, 100, 120, and 140 kV). The Electron Density Phantom was scanned using a routine abdominal protocol with collimation of 192 × 0.6 mm, rotation time of 0.5 s, pitch of 0.6, 90 mAs, and different kV values (70, 80, 100, 120, and 140 kV). All images were reconstructed using convolution kernel I41f and strength of 3 with a slice thickness of 5 mm. The CT images of the phantom were analyzed by an ABR-certified diagnostic medical physicist by drawing a circle on each of the eight different tissue equivalent electron density plugs.
Clinical study
The trauma registry database was queried for patients diagnosed with splenic injury between January 2015 and December 2016 (n = 412). This was done because the spleen is the most frequently injured solid parenchymal organ within the abdomen [11], and a definitive source of hemoperitoneum is necessary for the present study. A total of 200 patients between the dates of 1/16 and 12/16 were reviewed. Patients with preoperative CT abdomen and pelvis with intravenous contrast imaging and detectable hemoperitoneum were included in the study. Patients without detectable hemoperitoneum were excluded (n = 59). Patients imaged after splenectomy or laparotomy were excluded (n = 18). Duplicate patients and patients without a CT were excluded (n = 10). Three additional patients who were scanned at 80 kV were excluded for statistical purposes. The final cohort was 110 patients.
Multiple CT scanners were included in this study; however, most were obtained on a Siemens Edge Scanner using a standard trauma monoenergetic, weight-based kV protocol.
A second-year medical student (DH) reviewed all studies under the direct supervision of JL (Abdominal and Emergency Radiology Faculty, 7 years’ experience). Free fluid was measured in three different anatomic locations: the pelvic region, the perihepatic region, and the perisplenic region. The following criteria were used for anatomic locations for HU measurement: a measurable region of interest (ROI) of at least 50 mm2, up to 1 ROI per patient in the pelvic region, up to 3 ROIs (averaged) in the perihepatic region, and up to 2 ROIs in the perisplenic region. Measurements were made as large as possible without including surrounding tissues. Slice thickness of 1.5 mm was utilized to minimize the effects of volume averaging. For each anatomic location, effort was made to record measurements in different slices and different areas to obtain a more representative sample for a ROI.
The average HU numbers of hemoperitoneum found in the three different anatomic locations were measured at different kV values (80 kV, 100 kV, 120 kV, and 140 kV). The 80 kV value was excluded from comparison analysis due to insufficient number of just 3 patients imaged at this kV value to provide enough statistical power.
All analyses were completed in SAS 9.4 (SAS Institute Inc., Cary, NC, USA). First, ANOVA testing was performed at each anatomical region to ascertain differences in HU based on kV. Subsequent pairwise t testing was performed for anatomical regions that were found to have a statistically significant difference in kV subgroup means based on the ANOVA test.
Results
Phantom study
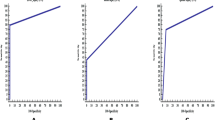
The CT images of the phantom were analyzed by an ABR-certified diagnostic medical physicist by drawing a circle on each of the eight different tissue equivalent electron density plugs. Figure 1 provides an example CT image of the phantom with sample measurement. Figure 2a represents image enhancement (HU) of the phantom for tissue densities of muscle, adipose, breast, and liver, and Figure 2b represents image enhancement of the phantom for tissue densities of lung (exhale), lung (inhale), trabecular bone, and dense bone.
The results from the phantom study indicate a trend that as kV increases, HU decreases, and vice versa. This is particularly true for the equivalent density plugs of dense bone and trabecular bone tissues.
Clinical study
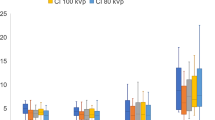
One hundred ten patients were retrospectively reviewed: 29 for 100 kV, 66 for 120 kV, and 15 for 140 kV. Figure 3a is an example of an HU measurement of large volume hemoperitoneum at 100 kV and Fig. 3b is an example of an HU measurement of small-volume hemoperitoneum at 140 kV. ANOVA testing was performed at each anatomical region to ascertain differences in HU based on kV within each group, i.e., pelvis, perihepatic, and perisplenic (Table 1).
Figure 4a–c below are graphical representations of the mean HU and standard error for each kV subgroup, using the data from Table 1.
Subsequent pairwise t test comparisons within the perihepatic and perisplenic groups were performed (Table 2). No pairwise comparison for the pelvic group was performed as this anatomical region was not found to have a statistically significant difference in kV subgroup means based on the ANOVA test.
As expected, HU measurement decreased as kV increased, and vice versa. For the perihepatic group, significant differences were observed in average HU in the following pairwise comparisons: 100/140 kV (average difference 13.7 (5.3), p < 0.05) and 120/140 kV (10.3 (4.5), p < 0.05). For the perisplenic group, significant differences were observed in the 100/120 kV (7.0 (3.5), p < 0.05) and 100/140 kV (13.2 (4.9), p < 0.05) subgroups. There was no significant difference observed in the pelvic location.
Discussion
This study demonstrates that HU measurements of hemoperitoneum decreased as kV increased, and vice versa. This finding is in accordance with the general trend observed from the results of the phantom study as well. HU measurements of hemoperitoneum in patients with blunt splenic injuries varied significantly with different tube voltages for the perihepatic and perisplenic groups. The two greatest mean differences among subgroups were the 100/140 kV perihepatic and 100/140 kV perisplenic pairings which may be expected due to a 40 kV difference between subgroups. The lowest mean HU for each anatomical region was found in the 140 kV subgroup. In most cases, hemoperitoneum with HU numbers falling below a 30 HU average was not observed. Instances where a kV subgroup mean was below 30 HU were the 140 kV perihepatic group (average HU 20.8), 140 kV perisplenic group (29.3), and 140 kV pelvic group (29.7). These are all mean values so there were several instances in the present study where strict use of a 30 HU cutoff for indeterminate free fluid in patients would result in underdiagnosis of hemoperitoneum.
Not all kV subgroups demonstrated statistically significant differences in kV subgroup means, however. For the perihepatic group, there was no significant difference observed between the 100 and 120 kV subgroups. For the perisplenic group, there was no significant difference observed between the 120 and 140 kV subgroups. This is likely because of an insufficient number of patients imaged at these kV values to provide enough statistical power. A difference of only 20 kV (100/120 and 120/140) between subgroups is likely to demonstrate less of a difference in subgroup means compared with a difference of 40 kV between subgroups (100/140).
There was no significant difference observed in the pelvis, despite the significant differences observed in the other two anatomical regions. This is likely due to beam hardening present because of the bony pelvis, leading to preferential attenuation of lower-energy X-ray photons. This results in increased mean energy of the X-ray beam after passing through high-density tissue within the pelvis.
Intravenous contrast was used in all patients in this study. IV contrast is recommended for detection of hemoperitoneum in CT because rapid infusion of contrast material can maximize the difference in attenuation between hematoma and adjacent normal parenchyma [12]. Blood attenuation will increase with IV contrast, but the use of IV contrast material in CT may result in a relative decrease in attenuation of blood especially if blood is adjacent to well-enhanced organs [12]. This is a difference in the clinical study from the phantom study, which assumed no contrast in its tissue equivalent electron density plugs.
Limitations of this study include that the splenic injury patient population may include concomitant injuries, including bowel injuries which could alter the HU of the free fluid measured in the abdomen. This study did not utilize a pure splenic injury cohort, which we believe more accurately represents the trauma population in general. AAST grades of splenic injuries were not provided.
Additional limitations of the present study include a single-center retrospective study, analysis of other imaging variables such as patient size were not recorded, timing of imaging after contrast administration was not recorded, and patients who had other medical reasons for free fluid were not excluded from the study. Also, limitations related to exclusion criteria such as small number of the 80 kV subgroup restricted statistical analysis. Due to a limited population size in the clinical study, fewer kV values were analyzed in the clinical study (100, 120, and 140 kV) compared with the phantom study (70, 80, 100, 120, and 140 kV).
Conclusions
Our results demonstrate that altering kV varies tissue attenuation in a clinical environment. Specifically, HU measurements of hemoperitoneum in patients with blunt splenic injuries significantly varied with the use of different kV values. In some circumstances, this could alter patient care. Radiologists should be aware of the possible effects of altering kV on HU. Variation in HU secondary to change in kV may have other clinical implications especially with thresholding techniques utilized with computer-aided detection. Further studies are needed to determine if dose modulation techniques may influence other tissues.
References
Storrs C (2013) Do CT scans cause cancer? Sci Am 309(1):30–32
Rizzo S, Kalra M, Schmidt B, Dalal T, Suess C, Flohr T, Blake M, Saini S (2006) Comparison of angular and combined automatic tube current modulation techniques with constant tube current CT of the abdomen and pelvis. AJR Am J Roentgenol 186(3):673–679. https://doi.org/10.2214/AJR.04.1513
Hara AK, Paden RG, Silva AC, Kujak JL, Lawder HJ, Pavlicek W (2009) Iterative reconstruction technique for reducing body radiation dose at CT: feasibility study. AJR Am J Roentgenol 193(3):764–771. https://doi.org/10.2214/AJR.09.2397
Yu L, Fletcher JG, Grant KL, Carter RE, Hough DM, Barlow JM, Vrtiska TJ, Williamson EE, Young PM, Goss BC, Shiung M, Leng S, Raupach R, Schmidt B, Flohr T, McCollough CH (2013) Automatic selection of tube potential for radiation dose reduction in vascular and contrast-enhanced abdominopelvic CT. AJR Am J Roentgenol 201(2):W297–W306. https://doi.org/10.2214/AJR.12.9610
Hu L, Wang Y, Hou H, Wei F, Yang G, Chen Y (2014) Radiation dose and image quality with abdominal computed tomography with automated dose-optimized tube voltage selection. J Int Med Res 42(4):1011–1017. https://doi.org/10.1177/0300060513496173
Dunnick NR, Korobkin M, Francis I (1996) Adrenal radiology: distinguishing benign from malignant adrenal masses. AJR Am J Roentgenol 167(4):861–867. https://doi.org/10.2214/ajr.167.4.8819372
Gaerte SC, Meyer CA, Winer-Muram HT, Tarver RD, Conces DJ, Jr (2002) Fat-containing lesions of the chest. Radiographics 22 Spec No:S61–78. https://doi.org/10.1148/radiographics.22.suppl_1.g02oc08s61
Wood CG 3rd, Stromberg LJ 3rd, Harmath CB, Horowitz JM, Feng C, Hammond NA, Casalino DD, Goodhartz LA, Miller FH, Nikolaidis P (2015) CT and MR imaging for evaluation of cystic renal lesions and diseases. Radiographics 35(1):125–141. https://doi.org/10.1148/rg.351130016
Cressoni M, Gallazzi E, Chiurazzi C, Marino A, Brioni M, Menga F, Cigada I, Amini M, Lemos A, Lazzerini M, Carlesso E, Cadringher P, Chiumello D, Gattinoni L (2013) Limits of normality of quantitative thoracic CT analysis. Crit Care 17(3):R93. https://doi.org/10.1186/cc12738
Federle MP, Jeffrey RB Jr (1983) Hemoperitoneum studied by computed tomography. Radiology 148(1):187–192. https://doi.org/10.1148/radiology.148.1.6856833
Moore EE, Moore JB, Van Duzer-Moore S, Thompson JS (1980) Mandatory laparotomy for gunshot wounds penetrating the abdomen. Am J Surg 140(6):847–851
Roberts JL, Dalen K, Bosanko CM, Jafir SZ (1993) CT in abdominal and pelvic trauma. Radiographics 13(4):735–752. https://doi.org/10.1148/radiographics.13.4.8356265
Funding
The project described was partially supported by the National Center for Advancing Translational Sciences, UL1TR000117, and the Dean of the College of Medicine, University of Kentucky.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the University of Kentucky.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hickman, D., Zhang, J., McQuerry, K. et al. Should radiologists care about kV? Phantom and clinical study of effects of kV on hemoperitoneum HU in the setting of splenic injuries. Emerg Radiol 27, 135–140 (2020). https://doi.org/10.1007/s10140-019-01738-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10140-019-01738-4