Abstract
Purpose
Patients with acute pulmonary embolism (PE) can quickly deteriorate and the condition has high mortality due to right ventricular (RV) failure. Immediately available predictors of adverse outcome are of major interest to the treating physician in the acute setting. The purpose of the present study was to evaluate if easily attainable measurements of RV function from the diagnostic computed tomography pulmonary angiography (CTPA) provide information for fast risk stratification in patients with acute PE.
Methods
We retrospectively evaluated images from CTPA in 261 patients (age median 60 years, 50% females) enrolled in a prospective study. RV and left ventricular (LV) diameters and their ratio, the presence of septal bowing, contrast reflux in the inferior vena cava, and the diameter of the central pulmonary arteries (PA) were measured. The composite outcome was 5-day severe adverse events including death, acute decompensation, or need for emergent treatment. We used Wilcoxon rank sum test and Fischer’s exact test to test between groups and multivariate logistic regression for prediction.
Results
In multivariate analysis, increased diameter of the main PA (OR = 1.08 per 1 mm increase, p = 0.047) and the presence of septal bowing (OR = 2.23, p = 0.055) were associated with severe adverse events. RV/LV > 1 did not predict severe outcomes (OR = 0.73, p = 0.541).
Conclusions
Two easily attainable parameters of RV function on CTPA, septal bowing and main PA diameter, are associated with short-term adverse outcomes in patients with acute PE. Further study is required to determine whether these findings can be incorporated into clinical treatment algorithms.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Acute pulmonary embolism (PE) is a common but dangerous condition. It is the third most common cause of cardiovascular death [1]. Death from PE is due to right ventricular (RV) failure caused by increased RV afterload [2], and the majority of PE-related deaths occur within hours of the embolus [1, 3]. Therefore, it is critical to diagnose and risk-stratify patients with PE as quickly as possible. Because CT pulmonary angiography (CTPA) is the primary modality for the diagnosis of PE [4, 5], it would be time-saving and of high value to the treating physician if CTPA findings could also identify patients at risk for death or clinical decompensation [4].
CTPA obstruction indices quantify the clot burden, but clot burden is not consistently associated with outcomes [1, 5,6,7,8,9]. Moreover, some clot burden scores are time-consuming and can be difficult to apply to acute medical care [7, 10]. Single-image analysis of the RV to left ventricular (LV) ratio may be quicker and more clinically relevant than quantifying clot burden. However, studies of CTPA measurement of the RV/LV ratio have yielded conflicting results, and some studies show poor specificity compared to echocardiography [7, 9, 11,12,13,14,15]. Other measures of RV function (e.g., the reflux of contrast material into the inferior vena cave (IVC), the size of the pulmonary arteries, or the morphology of the right atrium) have not been as widely studied as the RV/LV ratio.
Therefore, the objective of the present study was to evaluate several CTPA measures of RV function and the pulmonary vasculature and assess their correlation to short-term outcomes in emergency department (ED) patients diagnosed with acute PE.
Methods
Study design
This was a secondary analysis of data from a prospective observational cohort study performed between 2008 and 2011 in the ED of Massachusetts General Hospital, a level 1 trauma, quaternary care hospital with 105,000 annual visits in the ED. The study was approved by the Human Research Committee of Partners HealthCare Inc. (2008-P-002001) and all patients provided written consent to participation. The original study was funded by a grant from the Harvard Milton Foundation.
Details from the study have been published previously [10, 16]. In short, adult (age ≥ 18) patients diagnosed with PE based on a CTPA performed within 24 h after registration in the ED were enrolled. Enrolment and reporting of the study was performed in accordance with the Strengthen the Reporting of Observational Studies in Epidemiology guidelines [17]. Exclusion criteria for this analysis were patients with CTPA images not available for review, with inadequate follow-up data, with signs of chronic PE, or non-thromboembolic cause of PE (e.g., tumor emboli). After initial enrollment, trained study staff visited the patients daily for the first 5 days of hospitalization and recorded follow-up data including all adverse events.
CTPA protocol
All CTPA were performed on either a 16- or 64-section multidetector-row CT scanner (GE Lightspeed 16, GE Lightspeed VCT, GE Healthcare, Milwaukee, Wisconsin). Patients were placed in supine position and ask to rest arms above their shoulders for CT scanning. Images were obtained with shallow breath-hold to avoid inadvertent Valsalva maneuver. Scanning was triggered using standard algorithm with bolus triggering timed at the level of the main pulmonary artery where region of interest was placed at a threshold of 100 HU. Patients received non-ionic intravenous contrast media (Isovue 370 mg%, iopamidol, Bracco Diagnostics, Princeton, New Jersey) at 3–4 ml/s for a volume of 65–90 ml based on their weight followed by a 40-ml bolus of isotonic saline. All patients were scanned in caudocranial direction with helical scan mode using 100–120 kV, 1.375:1 pitch, 0.5 s gantry rotation time, specific detector configurations (16-section CT, 16 × 1.25 mm; 64-section CT, 64 × 0.625 mm), and longitudinal automatic tube current modulation (Auto mA, GE Healthcare) at noise index of 10–15 based on the patient weight. Images were reconstructed with standard reconstruction algorithm at 2.5 and 1.25 mm section thickness. Coronal and sagittal multiplanar reformatted (MPR) images were generated at 1.25 mm section thickness.
Image review
CTPA images were interpreted using 1.25 mm images and coronal and sagittal MPR reformations on the image processing workstation. Two independent radiologists (PM and FH with 5 years of experience) reviewed images for predefined measures of RV function. The readers were blinded to the clinical features, biomarkers, echocardiogram results, and outcome of patients to minimize risks of bias.
Acute PE was defined by as presence of an endoluminal, low-attenuation filling defect with partial or total obstruction of a pulmonary artery. Cardiac trans-sectional measurements were performed from standard axial source imaging of the heart generated on the image processing workstation. RV and LV dimensions were recorded as the distance between the endocardium of the septum and the free wall of each ventricle just below the atrial-ventricular leaflets at the level of widest luminal dimension [18]. The RV/LV ratio was calculated and a predefined cutoff of RV/LV > 1.0 was considered RV dilatation. Interventricular septal bowing was evaluated subjectively on transverse CT scans and reported as dichotomous outcome (present vs. absent) if any image showed septal bowing [18]. The presence of contrast medium in the IVC was noted as dichotomous outcome (present vs. absent). Vascular measurements were obtained on multiplanar reformatted images with a plane perpendicular to the long axis of the vessel [18]. We recorded the diameter of the main pulmonary artery (PA) and the right and left PA.
Outcome
Our primary endpoint was clinical deterioration within 5 days consistent. We focused on 5-day outcomes to identify events most likely to be associated with the acute PE (rather than events associated with underlying illness) and to capture events occurring in a timeframe consistent with the median length of hospitalization for PE in our hospital (3 days) [16, 19]. The endpoint was a composite of all-cause mortality, need for rescue interventions (systemic or catheter-directed thrombolysis, percutaneous or surgical embolectomy), unstable dysrhythmias (new ventricular tachycardia or fibrillation), need for hemodynamic support (inotropes or vasopressors), need for respiratory support (positive pressure ventilation or mechanical ventilation), and need for advanced cardiac life support. To identify adverse outcomes, trained study staff reviewed medical records, visited with hospitalized patients, and interviewed their providers daily during the first 5 days of hospitalization. Adverse outcomes in patients who were discharged from the hospital before 5 days were ascertained by a combination of telephone calls and review of medical records [16].
Statistical analysis
Data was analyzed using SAS software version 9.3 (SAS Institute, Cary, NC, USA). As two radiologists reviewed all images, we used the average of their measurements for all continuous data. Tests for normality were conducted (Kolmogorov–Smirnov test) and as the majority of data were not normally distributed, all data are presented as median and interquartile [IQ] range for interpretation and comparison. Differences between outcome groups in CT findings and other continuous variables of interest were compared using the Wilcoxon rank sum test. Fischer’s exact test was used for dichotomous variables. Multivariable logistic regression was conducted for variables showing strong relationship with outcome and those of clinical importance, i.e., adjusting for main PA diameter, presence of septal bowing, and RV/LV ratio. Because RV dysfunction is in the causal chain between clot burden and adverse outcomes, we did not adjust for clot burden in our primary multivariable model. Interobserver agreement was analyzed by correlation with Pearson coefficients for continuous variables and Kappa coefficient for binary. A p value < 0.05 was considered statistically significant [20].
Results
We retrospectively analyzed 302 patients. Forty-one patients were excluded (no CTPA performed: n = 9, CTPA performed but images not available in medical record for review: n = 15, false positives: n = 8, chronic PE n = 3, tumor n = 3, lost to follow-up n = 3). A total of 261 patients were included in the analyses.
Baseline characteristics are shown in Table 1 for the total population and stratified by outcome. Demographics and comorbid illnesses were comparable across the two groups, but consistent with our definition of adverse outcomes, patients with adverse outcomes had more severe clinical presentations (e.g., lower systolic blood pressure).
Interobserver variation analysis of CTPA measurements showed high correlation with Pearson correlation coefficients of 0.86 for the main PA, 0.86 for the right PA, 0.90 for the left PA, and 0.81 for the RV/LV ratio (all p < 0.0001). Kappa value was 0.45 for the presence of septal bowing and 0.83 for the presence of contrast reflux.
Cardiac and vascular measurements are presented in Table 2. The central pulmonary arteries were generally larger in patients with clinical deterioration. The diameters of the ventricles were comparable, whereas the presence of septal bowing was more common in patients with worse outcomes.
In univariate analysis, main PA diameter was associated with outcomes (OR 1.08, 95%CI 1.01–1.16, p = 0.027) but not the right PA diameter (OR 1.07, 95%CI 0.099–1.15, p = 0.090) or the left PA diameter (OR 1.07, 95%CI 0.098–1.17, p = 0.116). RV/LV ratio yielded an OR of 1.32 (95%CI 0.58–3.04, p = 0.508). The presence of septal bowing (single rater) was associated with adverse outcomes (OR 2.21, 95%CI 1.06–4.53, p = 0.031), whereas septal bowing (both raters) or the presence of contrast reflux in IVC were not associated (data not shown). Table 3 shows the multivariate regression analyses including main PA diameter, RV/LV ratio and septal bowing with odds ratios (OR) between cardiac and vascular measures and outcome. The presence of septal bowing showed the highest OR (2.23, 95%CI 0.98–5.04) and nearly achieved statistical significance (p = 0.055). The statistically strongest correlation was seen for main PA diameter with an OR = 1.08 (95%CI 1.00–1.16, p = 0.047).
Discussion
Patients with PE can deteriorate quickly, so there is a clinical need for rapid risk stratification after diagnosis. Because CPTA images are readily available for most patients diagnosed with PE, identifying features on CTPA associated with clinical deterioration or death could aid in risk stratification. We previously developed a rapid, simple method for quantifying clot burden and correlated this with clinical outcomes [10]. However, RV function is generally thought to be a more consistent predictor of adverse outcomes in PE than clot burden [1, 5, 6]. In the current study, we sought to investigate cardiovascular CTPA measurements reflective of RV function.
We found that the diameter of the main pulmonary artery and the presence of intraventricular septal bowing were associated with short-term clinical deterioration after PE (Table 3, Figs. 1 and 2). These are fast and simple measurements of cardiac and vascular dimensions available from any CTPA and our results are generally consistent with other studies [7, 9, 19, 21,22,23,24,25]. However, other studies evaluating CTPA measures of RV function have used different follow-up periods or strategies of measurement. For example, Moroni et al. followed patients for 3 months [22], and Apfaltrer et al. [9] measured RV and LV diameters at their broadest and not at the level of the leaflets or at least at the same level in the respective ventricles. Our follow-up period reflects short-term clinical deterioration that (A) is more likely to be directly related to the PE than longer term outcomes and (B) occurs during a typical hospitalization for PE and during a time period where acute intervention (e.g., thrombolysis) might be considered.
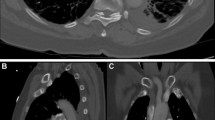
Representative images of pulmonary embolism with and without main PA dilatation. a CTPA from a patient, that despite PE has a normal, narrow main PA of 22.3 mm. b How PE has caused dilatation of the main PA to 30.2 mm. PA, pulmonary artery; CTPA, computed tomography pulmonary angiography; PE, pulmonary embolism; Ao, aorta
Signs of RV dysfunction on CT. Panels a and b stem from the same two patients as in Fig. 1. a A normal interventricular septum bowing towards the RV. b An enlarged RV with high intraventricular pressure causing the leftward shift and bowing of the septum. This may compromise LV preload, LV function, and the systemic circulation, too. See text for further details. CT, computed tomography; RV, right ventricle; LV, left ventricle
Acute PE causes mechanical obstruction and release of pulmonary vasoconstrictors [3]. The combination of obstruction and vasoconstriction increases pulmonary vascular resistance and causes blood to pool in the (normally compliant) pulmonary arteries, thereby causing PA distension (Fig. 1). However, the main pulmonary artery loses compliance the more it is stretched and does have a limit of distensibility. The value of 29.0 mm has been established as the maximal normal value of main PA diameter and suggested as cutoff to detect pulmonary hypertension (PH) [26, 27]. In our study, the median PA diameter in patients with adverse outcomes was 29.9 mm with a broad IQ range. This may limit its clinical utility. Furthermore, one might expect the progress of PA dilatation to be different between acute PE and slowly progressing PH. Therefore, while we do see a statistically significant association between PA diameter and short-term clinical deterioration, the limited total distensibility of the main PA in acute PE may mean that the OR (1.08, per 1 mm increase) we found is of limited clinical relevance. Other studies also find the PA diameter only to be a weak predictor [7, 8, 22, 25, 28, 29].
We also found that bowing of the interventricular septum was associated with a more than twofold risk of clinical deterioration, with a p value bordering statistical significance (p = 0.055). Septal bowing is a sign of high RV intraventricular pressure with a decreasing trans-septal pressure gradient. Leftward shift of the septum and RV output failure limits LV filling and compromises LV output and, accordingly, cardiac output and mean blood pressure. Decreased coronary perfusion pressure worsens RV oxygen supply, causing RV ischemia and will further worsen RV function [2] (see Fig. 2b). Abnormal septal positioning has been shown to be a negative predictor in both CT and echocardiography [8, 13] but not consistently [24, 25]. This discordance may be explained by a relatively poor interobserver agreement in the detection of septal bowing [30], which corresponds to the low kappa value in the present study. The limited reliability in the interpretation of septal bowing may need to be acknowledged in clinical practice. On the contrary, as septal movements vary through the cardiac cycle [31], non-gated CT may produce false-negative measurements yielding an even higher OR. Collectively, despite the kappa value and due to the high OR, we believe that finding of septal bowing on CTPA is the most clinically relevant finding of the present study, and emergency physicians and radiologists may find this observation useful.
The RV/LV ratio is a well-established measure of RV dilatation on CT. The most widely accepted cutoffs are 0.9 and 1.0 [10, 12, 14, 22, 32, 33]. Our study did not show a correlation with outcome using a predefined cutoff of 1.0. Previous studies with a positive correlation have used higher cutoffs and thereby limited their analyses to patients with more dilated RVs [8, 11, 28, 29, 34]. For example, Kang et al. [34] found RV/LV ratio to be an independent predictor for mortality using a cutoff of RV/LV > 1.5, and Hefeda and Elmasry [8] found RV/LV > 1.2 to be correlated with 30 day mortality. In opposition to our results, Singanayagam et al. showed independent association between the RV/LV ratio and short-term outcome with the same cutoff RV/LV > 1.0 we used. However, while their analysis only adjusted for pulmonary embolism severity index (PESI), patients with RV dilatation had significantly higher heart rates and respiratory rates and lower blood pressure and oxygen saturation. These unadjusted differences may explain their observed association [12].
Studies have established correlation between clot burden and RV dilatation [8, 21, 35]. While some studies have found a correlation between clot burden and adverse outcomes [8, 36], most studies support no correlation [6, 21, 22, 25, 28, 29]. We speculate that the discrepancy may be caused by a lack of adjustment for RV dysfunction.
We did not find a correlation between contrast medium reflux to the IVC, which would be a sign of RV dysfunction with blood stasis in the central veins and low forward flow from the RV. Previous studies including IVC reflux have reported conflicting results [8, 13, 19, 24, 25, 34] suggesting that this is a less reliable predictor. Besides PE, several other cardiac conditions and the contrast injection rate can cause IVC reflux [37, 38] which might explain the discrepancy.
Our study has several limitations. First, the analyses were done retrospectively. However, the patients were enrolled prospectively and both radiologists reading the CTPA were blinded to all clinical data and outcomes. Second, a major uncertainty in the measurement of RV/LV ratio is the use of non-gated CT acquisition as images are obtained at inconsistent times of the cardiac cycle. This might explain why we found no association between RV/LV ratio and short-term outcomes. Gated CT scanning does, on the other hand, require more radiation exposure to the patients and is not the clinical standard for CTPA in the ED. We recognize that RV/LV ratio can be measured volumetrically, and these measurements have been correlated with outcomes [9, 11, 13, 39]. Our method of measuring the RV/LV ratio in cross section may have led to inaccuracy and limited our ability to see a correlation with outcomes. However, cross-sectional measurements are more common clinically and can be done more rapidly, so may be more relevant to emergency radiology practice [39]. Lastly, we only measured few cardiac and vascular dimensions and did not adjust for clot burden or equally time-consuming measures. However, the aim of the study was to identify rapidly obtained measurements relevant to emergency settings. More complex measurements may predict outcomes, but if they are overly complex or time-consuming, they are unlikely to be adopted into clinical practice.
Conclusion
The presence of septal bowing and greater main PA diameter were associated with short-term adverse clinical outcomes in ED patients diagnosed with acute PE. These measures can be easily and quickly measured in the ED. The main PA diameter was only a weak predictor and may be of limited clinical relevance. Further research should continue to focus on RV function and pulmonary perfusion and could include novel CT parameters to identify patients who need aggressive therapy or intensive observation.
Change history
29 August 2019
The above article was published online with an error in an author’s last name: It should be Muzikansky (and not Muzikanski). The correct name is presented here. The original article has been corrected.
References
Konstantinides SV, Torbicki A, Agnelli G et al (2014) 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. European Heart Journal 35:3033–69–3069a–3069k. https://doi.org/10.1093/eurheartj/ehu283
Konstam MA, Kiernan MS, Bernstein D, Bozkurt B, Jacob M, Kapur NK, Kociol RD, Lewis EF, Mehra MR, Pagani FD, Raval AN, Ward C (2018) Evaluation and management of right-sided heart failure: a scientific statement from the American Heart Association. Circulation 137:e578–e622. https://doi.org/10.1161/CIR.0000000000000560
Smulders YM (2000) Pathophysiology and treatment of haemodynamic instability in acute pulmonary embolism: the pivotal role of pulmonary vasoconstriction. Cardiovasc Res 48:23–33
Zhang LJ, Lu GM, Meinel FG, McQuiston AD, Ravenel JG, Schoepf UJ (2015) Computed tomography of acute pulmonary embolism: state-of-the-art. Eur Radiol 25:2547–2557. https://doi.org/10.1007/s00330-015-3679-2
Sista AK, Kuo WT, Schiebler M, Madoff DC (2017) Stratification, imaging, and management of acute massive and submassive pulmonary embolism. Radiology 284:5–24. https://doi.org/10.1148/radiol.2017151978
Vedovati MC, Germini F, Agnelli G, Becattini C (2013) Prognostic role of embolic burden assessed at computed tomography angiography in patients with acute pulmonary embolism: systematic review and meta-analysis. J Thromb Haemost 11:2092–2102. https://doi.org/10.1111/jth.12429
Bach AG, Nansalmaa B, Kranz J, Taute BM, Wienke A, Schramm D, Surov A (2015) CT pulmonary angiography findings that predict 30-day mortality in patients with acute pulmonary embolism. Eur J Radiol 84:332–337. https://doi.org/10.1016/j.ejrad.2014.11.023
Hefeda MM, Elmasry MM (2014) Prediction of short term outcome of pulmonary embolism: parameters at 16 multi-detector CT pulmonary angiography. Egypt J Radiol Nucl Med 45:1089–1098. https://doi.org/10.1016/j.ejrnm.2014.05.011
Apfaltrer P, Henzler T, Meyer M, Roeger S, Haghi D, Gruettner J, Süselbeck T, Wilson RB, Schoepf UJ, Schoenberg SO, Fink C (2012) Correlation of CT angiographic pulmonary artery obstruction scores with right ventricular dysfunction and clinical outcome in patients with acute pulmonary embolism. Eur J Radiol 81:2867–2871. https://doi.org/10.1016/j.ejrad.2011.08.014
Hariharan P, Dudzinski DM, Rosovsky R, Haddad F, MacMahon P, Parry B, Chang Y, Kabrhel C (2016) Relation among clot burden, right-sided heart strain, and adverse events after acute pulmonary embolism. Am J Cardiol 118:1568–1573. https://doi.org/10.1016/j.amjcard.2016.08.025
Apfaltrer P, Walter T, Gruettner J, Weilbacher F, Meyer M, Henzler T, Neumaier M, Schoenberg SO, Fink C (2013) Prediction of adverse clinical outcome in patients with acute pulmonary embolism: evaluation of high-sensitivity troponin I and quantitative CT parameters. Eur J Radiol 82:563–567. https://doi.org/10.1016/j.ejrad.2012.11.009
Singanayagam A, Chalmers JD, Scally C, Akram AR, al-Khairalla MZ, Leitch L, Hill LE, Hill AT (2010) Right ventricular dilation on CT pulmonary angiogram independently predicts mortality in pulmonary embolism. Respir Med 104:1057–1062. https://doi.org/10.1016/j.rmed.2010.02.004
Kang DK, Thilo C, Schoepf UJ, Barraza JM Jr, Nance JW Jr, Bastarrika G, Abro JA, Ravenel JG, Costello P, Goldhaber SZ (2011) CT signs of right ventricular dysfunction: prognostic role in acute pulmonary embolism. JACC Cardiovasc Imaging 4:841–849. https://doi.org/10.1016/j.jcmg.2011.04.013
Trujillo-Santos J, den Exter PL, Gómez V et al (2013) Computed tomography-assessed right ventricular dysfunction and risk stratification of patients with acute non-massive pulmonary embolism: systematic review and meta-analysis. J Thromb Haemost 11:1823–1832. https://doi.org/10.1111/jth.12393
Dudzinski DM, Hariharan P, Parry BA, Chang Y, Kabrhel C (2017) Assessment of right ventricular strain by computed tomography versus echocardiography in acute pulmonary embolism. Acad Emerg Med 24:337–343. https://doi.org/10.1111/acem.13108
Kabrhel C, Okechukwu I, Hariharan P, Takayesu JK, MacMahon P, Haddad F, Chang Y (2014) Factors associated with clinical deterioration shortly after PE. Thorax 69:835–842. https://doi.org/10.1136/thoraxjnl-2013-204762
Vandenbroucke JP, von Elm E, Altman DG et al (2007) Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. PLoS Med 4:e297. https://doi.org/10.1371/journal.pmed.0040297
Heyer CM, Lemburg SP, Knoop H, Holland-Letz T, Nicolas V, Roggenland D (2011) Multidetector-CT angiography in pulmonary embolism—can image parameters predict clinical outcome? Eur Radiol 21:1928–1937. https://doi.org/10.1007/s00330-011-2125-3
Kumamaru KK, Saboo SS, Aghayev A, Cai P, Quesada CG, George E, Hussain Z, Cai T, Rybicki FJ (2016) CT pulmonary angiography-based scoring system to predict the prognosis of acute pulmonary embolism. J Cardiovasc Comput Tomogr 10:473–479. https://doi.org/10.1016/j.jcct.2016.08.007
Amrhein V, Greenland S, McShane B (2019) Scientists rise up against statistical significance. Nature 567:305–307. https://doi.org/10.1038/d41586-019-00857-9
Ceylan N, Tasbakan S, Bayraktaroglu S, Cok G, Simsek T, Duman S, Savaş R (2011) Predictors of clinical outcome in acute pulmonary embolism: correlation of CT pulmonary angiography with clinical, echocardiography and laboratory findings. Acad Radiol 18:47–53. https://doi.org/10.1016/j.acra.2010.08.024
Moroni A-L, Bosson J-L, Hohn N, Carpentier F, Pernod G, Ferretti GR (2011) Non-severe pulmonary embolism: prognostic CT findings. Eur J Radiol 79:452–458. https://doi.org/10.1016/j.ejrad.2010.03.013
Ozsu S, Karaman K, Mentese A, Ozsu A, Karahan SC, Durmus I, Oztuna F, Kosucu P, Bulbul Y, Ozlu T (2010) Combined risk stratification with computerized tomography /echocardiography and biomarkers in patients with normotensive pulmonary embolism. Thromb Res 126:486–492. https://doi.org/10.1016/j.thromres.2010.08.021
Araoz PA, Gotway MB, Trowbridge RL, Bailey RA, Auerbach AD, Reddy GP, Dawn SK, Webb WR, Higgins CB (2003) Helical CT pulmonary angiography predictors of in-hospital morbidity and mortality in patients with acute pulmonary embolism. J Thorac Imaging 18:207–216
Atasoy MM, Sariman N, Levent E, Çubuk R, Çelik Ö, Saygi A, Atasoy I, Şahin S (2015) Nonsevere acute pulmonary embolism: prognostic CT pulmonary angiography findings. J Comput Assist Tomogr 39:166–170. https://doi.org/10.1097/RCT.0000000000000201
Truong QA, Massaro JM, Rogers IS, Mahabadi AA, Kriegel MF, Fox CS, O'Donnell CJ, Hoffmann U (2012) Reference values for normal pulmonary artery dimensions by noncontrast cardiac computed tomography: the Framingham Heart Study. Circ Cardiovasc Imaging 5:147–154. https://doi.org/10.1161/CIRCIMAGING.111.968610
Mahammedi A, Oshmyansky A, Hassoun PM, Thiemann DR, Siegelman SS (2013) Pulmonary artery measurements in pulmonary hypertension: the role of computed tomography. J Thorac Imaging 28:96–103. https://doi.org/10.1097/RTI.0b013e318271c2eb
Baptista R, Santiago I, Jorge E, Teixeira R, Mendes P, Curvo-Semedo L, Castro G, Monteiro P, Caseiro-Alves F, Providência LA (2013) One-shot diagnostic and prognostic assessment in intermediate- to high-risk acute pulmonary embolism: the role of multidetector computed tomography. Rev Port Cardiol 32:7–13. https://doi.org/10.1016/j.repc.2012.05.020
Oz II, Altınsoy B, Serifoglu I, Sayın R, Buyukuysal MC, Erboy F, Akduman EI (2015) Evaluation of right atrium-to-right ventricle diameter ratio on computed tomography pulmonary angiography: prediction of adverse outcome and 30-day mortality. Eur J Radiol 84:2526–2532. https://doi.org/10.1016/j.ejrad.2015.08.019
John G, Marti C, Poletti P-A, Perrier A (2014) Hemodynamic indexes derived from computed tomography angiography to predict pulmonary embolism related mortality. Biomed Res Int 2014:363756–363758. https://doi.org/10.1155/2014/363756
Rudski LG, Lai WW, Afilalo J et al (2010) Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 23:685–713– quiz 786–8. https://doi.org/10.1016/j.echo.2010.05.010
Attia NM, Seifeldein GS, Hasan AA, Hasan A (2015) Evaluation of acute pulmonary embolism by sixty-four slice multidetector CT angiography: correlation between obstruction index, right ventricular dysfunction and clinical presentation. Egypt J Radiol Nucl Med 46:25–32. https://doi.org/10.1016/j.ejrnm.2014.10.007
Gutte H, Mortensen J, Mørk ML, Kristoffersen US, Jensen CV, Petersen CL, von der Recke P, Kjaer A (2017) Non-ECG-gated CT pulmonary angiography and the prediction of right ventricular dysfunction in patients suspected of pulmonary embolism. Clin Physiol Funct Imaging 37:575–581. https://doi.org/10.1111/cpf.12325
Kang DK, Sun JS, Park KJ, Lim HS (2011) Usefulness of combined assessment with computed tomographic signs of right ventricular dysfunction and cardiac troponin T for risk stratification of acute pulmonary embolism. Am J Cardiol 108:133–140. https://doi.org/10.1016/j.amjcard.2011.03.009
Guo Z-J, Liu H-T, Bai Z-M, Lin Q, Zhao BH, Xu Q, Zeng YH, Feng WQ, Zhou HT, Liang F, Cui JY (2018) A new method of CT for the cardiac measurement: correlation of computed tomography measured cardiac parameters and pulmonary obstruction index to assess cardiac morphological changes in acute pulmonary embolism patients. J Thromb Thrombolysis 45:410–416. https://doi.org/10.1007/s11239-018-1628-z
Chaosuwannakit N, Makarawate P (2012) Prognostic value of right ventricular dysfunction and pulmonary obstruction index by computed tomographic pulmonary angiography in patients with acute pulmonary embolism. J Med Assoc Thail 95:1457–1465
Gosselin MV, Rubin GD (1997) Altered intravascular contrast material flow dynamics: clues for refining thoracic CT diagnosis. Am J Roentgenol 169:1597–1603. https://doi.org/10.2214/ajr.169.6.9393173
Ramos-Duran LR, Kalafut JF, Hanley M, Schoepf UJ (2010) Current contrast media delivery strategies for cardiac and pulmonary multidetector-row computed tomography angiography. J Thorac Imaging 25:270–277. https://doi.org/10.1097/RTI.0b013e3181efe8b0
Henzler T, Krissak R, Reichert M, Sueselbeck T, Schoenberg SO, Fink C (2010) Volumetric analysis of pulmonary CTA for the assessment of right ventricular dysfunction in patients with acute pulmonary embolism. Acad Radiol 17:309–315. https://doi.org/10.1016/j.acra.2009.10.022
Funding
No funding was received for the present analysis, but the original prospective study was funded by Harvard Milton Fund.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised: The above article was published online with an error in an author’s last name: It should be Muzikansky (and not Muzikanski).
Rights and permissions
About this article
Cite this article
Lyhne, M.D., Schultz, J.G., MacMahon, P.J. et al. Septal bowing and pulmonary artery diameter on computed tomography pulmonary angiography are associated with short-term outcomes in patients with acute pulmonary embolism. Emerg Radiol 26, 623–630 (2019). https://doi.org/10.1007/s10140-019-01709-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10140-019-01709-9