Abstract
The aim of our study was to discuss the option of endovascular treatment compared to surgery for patients with endoscopically unmanageable nonvariceal hemorrhage of the upper gastrointestinal tract. From 2000 to 2006, 23 patients (male, 15 male; female, 8; mean age, 69 years) who failed endoscopic therapy for upper gastrointestinal hemorrhage were retrospectively evaluated. Twelve patients were operated on (SG), whereas 11 patients had an endovascular intervention (IG). Technical and primary clinical success rates and complications rates were calculated. Clinical parameters and comorbidities were related to outcome. The surgical group suffered less frequently from pre-existing pulmonary diseases (SG, 17%; IG, 55%; p = 0.05) and had a higher incidence of shock requiring catecholamines (p < 0.01) or plasma expander therapy (p < 0.01). There was no significant difference in the incidence of recurrent bleeding episodes (SG, 17%; IG, 27%; p = 0.35) and mortality rates (SG, 17%; IG, 27%, p = 0.35). Deaths in the IG were due to recurrent bleeding. In patients with unsuccessful endoscopic control of nonvariceal bleeding of the upper GI tract, surgery remains a very effective treatment. However, in patients with a high surgical risk due to unknown bleeding sources and/or severe pre-existing diseases/comorbidities, endovascular therapy offers an excellent treatment option. These patients should then be operated on as early as possible to minimize the risk of recurrent bleeding episodes, which are associated with high morbidity and mortality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nonvariceal hemorrhage of the upper gastrointestinal tract is commonly caused by duodenal or gastric ulcers [1] and may present as a life-threatening event. The incidence of these cases has been reported to vary from 40 to 150/100,000 annually [2, 3]. The overall incidence has remained stable within the past 25 years. It is associated with increased age and medication of nonsteroidal anti-inflammatory drugs (NSAIDs), thrombocyte aggregation inhibitors, and anticoagulation drugs [4–6]. Similarly, mortality rates have not decreased significantly, remaining fairly unchanged at 10% despite advanced treatment regimens [7].
Emergency endoscopy to control bleeding has become the gold standard of therapy [8]. Several techniques have been developed including local injection of vasoactive substances, sclerosing agents, or coagulation-inducing substances as well as local application of clips or laser therapy (Fig. 1). This is followed by eradication of Helicobacter pylori, if present, and medication with proton pump inhibitors at high dosages to minimize the risk of recurrent bleeding episodes [9, 10].
This approach has remarkably reduced the need for emergency surgery [8]. However, depending on certain risk factors such as age, shock, comorbidities, localization of the bleeding, and endoscopic findings [11], endoscopy may not control the bleeding effectively in up to 10% of patients [12, 13], and early ulcerous hemorrhage is observed in 15–30% after initially effective treatment. In these cases, bleeding may recur or even continue resulting in emergency surgery [11]. Commonly, afferent vessels are ligated and ulcers are safely stitched centrally. Surgery is associated with a morbidity of up to 30% (36/7) and mortality rates of up to 50% in patients with one or several concomitant comorbidities. For patients with an increased surgical risk, an endovascular intervention may provide a therapeutic option. This approach was developed during the 1970s [14, 15]. The introduction of coils or certain particles into the vessels that supply the bleeding ulcer can effectively control the bleeding by embolizing the vessel. At the same time, this approach allows exact localization of the bleeding source in patients with previously unknown bleeding site. Successful control of bleeding has been achieved by this intervention in 80–95% of patients [16, 17] with mortality rates ranging from 8–35% [18, 19]. In contrast, mortality rates in patients undergoing emergency surgery have been described to be approximately 20% [20].
The main objective of our retrospective single-center study was to discuss the option of endovascular treatment compared to surgery for patients with endoscopically unmanageable nonvariceal hemorrhage of the upper gastrointestinal tract with regard to primary clinical and technical success rates and complications, as well as to evaluate factors that may influence these aspects.
Materials and methods
Patients
Between January 2001 and January 2006, 23 patients suffering from acute nonvariceal hemorrhages of the upper gastrointestinal (GI) tract with previous unsuccessful endoscopic treatment were referred to our clinic. The study group included 15 men and eight women with a mean age of 69 years (range, 43 to 93 years). Of these patients, 18 (78%) suffered from bleeding duodenal ulcers; in five patients (22%), the source of bleeding was localized within the duodenum proximal to the ligament of Treitz. There were no cases of gastric or transpapillary bleeding. Hemorrhage was classified as acute if endoscopic signs of active bleeding were noted and/or the patient was hemodynamically unstable. Informed consent for embolization or surgery was obtained from conscious patients as far as the emergency permitted. Otherwise, the immediate relatives were informed.
The retrospective study was approved by the Institutional Review Board.
Technical procedure
Depending on the patients’ surgical risk factors (age and comorbidities) and the overall clinical situation (e.g., symptoms of shock), further treatment—surgery or endovascular intervention—was planned in agreement with the involved surgeon, endoscopist, and interventional radiologist.
Surgery was done using standardized procedures. Median laparotomy was followed by exploration of the abdomen. The gastroduodenal, the superior pancreaticoduodenal, and the right gastroepiploic arteries were identified and ligated. A purse-string ligature at the bottom of the ulcer accomplished via a longitudinal duodenotomy and exterritorialization of the posterior wall of the ulcer completed the procedure (Fig. 2). If necessary, the procedure was modified by resection of a short segment of the duodenum.
Celiac and superior mesenteric angiography was performed using a femoral approach with a 4- to 5-F Cobra or Simmons 2 catheter (Optitorque ®, Terumo, Leuven, Belgium) and with 30–40 ml of a nonionic contrast medium (iomeprol, Imeron 350 ®, Bracco Altana, Germany) injected at a flow rate of 4–6 ml/s. If necessary, 20–40 mg of butylscopolamine bromide (Buscopan ®, Boehringer Ingelheim, Germany) was infused as a bolus to prevent bowel motion artifacts. Pharmacoangiography with intra-arterial injections of vasodilators, anticoagulants, or fibrinolytic agents to provoke contrast extravasation was not performed. Active gastrointestinal hemorrhage (GIH) was proven and localized by demonstrating extravasation of contrast agent. Vascular anomalies, such as arteriovenous malformations or angiodysplasia, were considered to be definitive bleeding sources. Suggestive but less specific findings were larger mucosal blushes with abnormal vessels and prolonged mucosal contrast spots indicating a mucosal focus of inflammation or small vascular malformations.
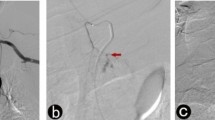
Two major principles of embolization were respected. First, when an anomaly was demonstrated, the feeding artery was catheterized as close as possible to the anomaly with a 2.7-F coaxial microcatheters (2.7F Progreat®, Terumo), introduced into the 4- or 5-F catheter. When the angiographic anomaly was fed by several arterial branches, it was attempted to occlude all of them to prevent secondary revascularization. Second, if no angiographic defect was detected, blind embolization of the gastroduodenal artery was performed involving the hemorrhagic area identified by endoscopy. If distal catheterization of the gastroduodenal artery failed, then a “sandwich” technique was used to synchronously occlude the lower arcade and the inferior gastroduodenal artery via the superior mesenteric artery. Embolic agents were microcoils in all cases (Target Coil®, Boston Scientific, Fremont, USA). The use of fibered of non-fibered coils was at the discretion of the performing radiologist. Successful embolization was documented by a follow-up angiography obtained 30 min later (Fig. 3). Then, the catheter was removed while the introducer sheath was kept in place until the next day. Patients were then transferred to the intensive care unit for further treatment. All patients were carefully monitored with special attention paid to possible ischemic complications and recurrent bleeding episodes. All patients treated by gastroduodenal embolization also underwent follow-up endoscopy 2–3 days later. In cases of recurrent bleeding episodes, patients were then treated employing the previously not used method, i.e., surgery in previously embolized patients and vice versa.
Definitions and study endpoints
All clinical, laboratory, and endoscopic data as well as outcome data and the care given were obtained from the medical records.
Each group [surgical group (SG) and interventional group (IG)] was retrospectively analyzed regarding:
-
Sex and age at admission
-
Comorbidities and risk factors
-
Current oral anticoagulation therapy or medication with thrombocyte aggregation inhibitors or NSAIDs
Comorbidities were analyzed in number (coexisting diseases per patient) and quality (severity of any particular coexisting disease per group). Cardiac disease/risk factors were present if the patient had a past history of myocardial infarction or cardiac revascularization therapy as well as antihypertensive or antiarrhythmic medication. Pulmonary disease/risk factors were present if the patient had a past history of chronic obstructive pulmonary disease, a history of pulmonary insufficiency during episodes of respiratory tract infections, or a long-standing history of smoking (>20 cigarettes/day). Hepatic disease/risk factors were present in patients with liver cirrhosis or established liver disease (at least Child stage A). Neurological disease/risk factors were present in patients with a history of a stroke or epileptic seizures. In addition, a past or present history of malignancy was regarded as an additional risk factor/comorbidity.
The overall clinical condition of the patient was retrospectively assessed on the basis of the patient’s hemoglobin on admission and the need for treatment with catecholamines and/or plasma expanders. The latter two therapies were sufficient to define shock as being present. Coagulopathy was defined as a platelet count less than 50,000/cm3 and/or a prothrombin time less than 50% of coagulation activity of reference plasma.
Statistical analysis
Success rates were analyzed according to the guidelines published by the Society of Cardiovascular and Interventional Radiology in 2003 [21].
Clinical success was defined as survival of the patient followed by complete recovery either after surgical or endovascular treatment. Technical success of surgery was defined as cessation of bleeding during the operation. Technical success of embolotherapy was defined as the impossibility to visualize the embolized artery by contrast medium or cessation of contrast leakage from a previously bleeding vessel. We included an early observation period from the day of intervention until day 3 to detect therapy-related failures. Recurrent bleeding episodes were excluded clinically in the absence of signs of shock, melena, hematemesis, and hematochezia and on hematological parameters.
Statistical analyses of qualitative variables were done by the χ 2-test (p < 0.05), those of quantitative variables by the Mann–Whitney-Wilcoxon test (confidence interval, 0.975).
Results
Of the 23 patients enrolled in this study, 12 patients (52%) were treated by surgery (SG) and 11 patients (48%) by an endovascular intervention/arterial embolotherapy (IG) (Table 1).
There were no significant differences between the two groups regarding age (SG, 71 years; IG, 68 years), sex (SG, 67% males; IG, 64% males), and hemoglobin on admission (SG, 5.2 mmol/l; IG, 4.9 mmol/l) or oral anticoagulant therapy, intake of thrombocyte aggregation inhibitors or NSAIDs (SG, 36%; IG, 25%, p = 0.36). On average, patients had two and three coexisting diseases (SG, 2.5; IG, 3.0). However, patients of the IG had a significantly higher incidence of pulmonary diseases (SG, 55%; IG, 17%, p < 0.005).
On admission, the SG displayed significantly more often signs of hypovolemic shock requiring catecholamine therapy (SG, 69%; IG, 27%, p < 0.01) or plasma expander solutions (SG, 92%; IG, 65%, p < 0.01).
There was no significant difference between the groups concerning mass transfusion of packed red cells (>10 units of packed cells per 24 h) before definitive treatment (SG, 58%, IG, 64%, p = 0.61). Incidence of coagulopathy (SG, 8%; IG, 18%, p = 0.59) was not significantly different.
Peptic ulcer disease was the most frequent cause of hemorrhage and was present in 18 patients (78%) of the total study population, ten patients (83%) of the surgery group and eight patients (73%) of the interventional group. The other two patients (17%) of the surgical group had upper GIH complicating chronic pancreatitis. In the IG, one patient (9%) had pancreatitis-related hemorrhage, and in two cases (18%), the etiology remained unknown. The hemorrhagic source was duodenal in all cases.
Before hemostatic treatment, the source of bleeding was known in all patients of the surgical group (12/12 = 100%) but in only 9 of 11 patients (82%) of the interventionally treated group.
In the interventional group, an anomaly was demonstrated by angiography in ten patients (91%) and consisted in extravasation of contrast material in six cases (60%) and localized hyperemia in four cases (40%). Treatment was highly selective in most cases (9/10; 90%). Blind embolization was performed in one case. In this case, the source of bleeding was not detected during angiography, but the preceding endoscopy had clearly identified the bleeding site, so blind embolization of the supplying arteries was performed.
Technical success was observed in all patients of both groups. There were no ischemic complications in the interventional group during follow-up.
The amounts of episodes of recurrent bleeding requiring further treatment differed slightly but not significantly between the two groups (SG, 17%; IG, 27%, p = 0.35).
Recurrent bleeding occurred in two patients of the surgical group and was successfully treated by endoscopy (n = 1) or arterial embolization (n = 1). Three instances of rebleeding were observed in the interventional group. Two patients required emergency surgery. Bleeding was technically stopped, but the patients did not survive due to progressive multiorgan failure resulting from hemorrhagic shock. In one patient, bleeding was successfully treated by endoscopy. Later on, this patient died because of progressive pulmonary insuffiency. This patient had a pre-existing severe pulmonary obstructive disease and also suffered from pre-existing cardiac disease and subcortical atherosclerotic encephalopathy (=2 comorbidities). The other two patients had pre-existing liver, cardiac, pulmonary and neurological diseases (= 4 comorbidities) and pre-existing gastric ulcers, liver, pulmonary, and malignant disease (=4 comorbidities), respectively. All three patients required mass transfusions, but only one patient had a coagulopathy at the time of treatment.
Mortality rates showed a small but not significant difference between the groups (SG, 17%; IG, 27%, p = 0.35). The two patients of the SG who died suffered from septic multiorgan failure (n = 1) or postoperative myocardial infarction (n = 1). Both patients had four pre-existing comorbidities.
Discussion
Endoscopic therapy has evolved to be the gold standard for treating nonvariceal hemorrhage of the upper gastrointestinal tract [12]. The endoscopic technique and success rates of endoscopic treatment in stopping hemorrhage have greatly improved over the last decade and have led to a decline in mortality rates. However, in up to 10% percent of patients, endoscopy fails to control hemorrhage [13]. So far, the gold standard for these patients has been emergency surgery. Not surprisingly, these patients have a higher surgical risk with increased mortality rates of up to 35%. This is mainly due to prolonged or recurrent anemia, pre-existing comorbidities, and advanced age [9–12, 22–24]. Therefore, in the 1980s, Siewert et al. [25] suggested the concept of early planned surgery. First, this concept was not supported by further studies, as elderly patients had an unchanged risk for surgery and, consecutively, unchanged mortality rates. On the other hand, younger patients with a low risk of recurrent bleeding episodes underwent unnecessary surgical procedures [26–28]. Later studies investigated the concept of early planned surgery 6–12 h after successful endoscopic treatment in patients at risk for recurrent bleeding episodes. Although the numbers of patients enrolled in these studies were small, a decrease in recurrent bleeding episodes and a decrease in mortality rates was demonstrated [29, 30]. Hence, this concept is currently suggested for the treatment of nonvariceal hemorrhage of the upper gastrointestinal tract [31].
The introduction and further development of endovascular interventional therapies, in particular arterial embolotherapy, has significantly changed the treatment of patients with acute hemorrhage, especially those with unknown bleeding sources. Yet, there is only little data confirming the value of embolotherapy compared to surgery in nonvariceal hemorrhage of the upper gastrointestinal tract, particularly in emergency settings. In this study, we have addressed this question in a retrospective study. Patients who entered the study were treated either by surgery or by endovascular intervention. There were no significant differences between the two treatment groups regarding age, hemoglobin on admission, or current medication of NSAIDs, anticoagulation therapy, or thrombocyte aggregation inhibitors. While the latter medication as such is no risk factor for recurrent bleeding episodes after endoscopy [32], patients taking thrombocyte aggregation inhibitors generally have a greater number of and more severe coexisting diseases. Therefore, these patients have a higher morbidity and mortality [1, 11, 32] in an emergency surgical setting [24]. In a retrospective study comparing arterial embolotherapy with emergency surgery, Ripoll et al. [33] found that, in the first group, patients took more often anticoagulation drugs, were older, and had a higher incidence of cardiac diseases. In our study, patients of the interventional group suffered more frequently from pulmonary diseases, whereas there was no significant difference in age.
Before hemostatic treatment, the source of bleeding was known in all patients of the surgical group but in only 82% of the interventional group. Therefore, the radiological intervention was also a diagnostic procedure in these patients.
In our study, the amounts of episodes of recurrent bleedings requiring further treatment differed slightly but not significantly between the two groups. Similarly, mortality rates showed a small but not significant difference between the groups. These findings confirm data by Ripoll et al., although their data were collected over a period of 15 years. The comparability of the two studies might therefore be questionable [18, 33, 34].
A previous report on embolization of upper gastrointestinal bleeding found that embolization was 2.9 times more likely to fail in patients with coagulopathy, and death from bleeding after embolization was 9.6 times more likely to occur in these patients [35]. These findings are not confirmed by our data. In the interventional group, only one patient with coagulopathy suffered from recurrent hemorrhage and died. However, the number of patients included in our study is too small to calculate odds ratios. In the surgical group, no patient with coagulopathy died.
Recent studies of arterial embolotherapy in nonvariceal hemorrhage of the upper GI tract report technical success rates of about 95% [34, 36]. This is in line with our technical success rate of 100%. Although problems related to arterial occlusion or endovascular intervention have been described in the literature [18, 33, 34], we did not see any of those in our study. However, patients of the interventional group requiring further treatment because of recurrent bleeding episodes did not survive the hospital stay. Pre-existing comorbidities may well have added to the unfavorable outcome in these patients but also recurrent bleeding episodes.
Finally, it remains unclear whether the two patients of the surgical group who died may have survived had they been treated initially by embolotherapy. However, surgery was not the immediate cause of death. Moreover, both patients were old (84 and 98 years) and suffered from atherosclerosis, signs of shock on admission, and severe pre-existing comorbidities making them highly prone to all kinds of medical problems. Surgery had been chosen as first-line treatment to decrease the risk of recurrent bleeding episodes in case of unsuccessful embolotherapy.
The retrospective nature of our study limits its relevance because patient management was not standardized. However, surgical and interventional treatments for upper gastrointestinal bleeding are quite uniform. In addition, the statistical results must be interpreted with caution due to the small number of patients included. Finally, the variety of etiologies, each with its own prognosis and pathophysiology, may have influenced the results presented in this paper.
Conclusion
In patients with unsuccessful endoscopic control of nonvariceal bleeding of the upper GI tract, surgery remains a very effective treatment. However, in patients with a high surgical risk due to unknown bleeding sources and/or severe pre-existing diseases/comorbidities, endovascular therapy offers an excellent treatment option. These patients should then be operated on as early as possible to minimize the risk of recurrent bleeding episodes, which are associated with high morbidity and mortality. Future prospective randomized trials have to clarify whether this treatment regimen can reduce mortality rates in patients with a high risk for surgery.
References
Rockall TA, Logan RF, Devlin HB, Northfield TC (1995) Incidence of and mortality from acute upper gastrointestinal haemorrhage in the United Kingdom. Steering committee and members of the national audit of acute upper gastrointestinal haemorrhage. BMJ 311(6999):222–226
Branicki FJ, Coleman SY, Fok PJ, Pritchett CJ, Fan ST, Lai EC, Mok FP, Cheung WL, Lau PW, Tuen HH et al (1990) Bleeding peptic ulcer: a prospective evaluation of risk factors for rebleeding and mortality. World J Surg 14(2):262–269 discussion 269–270
Gilbert DA (1990) Epidemiology of upper gastrointestinal bleeding. Gastrointest Endosc 36(5 Suppl):S8–S13
Koelz HR, Arn M (2006) [New epidemiology of acute gastrointestinal hemorrhage]. Chirurg 77(2):103–110
Shorr RI, Ray WA, Daugherty JR, Griffin MR (1993) Concurrent use of nonsteroidal anti-inflammatory drugs and oral anticoagulants places elderly persons at high risk for hemorrhagic peptic ulcer disease. Arch Intern Med 153(14):1665–1670
Sonnenberg A, Everhart JE (1996) The prevalence of self-reported peptic ulcer in the United States. Am J Public Health 86(2):200–205
Committee BSoGE (2002) Non-variceal upper gastrointestinal haemorrhage: guidelines. Gut 51(Suppl 4):iv1–iv6
Cook DJ, Guyatt GH, Salena BJ, Laine LA (1992) Endoscopic therapy for acute nonvariceal upper gastrointestinal hemorrhage: a meta-analysis. Gastroenterology 102(1):139–148
Gabriel SE, Jaakkimainen L, Bombardier C (1991) Risk for serious gastrointestinal complications related to use of nonsteroidal anti-inflammatory drugs. A meta-analysis. Ann Intern Med 115(10):787–796
Graham DY, Hepps KS, Ramirez FC, Lew GM, Saeed ZA (1993) Treatment of Helicobacter pylori reduces the rate of rebleeding in peptic ulcer disease. Scand J Gastroenterol 28(11):939–942
Rockall TA, Logan RF, Devlin HB, Northfield TC (1996) Risk assessment after acute upper gastrointestinal haemorrhage. Gut 38(3):316–321
Qvist P, Arnesen KE, Jacobsen CD, Rosseland AR (1994) Endoscopic treatment and restrictive surgical policy in the management of peptic ulcer bleeding. Five years’ experience in a central hospital. Scand J Gastroenterol 29(6):569–576
Schoenberg MH (2001) Surgical therapy for peptic ulcer and nonvariceal bleeding. Langenbecks Arch Surg 386(2):98–103
Rosch J, Dotter CT, Brown MJ (1972) Selective arterial embolization. A new method for control of acute gastrointestinal bleeding. Radiology 102(2):303–306
Wingen M, Gunther RW (2006) [Gastrointestinal bleeding. Diagnostics and therapy by interventional radiology]. Chirurg 77(2):117–125
Okazaki M, Higashihara H, Ono H, Koganemaru F, Sato S, Kimura S, Furui S (1992) Embolotherapy of massive duodenal hemorrhage. Gastrointest Radiol 17(4):319–323
Toyoda H, Nakano S, Takeda I, Kumada T, Sugiyama K, Osada T, Kiriyama S, Suga T (1995) Transcatheter arterial embolization for massive bleeding from duodenal ulcers not controlled by endoscopic hemostasis. Endoscopy 27(4):304–307
Rafique MZ, Ul Haq T, Ud Din GN, Ud Din MA, Chisty IA, Usman MU (2005) Transcatheter embolization of acute non-variceal gastrointestinal hemorrhage. J Coll Physicians Surg Pak 15(2):81–84
Schenker MP, Duszak R Jr., Soulen MC, Smith KP, Baum RA, Cope C, Freiman DB, Roberts DA, Shlansky-Goldberg RD (2001) Upper gastrointestinal hemorrhage and transcatheter embolotherapy: clinical and technical factors impacting success and survival. J Vasc Interv Radiol 12(11):1263–1271
Vellacott KD, Dronfield MW, Atkinson M, Langman MJ (1982) Comparison of surgical and medical management of bleeding peptic ulcers. Br Med J (Clin Res Ed) 284(6315):548–550
Drooz AT, Lewis CA, Allen TE, Citron SJ, Cole PE, Freeman NJ, Husted JW, Malloy PC, Martin LG, Van Moore A, Neithamer CD, Roberts AC, Sacks D, Sanchez O, Venbrux AC, Bakal CW (2003) Quality improvement guidelines for percutaneous transcatheter embolization. J Vasc Interv Radiol 14(9 Pt 2):S237–S242
Ell C, Hagenmuller F, Schmitt W, Riemann JF, Hahn EG, Hohenberger W (1995) [Multicenter prospective study of the current status of treatment for bleeding ulcer in Germany]. Dtsch Med Wochenschr 120(1–2):3–9
Park KG, Steele RJ, Mollison J, Crofts TJ (1994) Prediction of recurrent bleeding after endoscopic haemostasis in non-variceal upper gastrointestinal haemorrhage. Br J Surg 81(10):1465–1468
Read RC, Huebl HC, Thal AP (1970) Randomized study of massive bleeding from peptic ulceration. Ann Surg 162(4):561–577
Siewert JR, Bumm R, Holscher AH, Dittler HJ (1989) [Upper gastrointestinal bleeding from ulcer: reduction of mortality by early elective surgical therapy of patients at risk]. Dtsch Med Wochenschr 114(12):447–452
Dronfield MW, Atkinson M, Langman MJ (1979) Effect of different operation policies on mortality from bleeding peptic ulcer. Lancet 1(8126):1126–1128
Morris DL, Hawker PC, Brearley S, Simms M, Dykes PW, Keighley MR (1984) Optimal timing of operation for bleeding peptic ulcer: prospective randomised trial. Br Med J (Clin Res Ed) 288(6426):1277–1280
Saperas E, Pique JM, Perez Ayuso R, Bordas JM, Teres J, Pera C (1987) Conservative management of bleeding duodenal ulcer without a visible vessel: prospective randomized trial. Br J Surg 74(9):784–786
Imhof M, Schroders C, Ohmann C, Roher H (1998) Impact of early operation on the mortality from bleeding peptic ulcer—ten years’ experience. Dig Surg 15(4):308–314
Monig SP, Lubke T, Baldus SE, Schafer H, Holscher AH (2002) Early elective surgery for bleeding ulcer in the posterior duodenal bulb. Own results and review of the literature. Hepatogastroenterology 49(44):416–418
Knoefel WT, Rehders A (2006) [Gastrointestinal bleeding—concepts of surgical therapy in the upper gastrointestinal tract]. Chirurg 77(2):126–132
Thomopoulos KC, Vagenas KA, Vagianos CE, Margaritis VG, Blikas AP, Katsakoulis EC, Nikolopoulou VN (2004) Changes in aetiology and clinical outcome of acute upper gastrointestinal bleeding during the last 15 years. Eur J Gastroenterol Hepatol 16(2):177–182
Ripoll C, Banares R, Beceiro I, Menchen P, Catalina MV, Echenagusia A, Turegano F (2004) Comparison of transcatheter arterial embolization and surgery for treatment of bleeding peptic ulcer after endoscopic treatment failure. J Vasc Interv Radiol 15(5):447–450
Aina R, Oliva VL, Therasse E, Perreault P, Bui BT, Dufresne MP, Soulez G (2001) Arterial embolotherapy for upper gastrointestinal hemorrhage: outcome assessment. J Vasc Interv Radiol 12(2):195–200
Encarnacion CE, Kadir S, Beam CA, Payne CS (1992) Gastrointestinal bleeding: treatment with gastrointestinal arterial embolization. Radiology 183(2):505–508
Defreyne L, Vanlangenhove P, De Vos M, Pattyn P, Van Maele G, Decruyenaere J, Troisi R, Kunnen M (2001) Embolization as a first approach with endoscopically unmanageable acute nonvariceal gastrointestinal hemorrhage. Radiology 218(3):739–748
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Langner, I., Langner, S., Partecke, L.I. et al. Acute upper gastrointestinal hemorrhage: is a radiological interventional approach an alternative to emergency surgery?. Emerg Radiol 15, 413–419 (2008). https://doi.org/10.1007/s10140-008-0736-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10140-008-0736-z