Abstract
Nacre, also called mother-of-pearl, is a naturally occurring biomineral, largely studied by chemists, structural biologists, and physicists to understand its outstanding and diverse properties. Nacre is constituted of aragonite nanograins surrounded by organic matrix, and it has been established that the organic matrix is responsible for initiating and guiding the biomineralization process. The first challenge to study the organic matrix of nacre lays in its separation from the biomineral. Several extraction methods have been developed so far. They are categorized as either strong (e.g., decalcification) or soft (e.g., water, ethanol) and they allow specific extractions of targeted compounds. The structure of the nacreous organic matrix is complex, and it provides interesting clues to describe the mineralization process. Proteins, sugars, lipids, peptides, and other molecules have been identified and their role in mineralization investigated. Moreover, the organic matrix of nacre has shown interesting properties for human health. Several studies are investigating its activity on bone mineralization and its properties for skin care. In this review, we focus on the organic constituents, as lipids, sugars, and small metabolites which are less studied since present in small quantities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
How is mineral material included in living organisms? How are so many different mineralized structures created with the most common mineral building blocks (calcium carbonate CaCO3, silica SiO2, and phosphate calcium CaPO4) and combined with organic material (proteins, carbohydrates, lipids, secondary metabolites)? What is the secret of the diversity of the necessary biominerals produced by living organisms? This is the marvel of biomineralization: a dynamic physiological process by which a living organism synthesizes a mineralized structure (Marin et al. 2007). Interestingly, the nature of the organic molecules influences or even controls the calcium carbonate crystallization towards one of the possible polymorphs such as calcite, aragonite, or vaterite forms.
In the marine environment, biomineralization is performed by a wide range of living systems going from mollusks, brachiopods, or sponges to corals, sea urchin, coccoliths, and foraminifera (Cusack and Freer 2008). It is used to build a skeleton or a protective structure, as in the case of embryonic eggshells, to perform photosynthesis like coccoliths (Gordon and Du 2001), to detect the earth magnetic field for magnetotactic bacteria (Scheffel et al. 2006), or to protect cells from intense UV radiations (Price et al. 1998). The most common bricks involved in the elaboration of the mineral component are calcium phosphate, calcium carbonate, and silica. The variety of the known biomineral ultrastructures is driven by the diversity of organic matrix. Organic matrix is a minor constituent in terms of weight percentage, but is controlling important structural aspects and allowing the skeletal formation in organisms (Cusack and Freer 2008). In contrast with the high molecular weight molecules of the skeletal part that have been extensively studied, the small ones are poorly investigated.
Mollusks belong to the second-largest phylum in invertebrates (Mollusca) and are of great interest for the study of biomineralization process. The Mollusca phylum shows a significant diversity of morphologies and living places (e.g., sea water, fresh water, or earth) (Lowenstam and Weiner 1989). The shell or exoskeleton is the product of a biomineralization process. The structure of shell biominerals is studied applying several approaches including micro and ultrastructural observations by electron microscopy (SEM, TEM), physical measurements (X-ray diffraction), and biochemical characterizations. The results from these studies open to promising perspectives, due to the structure and mechanical properties of molluscan nacre (Mayer 2005) and for the use of bioactive corals- and mollusk-based implants as bone substitutes (Begley et al. 1995; Westbroek and Marin 1998; Zhang et al. 2017).
Among mollusk shell structures, nacre is widely studied for its attractive mechanical properties as it is a tough and strong (Sun and Bhushan 2012), yet light material. Nacre is also worthy of interest for application in the medicinal domain: it has been shown that it is osteogenic, osteoconductive, biocompatible, and biodegradable. A review by Zhang et al. relates the challenges and advances in using nacre as bone substitutes (Zhang et al. 2017). From the structural point of view, nacre is formed by alternating porous organic films and calcium carbonate layers (Grégoire et al. 1954). The organic matrix is studied to understand the mechanism of biomineralization and its structural function as well as for its medicinal properties. To perform these studies, nacre is extracted from the shell of different species of pearl oysters, mainly Pinctada margaritifera, Pinctada maxima, and Pinctada fucata collected in the Pacific Ocean (Polynesia, north Australia, and Japanese coast, respectively) (Southgate and Lucas 2008).
Until now, the study of the organic matrix has as its main objective the identification of the structural proteins composing it. These studies are undertaken either to understand the functions of the matrix and the mechanisms of biomineralization (Addadi et al. 2006) or to develop innovative, biomimetic applications (e.g., nanotechnologies, semiconductors, or natural bioactive factor) (Marin et al. 2007). The organic–inorganic assembly in nacre is also a matter of intensive research to perform the synthesis of artificial nacre from cheap abundant materials (Luz and Mano 2009; Gerhard et al. 2017; Huang et al. 2019). The integration of the organic matrix in nacre plays a key role in its formation, structure, and for its properties, despite being less than 3% of the shell weight.
Nacre is integrated in the mollusk shell structure, composed of several layers. The outer part, or periostracum, is mainly organic and the intermediary layer is calcitic, called prismatic layer. Then there is the nacreous layer covering the inner shell with its aragonitic phase. The mollusk body is directly in contact with the nacre, through an organic layer. The organic matrix is present in this structure at several levels: either assuring the interface between the layers or inside them. The primary structural components of nacre are pseudohexagonal aragonite tablets as shown in Fig. 1D. The intra-crystalline organic matrix is found inside the tablets while the inter-tabular (nm thick) and inter-lamellar (50–100 nm) matrix are found between individual tablets or layers (Rousseau et al. 2005).
The structure of the shell of the pearl-producing oyster, Pinctada margaritifera (redrawn from Rousseau et al. 2009). A An open shell with the soft tissue in place. The brighter nacreous layer of aragonite is clearly visible next to the animal. The nacreous layer coats the darker, prismatic shell of calcite that forms the outer shell. B Schematic representation of the shell structure and the overlying mantle of the animal. The mantle is an organic tissue acting as an interface between the organism and nacre tablets, secreting compounds involved in mineralization. C Electron microscopy image of the surface membrane covering the forming nacre tablets (white arrow) after mechanical fractures of the shell. D Electron microscopy image of the brick-and-mortar structure of the nacreous layer after fracture of the shell. Each tablet is about 0.5–1 μm thick. E SEM image of the cross-section of the nacreous to prismatic transition in the shell
In 1855, Fremy performed the first study on the organic matrix of mollusk shells and designated the insoluble matrix as “conchiolin” (Fremy 1855). Conchiolin was finally biochemically analyzed by Gregoire et al. in 1954 (Grégoire et al. 1954). In the last decades, many scientists have worked on the proteic components of nacre. A large number of shell matrix proteins involved in the nacreous layer formation have been identified, such as Pif (Suzuki et al. 2009), MSI60 (Sudo et al. 1997), lustrin A (Shen et al. 1997), N16/pearlin (Samata et al. 1999), perlucin, perlustrin (Weiss et al. 2000), N14, N66 (Kono et al. 2000), mucoperlin (Marin et al. 2000), AP7, AP24 (Michenfelder et al. 2003), P10, perlwapin (Treccani et al. 2006), perlinhibin (Mann et al. 2007), N19 (Yano et al. 2007, p. 19), N40 (Yan et al. 2007), and blue mussel shell protein (BMSP) (Suzuki et al. 2011). A recent review reports on the advances made in the characterization of shell matrix protein and cellular orchestration in marine molluscan shell biomineralization (Song et al. 2019).
The present review will focus on the other biomolecules extracted from the nacreous organic matrix such as sugars, lipids, peptides, and other small metabolites that are part of the organic matrix and are way less studied than proteins. Methods for their extraction and separation processing will be discussed.
Organic Matrix Structure
Following a consequent increase of calcium in the sea water, some mollusks evolved to grow shells (Marin et al. 1996). Biomineralization remains today an underexplored phenomenon. Many scientists are investigating the process of nacre formation to elucidate the role of the organic matrix. The organic structure is present prior to the formation of minerals as shown in Fig. 2. Proteins are instructing the process of biomineralization and are then incorporated in the mineral, conferring its mechanical properties to the nacre (Launspach et al. 2012).
Scheme of a suggested model for the process of nacre formation A before mineralization and B after mineralization (Addadi et al. 2006)
The nacreous organic matrix have been studied by TEM (transmission electron microscopy), AFM (atomic force microscopy) and SEM (scanning electron microscopy), mass spectrometry (e.g., nanoSIMS—nanoscale secondary ion mass spectrometry), IR (infra-red spectroscopy), or X-ray analysis to determine its physical organization around mineral phases and how the biomolecules are self-organized (Cartwright and Checa 2007). The mineral part of nacre is composed of aragonite tablets whereas that of the prismatic layer is composed of calcitic prisms, the two most common Ca-carbonate polymorphs in shells. The two scales of these structures can be clearly seen in Fig. 1E. In nacre, intra-crystalline, inter-tabular, and inter-lamellar matrix were identified. Rousseau et al. studied the composition of the inter-tabular and inter-lamellar matrix. The shell was prepared to access the surface between the mantle and the shell, where nacre is growing from the mantle. This surface was observed by SEM (scanning electron microscope) and nanoSIMS. They concluded that inter-tabular matrix is rich in aragonite nucleating proteins and carboxylates while inter-lamellar matrix is composed of chitin and other organic material (Rousseau et al. 2009). Organic envelopes or membranes are surrounding nacre, as shown in Fig. 1. In the prismatic layer, organic matrix is divided into three categories: interlayer membranes at the calcite-aragonite transition, interprismatic membranes, and intraprismatic membranes (Farre et al. 2011). These three kinds of organic matrix have revealed different compositions under TOF–SIMS (time of flight secondary ion mass spectrometry) analysis. Also, IR spectroscopy has allowed to show that the layers are of irregular thickness (Dauphin et al. 2010).
By studying nacre layers after incomplete decalcification, Weiner and Traub noticed a well-defined spatial relationship between chitin, proteins, and aragonite (Weiner and Traub 1980). These observations confirmed previous results representing the organic matrix as chitin fibrils lined up in a defined direction on a protein surface (Goffinet 1969). The organic matrix is described as a thin layer of β-chitin sandwiched between layers of glycine and alanine-rich proteins (Weiner and Traub 1984). In more recent studies, the way the chitin filament is associated with several proteins has been investigated (Mann et al. 2000, 2007; Weiss et al. 2001; Fu et al. 2005, p. 3). X-ray and electron diffraction pattern of organic matrices and mineral crystals have highlighted that the chitin fibers and the protein polypeptide chains are aligned with the a and b aragonite crystallographic axes respectively (Fig. 3) (Weiner et al. 1983).
Schematic interpretation of protein-decorated chitin matrix (Launspach et al. 2012). Chitin filaments (with a not specified acetylation degree) form a network with pores of varying diameter. The filaments are decorated with different kinds of proteins, which bind to the filaments with different strengths
This organization of nacre’s organic matrix clearly shows that organic molecules are implied in the process of biomineralization as well. This matrix is mainly composed of proteins and high molecular weight biomolecules, such as sugars or lipopeptides. The specific role of these compounds will be clarified in the following parts.
Extraction Methods
As organic matrix is only 2.3% of the nacre (Bourrat et al. 2007), extraction protocols remain a critical point to access the desired organic component. Demineralization is the most obvious method to extract organic compounds. It can be performed by acidification using hydrochloric acid (HCl) or in a softer way by using a chelating agent such as ethylenediaminetetraacetic acid (EDTA). A couple of soft extraction methods, which avoid demineralization, have been developed using either ethanol or water that have been shown to be efficient to solubilize a part of the organic matrix. The organic matrix is divided into two phases, soluble or insoluble in organic solvents.
Demineralization or Total Extraction Methods
Traditionally, the nacre organic matrix was extracted by demineralization. Demineralization by hydrochloric acid is considered to lead to a total extraction of the components of the organic matrix. This method has been used to study the structure and composition of mollusk shell organic matrix (Marin et al. 2001; Mann et al. 2007). To demineralize the nacreous layer, hydrochloric acid is added to powdered nacre to bring the suspension to pH 4 overnight. This method coupled with chromatography enabled to isolate proteins of the organic matrix. This drastic method certainly implies degradation of some acid-sensitive compounds.
A softer approach to demineralization uses ethylenediaminetetraacetic acid (EDTA). The demineralization can be performed by dialysis against a solution containing EDTA (3 weeks long) (Launspach et al. 2012) or by dissolving powdered nacre, directly into EDTA (stirring 72 h) (Takakura et al. 2008). EDTA-soluble and EDTA-insoluble fractions are thus separated. As proportions are very rarely indicated in the literature, it is difficult to give the yields and the extraction soluble/insoluble ratios.
According to Launspach et al. (2012), several different proteins have been identified to bind to chitin. As chitin is a polysaccharide building a complex intermolecular structure, it stays in the EDTA-insoluble phase after demineralization. Consequently, this demineralization approach does not allow to access the whole panel of proteins and molecules of the nacre organic matrix.
EDTA-based demineralization and further water extraction were compared in a study published by Pereira-Mouriès et al. (2002). Both methods gave around 0.05% (w/w) yield from powdered nacre. The proteins present in the two different extracts were analyzed (Whiteman Alcian blue binding technique, Fourier transformation infrared spectroscopy, electrophoresis, spectrophotometry) and shown to be different. The water-soluble extract seems to achieve a composition close to the EDTA-insoluble matrix. The EDTA-soluble matrix was aspartic-rich. Hydrophilic negatively charges molecules as aspartic-rich macromolecules are thought to interact specifically from solution to growing calcite crystals, suggesting that they are involved in the crystal formation process (Weiner and Addadi 1997). Importantly, the study of Pereira-Mouriès questions the widely accepted classification between soluble and non-soluble compounds in nacre organic matrix, as some of the EDTA-insoluble compounds are present in the water-soluble matrix (detailed below). In the EDTA-insoluble phase, there might be a crosslinking of proteins mediated by phenoloxidases, a well-known family of redox enzymes present also in the mollusk shell. This crosslinking would prevent proteins from dissolving in EDTA (Lowenstam et al. 1982).
Partial Extraction Methods
Methods to extract organic compounds from the powdered nacre without demineralizing have also been developed. A soft extraction by pure water of a very fine nacre powder was described for the first time by Almeida et al. in 2000 (Almeida et al. 2000). The powdered nacre was suspended in ultrapure water at room temperature for 20 h under continuous stirring. The water-soluble matrix (WSM) was then separated by size exclusion high-performance liquid chromatography to obtain a first rough separation of the polar compounds which were finally fractionated by anion exchange chromatography. This method seems promising to study molecules that are active on bone formation such as polyanionic polymers involved in mineral growth (Weiner and Addadi 1991).
In another method, organic matrix was extracted from the nacre powder using pure anhydrous ethanol containing 0.1% (v/v) of hydrochloric acid (Zhang et al. 2016). The polar protic organic solvent ethanol allows to better extract organic compounds from nacre. The ethanol-soluble matrix was then fractionated into cationic and anionic parts. Powdered nacre is suspended in ethanol to extract the molecules at the surface of the fine particles of nacre powder. The ethanol-soluble matrix activity on mineralization was tested in vitro and it was proved in this study that the cationic part of the extract is active in stimulating the mineralization of the osteoblast extracellular matrix. It is interesting to notice that while the anionic part containing polyanionic polymers is the one responsible of calcium carbonate mineral growth according to Weiner and Addadi (1991), it is the cationic part of the ethanol-soluble matrix which is promoting the formation of hydroxyapatite in the extracellular matrix of osteoblasts (Zhang et al. 2016).
A lipophilic extraction method was developed by Rousseau et al. (2006). Lipidic matrix was extracted by the mixture chloroform/methanol (2:1, v/v). This method allowed the identification of different classes of lipids in the nacreous organic matrix.
Finally, a protease-mediated hydrolysis was developed by Sasaki et al. to study proteins and peptides from Pinctada fucata nacre (Sasaki et al. 2019; Suzuki et al. 2019; Kintsu et al. 2020). This enzymatic hydrolysis was performed using the protease, nucleicin, and orientase 22 BF derived from Bacillus subtilis. Substrate and enzyme were mixed in a buffer solution and were incubated under specific optimal conditions (50 °C, pH 7.0 for nucleicin, and 60 °C, pH 9.2 for orientase). After the enzymatic reaction, the hydrolysate was boiled in water to inactivate the enzyme.
The extraction phase remains a challenge to the study of the nacreous organic matrix. Several methods have been developed to extract different compounds; however, most of these approaches are adapted to extract and study macromolecules and particularly proteins. The only published method adapted to extract specifically other biomolecules and small metabolites of the organic matrix is the lipid extraction method. Nevertheless, this method needs to be improved to selectively extract small molecules.
Biomolecules
Proteins
Proteins are nacre most studied compounds. Several of them have been isolated, identified, and tested for their biological activity. An exhaustive review about proteins in mollusks’ shell was recently published (Song et al. 2019), and therefore, they will not be further detailed here. Two older reviews are also available (Marin and Luquet 2004; Suzuki and Nagasawa 2013).
Sugars
Chitin
Chitin represent a huge part of the biomass on earth (Falini and Fermani 2004). It is a long chain polysaccharide, composed of β-(1–4)-linked N-acetyl-D-glucosamine subunits (Fig. 4) (Schönitzer and Weiss 2007; Muzzarelli et al. 2011) that is produced by many different species, such as mollusks, shrimps, insects, or fungi. Three forms of chitin have been identified, known as α-, β-, and γ-chitins. α- and β-chitins are well known. In mollusks shells, only β-chitin is present (Lowenstam and Weiner 1989). This form of chitin is widely studied and a complete crystallographic study is available (Gardner and Blackwell 1975). The chemical and physical properties of chitin are strongly influenced by the degree of polymerization, degree of deacetylation, and covalent or noncovalent protein modification (Weiss and Schönitzer 2006). A review by Falini and Fermani gives a comprehensive overview of chitin structure and role in biomineralization (Falini and Fermani 2004).
Chitin is the major constituent in the larval shell matrix. It seems to play an important function for the guidance of mineral deposition in the mollusk shell (Weiss and Schönitzer 2006). The impact of chitin synthesis and degradation in biomineralization was also highlighted (Weiss et al. 2006; Suzuki et al. 2007; Kintsu et al. 2017). As chitin structure is evolving between the larval and the adult shell, studying this component at the larval stage gives essential information about the role chitin plays in the material properties and development of the shell, by coordinating and guiding the mineralization process (Weiss and Schönitzer 2006). For example, Ps19 has been identified as a chitin binding protein and shows also calcium binding properties (Arroyo-Loranca et al. 2020, p. 19). To support these findings, it has been shown that a partial inhibition of chitin by nikkomycin Z (which imitate UDP-N-acetylglucosamine) leads to shell malformations (Schönitzer and Weiss 2007). Even if it cannot be a sufficient proof that chitin is inducing biomineralization, as nikkomycin Z is a non-selective inhibitor, this study shows that the mollusk chitin synthase plays an important enzymatic role in the coordinated formation of larval bivalve shells.
A very relevant study on biomineralization has been reported using a β-chitin-silk fibroin complex (Levi-Kalisman et al. 2001). β-Chitin from the pen of the squid Loligo and silk from the cocoons of silkworm Bombyx mori have been purified and were allowed to interact and stabilized in the β conformation by treatment with methanol. Crystallization was then induced by incubation of the substrate complex in a saturated solution of CaCO3. The results of this study showed that mineralization in aragonitic form or calcitic form is induced by the association of chitin and macromolecules. In vitro, chitin was also associated with soluble macromolecules in the absence of silk. In these conditions, calcite is formed if the macromolecules are from calcitic origin, but vaterite spherulites are formed inside the substrate if the macromolecules were from aragonitic origin. This study clearly highlights the central role of chitin in biomineralization. A schematic representation of organic matrix’ structure is shown in Fig. 5.
A schematic representation of the structure of the demineralized Atrina nacreous layer organic matrix (Levi-Kalisman et al. 2001)
Other Sugars
A few other kinds of sugars have been identified in the nacreous organic matrix from Pinctada fucata and Unio pictorum such as acidic glycosaminoglycans (GAGs), sulfated sugars, and glycoproteins (Marie et al. 2007; Takakura et al. 2008). Advanced studies of the composition of nacre acid soluble matrix demonstrated that a high ratio of the carbohydrates is sulfated and that the major proteins of this soluble matrix are heavily glycosylated (Marie et al. 2007). Marxen et al. proceeded to the isolation by gas chromatography and biochemical identification of the carbohydrates. Glucose, galactose, mannose, N-acetyl-glucosamine, and N-acetyl-galactosamine have been identified after hydrolysis as the major carbohydrates of the soluble and insoluble matrix (Marxen et al. 1998).
Carbohydrate moieties of glycoproteins have also been studied. N-linked oligosaccharides were enzymatically liberated from the nacreous organic matrix of Japanese pearl oyster Pinctada fucata. The structure of these carbohydrates covalently linked to the protein nacrein was identified by tandem mass spectrometry (MSMS) analysis (Takakura et al. 2008).
To investigate the role of carbohydrates in biomineralization, carboxylates of Asp-rich proteins and sulfated sugars were mapped on the surfaces of decalcified interlamellar matrices from the nacreous shell layer of the cephalopod Nautilus pompilius. This matrix was divided in four zones (Addadi et al. 2006). It had been demonstrated by Crenshaw and Ristedt that the highest concentration of sulfated sugars is in the central zone, where nucleation occurs (Crenshaw and Ristedt 1976; Marxen et al. 1998).
Dauphin and Marin studied cephalopod shell carbohydrates by FTIR (Fourier transform infrared spectrometry) and high-performance anion exchange chromatography. Four species were compared, including Nautilus macromphalus and Nautilus pompilius which are two nacreous species. They concluded that mannose, fructose, xylose, and ribose are absent from every studied matrix, while deoxyribose is specifically present in the Nautilus shell. Nautilus showed the highest content of glucose and galactose, and the lowest content in glucosamine (Dauphin and Marin 1995).
As for the role of polysaccharides in biomineralization, different hypotheses have been investigated. Sulfated polysaccharides, for example, may have a calcium binding role. Also, it has been shown that complete deglycosylation of the total organic matrix of freshwater mussel Unio pictorum leads to a loss of calcium binding ability of the two main matrix proteins and to a modification of the shape of the mineral (Marie et al. 2007). These observations are consistent with Takakura et al. who had concluded that nacrein is supplying Ca2+ and HCO3− through the carbonic anhydrase domain and N-glycan moieties (Takakura et al. 2008). To support these results, studies were driven on the PmCHST1 gene of Pinctada fucata martensii, encoding a protein which participates in the keratan sulfate synthesis. Keratan sulfate possesses considerable amounts of negatively charged sulfonic acid groups and contributes to biomineralization. The repression of this gene induced a decrease of sulfated polysaccharides in extrapallial fluid, the fluid located between the external epithelium of the mantle and the inner face of the shell, and caused disordered growth of nacre layer in shells (Hao et al. 2018). This gene possesses a sulfotransferase domain involved in the transfer of sulfate moieties to chondroitin sulfate disaccharides (Wang et al. 2017). The modification in mineralization due to the extinction of this gene supports the role of sulfated sugar in biomineralization.
Lipids and Terpenes
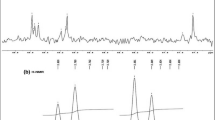
Even if lipids in nacre are way less studied than sugars or proteins, they are still part of the organic matrix. It is assumed that they represent 0.3 to 0.7% of the total weight of the shell (Rousseau et al. 2006; Saleuddin and Mukai 2017). Only the lipids of the soft part of the oyster have already been studied (Saito 2004). Rousseau et al. published the first results on the extraction of the lipids from the nacre organic matrix (Rousseau et al. 2006, p. 200). The precise lipidic composition of the nacreous organic matrix of two Pteriomorphia mollusks (Pinctada margaritifera and Pinna nobilis) was reported by Farre and Dauphin (2009). The organic matrix was studied by FTIR and TLC (thin layer chromatography) to detect lipids. Polar lipids (phospholipids), fatty acids (oleic acid), and waxes were identified as the major lipidic components. Sterols (cholesterol), triglycerides (triolein), and steroids (stearyl oleate) were also detected (Fig. 6). The presence of these compounds is consistent with the common lipids found in shells (Saleuddin and Mukai 2017). It has been shown that the lipidic composition of the two species differs, as these species are living in two very different environments, namely, the Pacific Ocean and the Mediterranean Sea.
It has to be noted that the same combination of lipids is found in several invertebrate skeletons. Lipids and sterols seem to be associated to the biomineralization process, as it was demonstrated that the removal of cholesterol and phospholipids from the shell matrix prevents biomineralization (Rousseau et al. 2006).
When lipids from nacre and flesh of Pinctada radiata oysters were studied in vitro (Ben Ammar et al. 2019), it was shown that they induce osteogenic MC3T3-E1 cell differentiation and in this way induce mineralization of the extracellular. These results are suggesting nacre lipids as good candidates in the quest of curing osteoporosis.
Peptides and Other Metabolites
Forty-two peptides from nacre are reported on uniprot.org database (Zhang 2017). Few of them have been studied in detail and some of the reported peptides are the product of protein’s hydrolysis as the two that will be detailed herein.
Three n16 proteins have been extracted from water insoluble matrix of Pinctada fucata. These three proteins are sharing 89% sequence identity, encoding between 129 and 131 amino acids with the 23 first amino acids apparently comprising a signal peptide (Samata et al. 1999). The 30 amino acid peptide N-terminal half (n16N) of the n16-1 protein was identified as the mineral-binding site (Metzler et al. 2010). This peptide has been recognized to preferentially nucleate aragonite once it is bound to β-chitin. A mutant of this peptide nucleates calcite (Keene et al. 2010). A second study noticed that the 15 N-terminal amino acids sequence of n16 protein promotes limited aragonite formation in vitro, while the 15 C-terminal amino acids part promotes calcite formation (Brown et al. 2014).
In another study, the nacreous organic matrix was hydrolyzed using two types of proteases to isolate a six amino acids peptide: GVGSPY. This peptide is an angiotensin I-converting enzyme inhibitory peptide and have shown activity in preventing high blood pressure (Sasaki et al. 2019).
Studies on nacre small molecules have been mostly undertaken on the whole water-soluble matrix mixture extracted from Pinctada margaritifera and Pinctada maxima. The molecules recovered from this matrix are first separated by size exclusion chromatography based on their molecular mass. It has been shown that between 110 and 300 different molecules ranging from 100 to 700 Da are present in the water-soluble matrix (Duplat et al. 2007; Rousseau et al. 2008). The organic water-soluble matrix presents only a low peptide content (10–12%, w/w).
Fractionation by dialysis, solvent extraction, reversed phase HPLC, or size exclusion chromatography allowed to study the water-soluble matrix in more details. Isolated fractions are then analyzed by mass spectrometry to identify the unknown compounds. Bédouet et al. identified four peptides: H-I/LGGI/L-OH, H-I/LGGGI/L-OH, H-G/KGAGI/L-OH, H-G/KGGGI/L-OH, and K/QGGI/L (Bédouet et al. 2006, 2007). Except for the five peptides mentioned, very few studies have been driven on the low molecular weight molecules extracted from nacre organic matrix.
The studies focusing on the small molecules present in nacre organic matrix aim above all to highlight their biological activity. In their studies, Bédouet et al. separated the low molecular weight components (< 8000 Da) from the water-soluble matrix. This fraction have shown a highly specific inhibitory action for the nacreous water-soluble matrix on both papain and cathepsin B (Bédouet et al. 2007; Duplat et al. 2007).
Earlier mineralization of MC3T3-E1 pre-osteoblasts was observed in the presence of the water-soluble matrix rather than in the presence of melatonin, a molecule known for its activity on cell differentiation (Rousseau 2003). In the same study, it was showed that a nacre fraction containing compounds with a molecular weight between 50 and 300 Da, comprising amino acids and peptides at trace level, was active on cell matrix mineralization. It was postulated that an ensemble of molecules is suggested to promote matrix mineralization, supposedly because the signal molecule of nacre might be the low molecular weight molecules (Rousseau et al. 2008).
These first studies are promising in the light of the suggested biological activities. It would be interesting to carry out additional chemical studies for the isolation of more small molecules from the nacreous organic matrix. Further chemical studies can turn out important to better understand the process of biomineralization and to potentially identify drug candidates to treat bone diseases.
Conclusion
Biomineralization is an interesting complex phenomenon whose studies are approached from various angles. Nacre is a well-studied biomineral produced by mollusks. An interesting way to investigate how nacre is formed and nacre’s mineral structure is to isolate and characterize the organic molecules of nacre. It was shown that the nacreous organic matrix is indeed initializing and guiding biomineralization. Studies were conducted about proteins mostly, but lipids, terpenes, sugars, or peptides are reported as well. Although our understanding of the structure of nacre has improved and the biomineralization processes leading to the formation of nacre have been partially enlightened, much remains to be explored. Understanding biomineralization remains a challenge in fundamental research and is also a point of interest for its applications.
Nacre organic matrix is also studied for its potential use for biomedical applications. Nacre extracts are indeed tested on diverse biological models, including mineralization properties or bones and skin barrier repairing. Small molecules among the secondary metabolites of this matrix remain very poorly studied. New extraction methods have to be developed to target them. The discovery of new small molecules could lead to a better understanding of biomineralization and to the development of new drug candidates.
References
Addadi L, Joester D, Nudelman F, Weiner S (2006) Mollusk shell formation: a source of new concepts for understanding biomineralization processes. Chem Eur J 12:980–987
Almeida MJ, Milet C, Peduzzi J et al (2000) Effect of water-soluble matrix fraction extracted from the nacre of Pinctada maxima on the alkaline phosphatase activity of cultured fibroblasts. J Exp Zool 288:327–334
Arroyo-Loranca RG, Hernandez-Saavedra NY, Hernandez-Adame L, Rivera-Perez C (2020) Ps19, a novel chitin binding protein from Pteria sterna capable to mineralize aragonite plates in vitro. PLoS ONE 15:e0230431
Bédouet L, Duplat D, Marie A et al (2007) Heterogeneity of proteinase inhibitors in the water-soluble organic matrix from the oyster nacre. Mar Biotechnol 9:437–449
Bédouet L, Rusconi F, Rousseau M et al (2006) Identification of low molecular weight molecules as new components of the nacre organic matrix. Comp Biochem Physiol B: Biochem Mol Biol 144:532–543
Begley CT, Doherty JM, Mollan RAB, Wilson DJ (1995) Comparative study of the osteoinductive properties of bioceramic, coral and processed bone graft substitutes. Biomaterials 16:1181–1185
Ben Ammar R, Piet M, Brion A et al (2019) Induction of osteogenic MC3T3-E1 cell differentiation by nacre and flesh lipids of Tunisian Pinctada radiata. Lipids 54:433–444
Bourrat X, Francke L, Lopez E et al (2007) Nacre biocrystal thermal behaviour. CrystEngComm 9:1205
Brown AH, Rodger PM, Evans JS, Walsh TR (2014) Equilibrium conformational ensemble of the intrinsically disordered peptide n16N: linking subdomain structures and function in nacre. Biomacromol 15:4467–4479
Cartwright JHE, Checa AG (2007) The dynamics of nacre self-assembly. J R Soc Interface 4:491–504
Crenshaw MA, Ristedt H (1976) The histological localization of reactive groups in septal nacre from Nautilus pompilius. The mechanisms of mineralization in the invertebrates and plants. University of South Carolina Press, Colombia, pp 355–367
Cusack M, Freer A (2008) Biomineralization: elemental and organic influence in carbonate systems. Chem Rev 108:4433–4454
Dauphin Y, Brunelle A, Cotte M et al (2010) A layered structure in the organic envelopes of the prismatic layer of the shell of the pearl oyster Pinctada margaritifera (Mollusca, Bivalvia). Microsc Microanal 16:91–98
Dauphin Y, Marin F (1995) The compositional analysis of recent cephalopod shell carbohydrates by Fourier transform infrared spectrometry and high performance anion exchange-pulsed amperometric detection. Experientia 51:278–283
Duplat D, Gallet M, Berland S et al (2007) The effect of molecules in mother-of-pearl on the decrease in bone resorption through the inhibition of osteoclast cathepsin K. Biomaterials 28:4769–4778
Falini G, Fermani S (2004) Chitin mineralization. Tissue Eng 10:1–6
Farre B, Brunelle A, Laprévote O et al (2011) Shell layers of the black-lip pearl oyster Pinctada margaritifera: matching microstructure and composition. Comp Biochem Physiol B: Biochem Mol Biol 159:131–139
Farre B, Dauphin Y (2009) Lipids from the nacreous and prismatic layers of two Pteriomorpha mollusc shells. Comp Biochem Physiol B: Biochem Mol Biol 152:103–109
Fremy M (1855) Recherches chimiques sur les os. Ann Chim Phys 443:95–97
Fu G, Valiyaveettil S, Wopenka B, Morse DE (2005) CaCO3 biomineralization: acidic 8-kDa proteins isolated from aragonitic abalone shell nacre can specifically modify calcite crystal morphology. Biomacromol 6:1289–1298
Gardner KH, Blackwell J (1975) Refinement of the structure of B-chitin. Biopolymers 14:1581–1595
Gerhard EM, Wang W, Li C et al (2017) Design strategies and applications of nacre-based biomaterials. Acta Biomater 54:21–34
Goffinet G (1969) Etude au microscope electronique de structures organisees des constituants de la conchioline de nacre du Nautilus macromphalus sowerby. Comp Biochem Physiol 29:835–839
Gordon HR, Du T (2001) Light scattering by nonspherical particles: application to coccoliths detached from Emiliania huxleyi. Limnol Oceanogr 46:1438–1454
Grégoire Ch, Duchateau G, Florkin M (1954) La trame protidique des nacres. Experientia 10:37–40
Hao R, Zheng Z, Wang Q et al (2018) Molecular and functional analysis of PmCHST1b in nacre formation of Pinctada fucata martensii. Comp Biochem Physiol B: Biochem Mol Biol 225:13–20
Huang W, Restrepo D, Jung J et al (2019) Multiscale toughening mechanisms in biological materials and bioinspired designs. Adv Mater 31:1901561
Keene EC, Evans JS, Estroff LA (2010) Matrix interactions in biomineralization: aragonite nucleation by an intrinsically disordered nacre polypeptide, n16N, associated with a β-chitin substrate. Cryst Growth Des 10:1383–1389
Kintsu H, Nishimura R, Negishi L et al (2020) Identification of methionine-rich insoluble proteins in the shell of the pearl oyster. Pinctada Fucata Sci Rep 10:18335
Kintsu H, Okumura T, Negishi L et al (2017) Crystal defects induced by chitin and chitinolytic enzymes in the prismatic layer of Pinctada fucata. Biochem Biophys Res Commun 489:89–95
Kono M, Hayashi N, Samata T (2000) Molecular mechanism of the nacreous layer formation in Pinctada maxima. Biochem Biophys Res Commun 269:213–218
Launspach M, Rückmann K, Gummich M et al (2012) Immobilisation and characterisation of the demineralised, fully hydrated organic matrix of nacre – an atomic force microscopy study. Micron 43:1351–1363
Levi-Kalisman Y, Falini G, Addadi L, Weiner S (2001) Structure of the nacreous organic matrix of a bivalve mollusk shell examined in the hydrated state using cryo-TEM. J Struct Biol 135:8–17
Lowenstam HA, Traub W, Weiner S (1982) Organic matrix in calcified exoskeletons. In: Westbroek P, Jong EW (eds) Biomineralization and biological metal accumulation. Springer, Netherlands, pp 204–225
Lowenstam HA, Weiner S (1989) On biomineralization. Oxford University Press, New York
Luz GM, Mano JF (2009) Biomimetic design of materials and biomaterials inspired by the structure of nacre. Phil Trans R Soc A 367:1587–1605
Mann K, Siedler F, Treccani L et al (2007) Perlinhibin, a cysteine-, histidine-, and arginine-rich miniprotein from abalone (Haliotis laevigata) nacre, inhibits in vitro calcium carbonate crystallization. Biophys J 93:1246–1254
Mann K, Weiss IM, André S et al (2000) The amino-acid sequence of the abalone (Haliotis laevigata) nacre protein perlucin: detection of a functional C-type lectin domain with galactose/mannose specificity. Eur J Biochem 267:5257–5264
Marie B, Luquet G, Pais De Barros J-P et al (2007) The shell matrix of the freshwater mussel Unio pictorum (Paleoheterodonta, Unionoida): involvement of acidic polysaccharides from glycoproteins in nacre mineralization. FEBS J 274:2933–2945
Marin F, Corstjens P, de Gaulejac B et al (2000) Mucins and molluscan calcification: molecular characterization of mucoperlin, a novel mucin-like protein from the nacreous shell layer of the fan mussel Pinna nobilis (Bivalvia, Pteriomorphia). J Biol Chem 275:20667–20675
Marin F, Luquet G (2004) Molluscan shell proteins. CR Palevol 3:469–492
Marin F, Luquet G, Marie B, Medakovic D (2007) Molluscan shell proteins: primary structure, origin, and evolution. In: Current topics in developmental biology. Elsevier, pp 209–276
Marin F, Pereira L, Westbroek P (2001) Large-scale fractionation of molluscan shell matrix. Protein Expr Purif 23:175–179
Marin F, Smith M, Isa Y et al (1996) Skeletal matrices, muci, and the origin of invertebrate calcification. Proc Natl Acad Sci 93:1554–1559
Marxen JC, Hammer M, Gehrke T, Becker W (1998) Carbohydrates of the organic shell matrix and the shell-forming tissue of the snail Biomphalaria glabrata (Say). Biol Bull 194:231–240
Mayer G (2005) Rigid biological systems as models for synthetic composites. Science 310:1144–1147
Metzler RA, Evans JS, Killian CE et al (2010) Nacre protein fragment templates lamellar aragonite growth. J Am Chem Soc 132:6329–6334
Michenfelder M, Fu G, Lawrence C et al (2003) Characterization of two molluscan crystal-modulating biomineralization proteins and identification of putative mineral binding domains. Biopolymers 70:522–533
Muzzarelli R, Jeuniaux C, Gooday GW (2011) Chitin in nature and technology. Springer Verlag
Pereira-Mouriès L, Almeida M-J, Ribeiro C et al (2002) Soluble silk-like organic matrix in the nacreous layer of the bivalve Pinctada maxima: a new insight in the biomineralization field. Eur J Biochem 269:4994–5003
Price LL, Yin K, Harrison PJ (1998) Influence of continuous light and L: D cycles on the growth and chemical composition of Prymnesiophyceae including coccolithophores. J Exp Mar Biol Ecol 223:223–234
Rousseau M (2003) The water-soluble matrix fraction from the nacre of Pinctada maxima produces earlier mineralization of MC3T3-E1 mouse pre-osteoblasts. Comp Biochem Physiol B: Biochem Mol Biol. https://doi.org/10.1016/S1096-4959(03)00032-0
Rousseau M, Bédouet L, Lati E et al (2006) Restoration of stratum corneum with nacre lipids. Comp Biochem Physiol B: Biochem Mol Biol 145:1–9
Rousseau M, Boulzaguet H, Biagianti J et al (2008) Low molecular weight molecules of oyster nacre induce mineralization of the MC3T3-E1 cells. J Biomed Mater Res 85A:487–497
Rousseau M, Lopez E, Stempflé P et al (2005) Multiscale structure of sheet nacre. Biomaterials 26:6254–6262
Rousseau M, Meibom A, Gèze M et al (2009) Dynamics of sheet nacre formation in bivalves. J Struct Biol 165:190–195
Saito H (2004) Lipid and FA composition of the pearl oyster Pinctada fucata martensii: influence of season and maturation. Lipids 39:997–1005
Saleuddin S, Mukai S (eds) (2017) Physiology of molluscs: a collection of selected reviews. Apple Academic Press Inc, New Jersey
Samata T, Hayashi N, Kono M et al (1999) A new matrix protein family related to the nacreous layer formation of Pinctada fucata. FEBS Lett 462:225–229
Sasaki C, Tamura S, Tohse R et al (2019) Isolation and identification of an angiotensin I-converting enzyme inhibitory peptide from pearl oyster (Pinctada fucata) shell protein hydrolysate. Process Biochem 77:137–142
Scheffel A, Gruska M, Faivre D et al (2006) An acidic protein aligns magnetosomes along a filamentous structure in magnetotactic bacteria. Nature 440:110–114
Schönitzer V, Weiss IM (2007) The structure of mollusc larval shells formed in the presence of the chitin synthase inhibitor nikkomycin Z. BMC Struct Biol 7:71
Shen X, Belcher AM, Hansma PK et al (1997) Molecular cloning and characterization of lustrin A, a matrix protein from shell and pearl nacre of Haliotis rufescens. J Biol Chem 272:32472–32481
Song X, Liu Z, Wang L, Song L (2019) Recent advances of shell matrix proteins and cellular orchestration in marine molluscan shell biomineralization. Front Mar Sci 6:41
Southgate PC, Lucas JS (eds) (2008) The pearl oyster. Elsevier Science, Amsterdam
Sudo S, Fujikawa T, Nagakura T et al (1997) Structures of mollusc shell framework proteins. Nature 387:563–564
Sun J, Bhushan B (2012) Hierarchical structure and mechanical properties of nacre: a review. RSC Adv 2:7617
Suzuki M, Iwashima A, Tsutsui N et al (2011) Identification and characterisation of a calcium carbonate-binding protein, blue mussel shell protein (BMSP), from the nacreous layer. ChemBioChem 12:2478–2487
Suzuki M, Kubota K, Nishimura R et al (2019) A unique methionine-rich protein–aragonite crystal complex: structure and mechanical functions of the Pinctada fucata bivalve hinge ligament. Acta Biomater 100:1–9
Suzuki M, Nagasawa H (2013) Mollusk shell structures and their formation mechanism. Can J Zool 91:349–366
Suzuki M, Sakuda S, Nagasawa H (2007) Identification of chitin in the prismatic layer of the shell and a chitin synthase gene from the Japanese pearl oyster, Pinctada fucata. Biosci Biotechnol Biochem 71:1735–1744
Suzuki M, Saruwatari K, Kogure T et al (2009) An acidic matrix protein, Pif, is a key macromolecule for nacre formation. Science 325:1388–1390
Takakura D, Norizuki M, Ishikawa F, Samata T (2008) Isolation and characterization of the N-linked oligosaccharides in nacrein from Pinctada fucata. Mar Biotechnol 10:290–296
Treccani L, Mann K, Heinemann F, Fritz M (2006) Perlwapin, an abalone nacre protein with three four-disulfide core (whey acidic protein) domains, inhibits the growth of calcium carbonate crystals. Biophys J 91:2601–2608
Wang Q, Yang C, Hao R et al (2017) Molecular characterization of CHST11 and its potential role in nacre formation in pearl oyster Pinctada fucata martensii. Electron J Biotechnol 28:113–119
Weiner S, Addadi L (1991) Acidic macromolecules of mineralized tissues: the controllers of crystal formation. Trends Biochem Sci 16:252–256
Weiner S, Addadi L (1997) Design strategies in mineralized biological materials. J Mater Chem 7:689–702
Weiner S, Talmon Y, Traub W (1983) Electron diffraction of mollusc shell organic matrices and their relationship to the mineral phase. Int J Biol Macromol 5:325–328
Weiner S, Traub W (1980) X-ray diffraction study of the insoluble organic matrix of mollusk shells. FEBS Lett 111:311–316
Weiner S, Traub W (1984) Macromolecules in mollusc shells and their functions in biomineralization. Phil Trans R Soc Lond B 304:425–434
Weiss IM, Göhring W, Fritz M, Mann K (2001) Perlustrin, a Haliotis laevigata (abalone) nacre protein, is homologous to the insulin-like growth factor binding protein N-terminal module of vertebrates. Biochem Biophys Res Commun 285:244–249
Weiss IM, Kaufmann S, Mann K, Fritz M (2000) Purification and characterization of perlucin and perlustrin, two new proteins from the shell of the mollusc Haliotis laevigata. Biochem Biophys Res Commun 267:17–21
Weiss IM, Schönitzer V (2006) The distribution of chitin in larval shells of the bivalve mollusk Mytilus galloprovincialis. J Struct Biol 153:264–277
Weiss IM, Schönitzer V, Eichner N, Sumper M (2006) The chitin synthase involved in marine bivalve mollusk shell formation contains a myosin domain. FEBS Lett 580:1846–1852
Westbroek P, Marin F (1998) A marriage of bone and nacre. Nature 392:861–862
Yan Z, Jing G, Gong N et al (2007) N40, a novel nonacidic matrix protein from pearl oyster nacre, facilitates nucleation of aragonite in vitro. Biomacromol 8:3597–3601
Yano M, Nagai K, Morimoto K, Miyamoto H (2007) A novel nacre protein N19 in the pearl oyster Pinctada fucata. Biochem Biophys Res Commun 362:158–163
Zhang G (2017) Tests des composés de nacre sur l’activité des ostéoblastes et leur identification
Zhang G, Brion A, Willemin A-S et al (2017) Nacre, a natural, multi-use, and timely biomaterial for bone graft substitution. J Biomed Mater Res 105:662–671
Zhang G, Willemin AS, Brion A et al (2016) A new method for the separation and purification of the osteogenic compounds of nacre ethanol soluble matrix. J Struct Biol 196:127–137
Funding
D.I. acknowledges the financial support by project IDEXLYON of the University of Lyon in the frame of the “Programne Investissements D’Avenir” (ANR-16-IDEX-0005, IDEX/ELAN/2019/01). The Agence Nationale de la Recherche awards the ANR JCJC 2018 project called NADO for financial support (N°ANR-18-CE18-0003–01).
Author information
Authors and Affiliations
Contributions
C.J., A.A., and M.R.: writing—original draft preparation; C.J., D.I., K.N., A.A., and M.R.: writing—review and editing; M.R.: idea and project administration. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
M.R. is one of the co-founders of the company Stansea that was created in 2013 and that commercialize nacre.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Muizon, C.J., Iandolo, D., Nguyen, D.K. et al. Organic Matrix and Secondary Metabolites in Nacre. Mar Biotechnol 24, 831–842 (2022). https://doi.org/10.1007/s10126-022-10145-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10126-022-10145-9