Abstract
High-pH tolerance and growth are important traits for the shrimp culture industry in areas with saline-alkali water. In the present study, an F1 full-sib family of Pacific white shrimp (Litopenaeus vannamei) was generated with a new “semidirectional cross” method, and double-digest restriction site-associated DNA sequencing (ddRAD-Seq) technology was applied to genotype the 2 parents and 148 progenies. A total of 3567 high-quality markers were constructed for the genetic linkage map, and the total map length was 4161.555 centimorgans (cM), showing 48 linkage groups (LGs) with an average interlocus length of 1.167 cM. With a constrained logarithm of odds (LOD) score ≥ 2.50, 12 high-pH tolerance and 2 growth (body weight) QTLs were located. L. vannamei genomic scaffolds were used to assist with the detection of 21 stress- and 5 growth-related scaffold genes. According to the high-pH transcriptome data of our previous study, 6 candidate high-pH response genes were discovered, and 5 of these 6 genes were consistently expressed with the high-pH transcriptome data, validating the locations of the high-pH tolerance trait-related QTLs in this study. This paper is the first report of fine-mapping high-pH tolerance and growth (body weight) trait QTLs in one L. vannamei genetic map. Our results will further benefit marker-assisted selection work and might be useful for promoting genomic research on the shrimp L. vannamei.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Pacific white shrimp, Litopenaeus vannamei (L. vannamei), is famous worldwide as a high-quality food in the human diet (FAO 2012). With the high economic value and good stress resistance of this species (Li et al. 2007, Wang et al. 2013a, b), L. vannamei has become one of the most important aquatic species for culturing, and L. vannamei farming areas have been widely introduced in nonnative environments worldwide (Huang et al. 2018). Total shrimp production reached 4 million tons, and the total output value achieved 24 billion US dollars in 2016 (Yu et al. 2019). Advances in shrimp breeding projects might further promote the high economic value of the shrimp culture industry.
Soil salinization significantly impacts crop yields and poses a threat to regional human life; therefore, the strategy of reusing and transferring saline-alkali lands as cultivated resources is becoming imperative (Li et al. 2014; Wang et al. 2017; Huang et al. 2018). The development of aquaculture industries in saline-alkali water areas, including inland lakes, rivers, and marine coasts in China, has been thought to be an effective method for taking full advantage of these territorial resources (Liang et al. 2013; Liu et al. 2016) and has economic (production of saline-alkali water reached USD$1.14 million) (Liu et al. 2016) and environmental (the top pH value of the saline-alkali lands dropped from 9.01 to 8.56) (Xue 2018) value in the Hebei and Shaanxi Provinces of China. L. vannamei is one of the most important aquatic species for culturing in saline-alkali water areas in China (Luan et al. 2003; Liu et al. 2008; Zhang 2016). However, compared with the farming of L. vannamei under normal seawater conditions, the immunity and production of the shrimp might be weakened when the species is cultured in high-pH environments (Li and Chen 2008; Wang et al. 2009; Huang et al. 2018). The high-pH tolerance trait of the shrimp has become an important economic trait for the breeding and culturing industries.
The development of marker-assisted selection (MAS) has substantially accelerated genetic breeding work (Yue 2014; Abdelrahman ElHady et al. 2017; Li et al. 2017; Yu et al. 2019; Li et al. 2019). Most targeted traits of species are governed by multiple genes or loci, and quantitative trait loci (QTLs) mapping with dense genetic linkage maps is employed to reveal the locations of the trait-related genes (Yue 2014; Das et al. 2015; Wan et al. 2017; Li et al. 2017; Kong et al. 2019; Li et al. 2019). Single nucleotide polymorphism (SNP) markers, which represent the most abundant source of variation in the genome, are increasingly utilized for the construction of high-density genetic linkage maps (Lien et al. 2011; Shao et al. 2015; Wan et al. 2017). High-resolution genetic maps with 12,712 high-confidence SNPs and 24 consensus linkage groups (LGs) were constructed for the Japanese flounder (Paralichthys olivaceus, P. olivaceus), and 9 positive QTLs and 4 major genes for Vibrio anguillarum disease resistance were detected (Shao et al. 2015). Yu et al. (2015) used a total of 6146 high-quality SNP markers for QTL mapping, and an average marker distance of 0.7 cM was obtained for the linkage map (Yu et al. 2015).
With the development of next generation sequencing (NGS) technology, high-throughput marker development and genetic map construction has become possible to finely map trait-related QTLs (Shao et al. 2015, Wan et al. 2017). In recent years, many advanced methods for mapping trait-related QTLs have been developed, such as genome-wide association studies (GWAS) (Abdelrahman ElHady et al. 2017), expression quantitative trait loci (eQTLs) (Imprialou et al. 2017), specific-locus amplified fragment sequencing (SLAF) (Miller et al. 2007), genotyping-by-sequencing (GBS) (Baird et al. 2008), and restriction site-associated DNA sequencing (RAD-seq) (Sun et al. 2013). In particular, as a reliable, affordable method to reduce genomic complexity, RAD-Seq has been very useful for SNP discovery and genotyping (Berthier-Schaad et al. 2007; Rowe et al. 2011; Wang et al. 2012; Shao et al. 2015; Fu et al. 2016; Wan et al. 2017). RAD-Seq technology has now been applied for the genetic study of various aquatic species, such as P. olivaceus (Shao et al. 2015), Hypophthalmichthys nobilis (Fu et al. 2016), Megalobrama amblycephala (Wan et al. 2017), Trachinotus blochii (Zhang et al. 2018), Larimichthys crocea (Kong et al. 2019), and Oreochromis spp. (Li et al. 2017; Li et al. 2019).
In previous studies on L. vannamei, the genetic mechanism of growth traits has been elucidated (Andriantahina et al. 2013; Yu et al. 2015, Yu et al. 2019), but few genes have been identified in association with growth traits (Yu et al. 2019). Further genetic studies on growth-related traits in this species are still needed. The high-pH tolerance trait of the shrimp is becoming an important economic trait for industrial breeding and culturing in saline-alkali water areas; however, the stress response QTLs are still unknown. In the present work, double-digest restriction site-associated DNA sequencing (ddRAD-Seq) technology was applied to genotype an F1 full-sib family of L. vannamei, and a high-resolution genetic map was constructed with high-quality SNP markers. High-pH tolerance and growth (body weight) trait-related QTLs were detected, high-pH response and growth-related candidate genes were discovered, and real-time PCR was carried out to validate the candidate high-pH response genes. Our results will further benefit MAS work and might be useful for promoting genomic research on the shrimp L. vannamei.
Materials and Methods
Shrimp Full-Sib Family Production
An F1 full-sib shrimp family was produced by inner species crossing at JingYang Tropical Biology Co., LTD in Maoming, Guangdong, China (August, 2017). The female shrimp were selected from the inbred line called “L. vannamei ZhongKe No.1” (a shrimp variety in China, variety registration no. GS-01-007-2010), and the candidate male shrimps were obtained from a commercial shrimp termed “Zheng Da.” According to the propagation characteristic (open thelycum) of L. vannamei, mating a single mature male shrimp with a mature female was difficult, and a full-sib shrimp family was thus produced with a new “semidirectional cross” method according to the following steps. A fertile female shrimp (containing yellow shrimp eggs) (Supplementary Fig. 1a) was placed in a seawater mating pond (5 m2, 1 m height) that contained 7–15 fertile male shrimps, whose spermatophores were packed with white spermatia (Supplementary Fig. 1b). The male shrimps in the mating pond took turns to chasing the female until mating occurred, then, the female shrimp whose thelycum contained the white spermatia (Supplementary Fig. 1c) was removed. The mated female shrimp was reared in an isolation tank (round, 70 cm in height, volume ≤ 1000 L) for spawning and served as the female parent of the F1 full-sib family. The male parent of the F1 full-sib family was visually determined by determining based on which male showed an empty spermatophore (without packed spermatium) (Supplementary Fig. 1b, d), as naturally, only one male shrimp could mate with the female shrimp. The F1 full-sib progenies were first nursed in the tank for 15 days, and the full-sib shrimps were then moved to an indoor culturing pond (5.0 m in length, 6.0 m in width, and 1.2 m in height) for further rearing.
High-pH Challenges and Preparation of the Shrimp Samples
After being reared in the culturing pond for 40 days, 35 of the full-sib progenies were randomly selected and treated in a high-pH gradient environment (gradient pH values of 8.1, 8.3, 8.5, 8.7, 8.9, 9.1, 9.3, 9.5, and 9.7) for 48 h. The pH values were maintained with Na2CO3 and NaHCO3 solutions according to the description by Huang et al. (2018). The survival statuses of the shrimp were evaluated by observing their ability to move spontaneously or after gentle prodding (Huang et al. 2017). The dead individuals were collected, and the endurance times were recorded for each pH level. The high-pH stress was determined by evaluating the number of survivors in each high-pH treatment group.
A total of 148 full-sib progenies were randomly selected from nearly 5000 whole full-sib family numbers in the rearing pond and treated in the stress high-pH environments. Afterward, the dead shrimps were collected and weighed. The data regarding the endurance times under the stress of the high-pH environment (high-pH tolerance trait, HP) and body weight (body weight, growth trait, BW) were collected. The muscle tissues of 2 parents and 148 offspring were separated for the extraction of genomic DNA, and the experiments were conducted with the TIANGEN Marine Animal DNA extraction kit (TIANGEN, Beijing, China) according to the manufacture’s protocol. The concentration of total DNA was determined with a NanoDrop 1000 spectrophotometer (NanoDrop, Wilmington, DE, USA), and the quality of each DNA sample was evaluated by gel electrophoresis (Yu et al. 2015).
RAD Library Construction and Sequencing
Genomic DNA from 2 parents and 148 offspring was used to construct the 150 L. vannamei ddRAD libraries (October 2017) using protocols described by Peterson et al. (2012) and Sun et al. (2017). Briefly, the DNA from each shrimp sample was double-digested using the restriction enzymes EcoRI and NlaIII (New England Biolabs, Ipswich, MA, USA) (Yu et al. 2015). Then, the digested samples were purified using a Qiagen MinElute Reaction Cleanup Kit (Qiagen, Valencia, CA, USA). The fragments were then ligated to adapters that included unique 4- to 8-bp multiplex identifiers (MIDs) that were used to distinguish each individual. The samples were pooled, size-selected (400 to 600 bp) on an agarose gel and subsequently purified with a Qiagen MinElute Gel Purification Kit. The paired-end (150 bp) sequencing of the ddRAD products was performed on an Illumina HiSeq 2500 platform (Illumina, Inc., San Diego, CA, USA).
SNP Discovery and Genotyping
The Illumina short reads that lacked the sample-specific MIDs and the expected restriction enzyme motifs were discarded. The raw data were then filtered using Trimmomatic software (v0.32) (Bolger et al. 2014) in three steps: removal of adapters; removal of reads with bases from the start or end of a read with a quality threshold below 3; and scanning reads with a 4-bp sliding window, removing those with an average Phred quality per base below 20 (Sun et al. 2017). The STACKS (v1.41) (Catchen et al. 2013) pipeline was employed for de novo assembly of the loci and for SNP discovery. The Ustacks program was used to cluster the enzyme cutting sequences of each individual, and the Cstacks program was applied to identify the congruent loci and make the alleles in the parental data uniform. The Sstacks program was utilized to compare the progeny and parent loci, confirming the genotype of each progeny locus; and the Genotype program was utilized to proofread the genotype data. The following parameters were used:
-
Ustacks: -t gzfastq -i -m 3 -M 9 -p 15 -d -r –f –o
-
Cstacks: -b 1 –o –s –n 2 –p 15
-
Sstacks: -b 1 –c –p 15
-
Genotype: -b 1 –P -r 1 -c -s -t CP
The miss rates (number of samples with no genotype information/number of total samples) were less than 10%, and biallelic SNPs were used to avoid sequencing errors and to advance the segregation analysis (Sun et al. 2017; Lu et al. 2016). The sequencing depth of the SNP loci was no less than 5.
Linkage Map Construction
Only biallelic SNPs were introduced to construct the genetic linkage map, and the SNP genotypes were defined as follows: (1) SNPs that were heterozygous in the maternal parent (lm) and homozygous in the paternal parent (ll); (2) SNPs that were heterozygous in the paternal parent (np) and homozygous in the maternal parent (nn); and (3) SNPs that were heterozygous in the maternal and paternal parent (hk) (Lu et al. 2016). Biallelic SNPs that presented significant segregation distortion in the χ2 goodness-of-fit tests (chi-square test, P < 0.05) were also eliminated in the linkage analysis (Liu et al. 2017). LG assignments were conducted with JoinMap 4.1 software (Stam 1993) using a logarithm of odds (LOD) score ≥ 6.0. A pseudo-testcross strategy (a mapping population was developed by hybridizing two unrelated highly heterozygous parents to produce a set of F1 progeny) was utilized to construct the linkage map (Shao et al. 2015; Sun et al. 2017). The regression mapping algorithm and Kosambi’s mapping function were used for map construction with the following settings: Rec = 0.4, LOD = 1.0, and Jump = 5. The genetic distance of 30 cM was set as the longest gap between two markers in one LG. The resulting linkage maps were drawn using R software (version of R x64 3.5.2, https://www.r-project.org/) with the package LinkageMapView (Ouellette et al. 2018).
QTL Mapping
QTL mapping of the HP and BW traits was performed with MapQTL 5.0 software (Van Ooijen 2011). The Multiple QTL Mapping (MQM) program was used to detect the QTL region and calculate the percentage of explained phenotypic variance (Jansen and Stam 1994); an LOD ≥ 2.50 was set for the trait-related QTL regions. A mapping step size of 1 cM and five neighboring markers were used in the QTL analysis (Louro et al. 2016; Liu et al. 2017). The genome-wide LOD threshold (significance level) or group-wide LOD threshold (suggestive level) was determined in MapQTL5.0 by a Permutation Test on the basis of 1000 permutations with a confidence interval of 95% (Piepho 2001; Lu et al. 2016).
Identification of the Candidate High-pH Response and Growth-Related Genes
To further reveal the high-pH response and growth-related genes, the tag sequences of the HP and BW QTL markers were searched in the Pacific white shrimp genome database (GenBank no. QCYY00000000.1), and the aligned shrimp genome scaffolds were identified. The genes contained in the genome scaffolds (called the scaffold genes) were obtained, and the stress- and growth-related scaffold genes were annotated and summarized according to previous studies.
All the stress-related scaffold genes were compared with the high-pH transcriptome data from our previous work (S7 Table in Huang et al. 2018), and the coexisting candidate genes for high-pH response were discovered. To verify the mRNA expression patterns of the candidate high-pH response genes, healthy cultured shrimp (supplied by Yuehai Feed Group Co., LTD, Zhanjiang, China; average weight, 11.09 ± 2.37 g) were selected, and the three shrimp groups were treated in pH 8.0 seawater (control pH), pH 9.0 seawater, and pH 9.3 seawater. The high-pH values of the seawater were maintained as described previously (Wang et al. 2009; Huang et al. 2018). Total RNA was extracted from the posttreated gill tissues of the shrimp at 0, 1, 6, 12, 24, and 48 h. Real-time PCR analysis was carried out using the primers listed in Supplementary Table 1. The relative expression levels of the candidate genes were obtained according to the 2−ΔΔCT method by normalizing to the expression of the L. vannamei β-actin gene (Livak and Schmittgen 2001).
Results
Summary of the Data
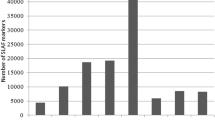
The high-pH challenge data were analyzed. In total, 21 shrimps (survival rate of 60.0%) survived after treatment in the high-pH environment of 9.1 for 48 h, 3 (survival rate of 8.6%) survived after treatment in the high-pH environment of 9.3 for 48 h, and all died after treatment in a high-pH environment of 9.5 for 12 h (Fig. 1a).
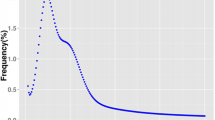
Information on the experimental and RAD-Seq libraries. a High-pH challenges in gradient high-pH environments for 48 h; the high-pH stress condition of the full-sib family is indicated. b Summary of the high-pH tolerance data of the full-sib offspring. c Summary of the body weight data of the full-sib offspring. d Quality control of the RAD-Seq libraries. The X axes represent the base position along the reads. The Y axes represent the base content percentages. e Summary of the SNP markers; high-quality markers are enclosed in a box
The high-pH value of 9.3 was set as the stress environment, and 148 progenies were treated in pH 9.3 seawater; with the ability or inability of the shrimp to resist a high-pH environment, 9 different stages were determined (Fig. 1b). Nine individuals (6.08%) died in the first stage (0–2 h), and 11 (7.43%) progenies survived beyond 48 h of treatment (Fig. 1b). The body weights were immediately obtained when the shrimp were dying under the pH 9.3 stress condition; the minimum shrimp body weight was 0.048 g, the maximum shrimp body weight was 1.100 g, the mean body weight was 0.319 ± 0.211 g, and the body weight values of the shrimp individuals were continuous (Fig. 1c).
Sequencing and Genotyping
A total of 150 ddRAD libraries were constructed from the two parents and their 148 offspring. The mean value of the GC% in the offspring was 43.67 ± 0.73% (Supplementary Table 2). The adenine bases (A) nearly overlapped the thymine bases (T), and the guanine bases (G) nearly overlapped the cytosine bases (C) (Fig. 1d, Supplementary Table 2). After filtering, the female parent had 2.17 Gb of clean data (GC rate of 42.16% and a Q30 rate of 90.08%), and the male parent had 2.58 Gb of clean data (GC rate of 43.21% and Q30 rate of 90.18%). The clean reads of the progenies ranged from 5.42 to 27.97 million, and the clean reads of the offspring ranged from 0.80 to 4.07 Gb (average GC rate of 43.61 ± 0.73 and mean Q30 value of 89.54 ± 1.12) (Supplementary Table 3). All clean data were deposited into the US National Center for Biotechnology Information (NCBI) Sequence Read Archive (SRA, http://www.ncbi.nlm.nih.gov/Traces/sra) under GenBank accession no. PRJNA532795. A total of 10,465 markers were detected from more than 90% of the progenies (Fig. 1e), and after a Mendelian fit test (P ≥ 0.05), 3894 high-quality markers (three types of markers including 2446 “lm × ll” markers, 1308 “nn × np” markers, and 140 hk × hk markers) were used to construct the consensus genetic map (Fig. 1e).
Construction of Linkage Maps
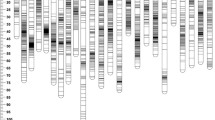
A ddRAD-based linkage map of L. vannamei was constructed with high-quality markers. The LGs containing fewer than 15 markers were discarded, and the final integrated map consisted of 48 LGs, including 3567 high-quality segregating SNP markers (327 high-quality markers were discarded or not located) (Table 1, Fig. 2). The total map length was 4161.555 cM, the average interlocus length was 1.167 cM, the genetic length of the LGs ranged from 14.612 cM (LG48) to 165.922 cM (LG 1), and the average interval length of the LGs ranged from 0.393 cM (LG 43) to 3.210 cM (LG 30) (Table 1, Fig. 2). Detailed information, including the marker names, tag sequences, and positions of the LGs, is provided in Supplementary Tables 4–5.
QTL Analysis of High-pH Tolerance and Growth Traits
For the HP trait, 12 QTLs were discovered (LOD value > 2.5) (Table 2) and were distributed in 5 different LGs (LG 4, LG 13, LG 18, LG 26, and LG 36) (Figs. 3 and 4). The most prominent QTL was HP-3, with an LOD value of 3.47; this QTL was 4.660 cM in length and explained 65.2% of the phenotypic variation. The detailed QTL information for the HP trait is available in Supplementary Table 4.
Genetic locations of high-pH tolerance-related and growth-related (body weight) QTLs on 48 LGs of L. vannamei. The red lines indicate the logarithm of odds (LOD) value of the high-pH trait. The green lines indicate the logarithm of odds (LOD) value of the body weight trait. The dashed lines indicate a cutting threshold of LOD at 2.5
For the BW trait, 2 QTLs were detected (LOD value > 2.50) (Table 2), and the BW QTLs were located in LG 1 (Figs. 3 and 4). BW-2 was the major QTL (interval length of 0.933 cM), and it had an LOD value of 2.92 and explained 10.3% of the phenotypic variation (Table 2). The major BW-related genes might be located between the SNP markers of R1_309702 and R1_418289. Another BW QTL is listed in Table 2 and Fig. 4, and the detailed QTL information for the BW trait is provided in Supplementary Table 5.
Identification of the High-pH Response Gene Candidates
In total 73 HP and BW QTL markers were aligned to 46 L. vannamei scaffolds (Table 3). In the HP-2 QTL, the marker “R1_60745,” with an LOD value of 2.68 in LG4, was aligned to the L. vannamei scaffold “LVANscaffold_2027,” and the marker “R1_247764” in this QTL was aligned to the same L. vannamei scaffold, indicating a close genetic distance between the two markers (Table 3).
The stress-related genes contained in these L. vannamei scaffolds were summarized according to previous studies (Table 4). The expression levels of the stress-related genes were investigated using high-pH transcriptome data from our previous work (Huang et al. 2018), and six candidate high-pH response genes were discovered: hypoxia inducible factor 1 beta (HIF1b), transcription initiation factor TFIID subunit 3 (TAF3), glutamyl aminopeptidase (ENPEP), discoidin domain-containing receptor 2-like (DDR2), phosphatidylcholine: ceramide cholinephosphotransferase 1-like (SGMS1) and histone H2A.V-like (H2AFV) (Table 4). With the same method, 5 growth-related genes were obtained (Table 5).
To further verify the results in this study, real-time PCR was carried out to identify the expression patterns of the high-pH response genes. The expression levels of the 6 candidate genes were significantly influenced by the high-pH treatment, and 5 (all except the ENPEP gene) of the 6 candidate genes were consistent with the high-pH transcriptome data (Fig. 5). Specifically, the HIF1b and SGMS1 genes were significantly upregulated for 6 h in the high-pH environment, and TAF3 and DDR2 were obviously downregulated in the first 6 h in response to the high-pH stress. The H2AFV gene was downregulated in the first few hours but was then substantially upregulated in the following hours in response to high-pH stress, and the results validate the candidate high-pH response genes (Fig. 5).
Transcriptional expression of the candidate high-pH response genes. a Expression pattern of the gene “hypoxia inducible factor 1 beta (HIF1b)”. b Expression pattern of the gene “transcription initiation factor TFIID subunit 3 (TAF3)”. c Expression pattern of the gene “glutamyl aminopeptidase (ENPEP)”. d Expression pattern of the gene “discoidin domain-containing receptor 2-like (DDR2)”. e Expression pattern of the gene “phosphatidylcholine: ceramide cholinephosphotransferase 1-like (SGMS1)”. f Expression pattern of the gene “histone H2A.V-like (H2AFV)”. Gill tissues of L. vannamei treated in high-pH environment for 0, 1, 6, 12, 24, and 48 h. The data are presented as the mean ± SE (n = 6), and the groups denoted by the same letter exhibit a similar expression levels (P > 0.05, two-way ANOVA followed by Fisher’s LSD test). Dotted lines indicate the expression pattern of the high-pH transcriptome data (the shrimp were treated in the pH 9.3 environment for 1 and 48 h) from our previous work (Huang et al. 2018)
Discussion
Full-sib families provide basic materials for genetic breeding programs in aquatic species, and they are particularly important for mapping trait-related QTLs based on RAD-Seq technologies. For example, F1 full-sib families (including the two parents) have been generated and used to build ddRAD libraries in several aquatic species, such as Oreochromis niloticus (Li et al. 2017), Trachinotus blochii (Zhang et al. 2018), and Larimichthys crocea (Kong et al. 2019). Generally, the F1 full-sib families were constructed with methods of directional crossing (a pair of parents for mating) (Fu et al. 2016; Li et al. 2017; Kong et al. 2019) or undirectional crossing (several or more pairs of parents for mating) (Shao et al. 2015). According to the propagation characteristic of the shrimp L. vannamei, mating a pair of fertile shrimp is difficult to achieve in nature. Yu et al. (2015, 2019) used the “undirectional cross” method to create four combined full-sib L. vannamei families and then used 10 microsatellite loci to select the mapping family; an F1 full-sib family of 205 progenies was constructed, and their parental genomic DNA was identified (Alcivar-Warren et al. 2007; Yu et al. 2015; Yu et al. 2019). In the present study, the “semidirectional cross” method was used to generate the L. vannamei F1 full-sib family, with 148 progenies and 2 parental individuals (not just the parental genomic DNA) of the mapping family being visually identified. This method may be a quick way to generate full-sib families, which would be beneficial for genetic research on the shrimp L. vannamei.
SNP markers are ideal for the construction of genetic linkage maps (Bourgeois et al. 2013; Stölting et al. 2013; Wang et al. 2013a, 2013b; Zhang et al. 2018). SNPs are the most common type of DNA polymorphism in the genome, have a low mutation rate and high genetic stability, and are amenable to high-throughput genotyping (Berthier-Schaad et al. 2007; Shao et al. 2015; Zhang et al. 2018; Kong et al. 2019). In total, 3567 high-quality SNP markers were constructed for the linkage map in the present work. The number of SNPs identified was less than that identified in the previous study on L. vannamei by Yu et al. (2015) (6359 SNPs were selected for mapping) but more than that identified in the study on Hypophthalmichthys nobilis by Fu et al. (2016) (3121 SNPs were used for mapping). Genetic linkage maps constructed by SNP markers are important resources for various genetic studies, including comparative genomics, functional gene mapping, candidate gene positional cloning, and genome assembly (Yue 2014; Xu et al. 2014; Kujur et al. 2015; Zhang et al. 2018; Kong et al. 2019). In total, 48 LGs with a total map length of 4161.555 cM and an average interlocus length of 1.167 cM were obtained in the present study. The number of LGs was close to those obtained in previous studies; examples include 44 pseudochromosomes (Zhang et al. 2019), 44 sex-averaged LGs (Yu et al. 2015), and 45 sex-averaged LGs (Du et al. 2010). Our results might be useful for future genomic assembly research on the shrimp L. vannamei.
With the help of a high-resolution genetic map, fine mapping of QTLs is an efficient approach for identifying genetic loci and the candidate genes underlying these quantitative traits of interest (Shao et al. 2015; Wang et al. 2018). Growth is a priority trait for genetic improvement, and mapping of growth-related QTLs has been widely performed in studies on various aquatic species (Andriantahina et al. 2013, Yue 2014, Tong and Sun 2015, Wang et al. 2018). In the present work, 2 growth-related (body weight) QTLs were detected, both of which were located in LG1 of the genetic map. Several growth-related QTL studies have been previously performed on L. vannamei. For example, Andriantahina et al. (2013) used amplified fragment length polymorphism (AFLP) and simple sequence repeat (SSR) markers to construct a genetic map, and 14 growth-related QTLs were identified (Andriantahina et al. 2013). Yu et al. (2015) detected several QTLs for body weight and body length based on a high-density linkage map (Yu et al. 2015); however, candidate gene associations with the growth trait were not identified due to limited genomic information (Yu et al. 2019). In the present study, 2 growth-related QTLs were detected in 55-day-old shrimp using L. vannamei genomic scaffolds (Zhang et al. 2019), and 5 growth-related candidate genes were detected and demonstrated to be associated with cell growth or energy metabolism. The gene “putative ATP-dependent RNA helicase DDX43” of BW-1 in this paper is thought to be required for tumor growth through the promotion of RAS protein expression (Linley et al. 2012). The Ras-related protein Rap-2a was identified as being significantly associated with the growth trait by applying GWAS in two independent populations of L. vannamei (Yu et al. 2019) and was discovered to be associated with head size regulation in catfish (Ictalurus punctatus) (Geng et al. 2016). The detection of the 5 candidate genes in this study might be relevant to the growth trait, the Ras-related proteins might be associated with the growth trait of L. vannamei at 55 days of age, and the location of the 2 growth-related QTLs (BW-1 and BW-2) in this study might be valid.
The balance of acidic-alkaline ions in water is important for aquatic crustaceans (Fehsenfeld et al. 2011; Roggatz et al. 2016; de Vries et al. 2016; Wu et al. 2017; Huang et al. 2018). The farming of L. vannamei in high-pH environments might affect the final production (Huang et al. 2018), and the high-pH tolerance of shrimp has become an important trait in the culturing industry in saline-alkali water areas. In the present study, the first genetic study on the high-pH tolerance trait for L. vannamei, 12 high-pH tolerance QTLs were discovered. With the development of genomic research on L. vannamei (Zhang et al. 2019), tag sequences of the SNP markers are now available that align with the genomic scaffolds of the shrimp; in the present study, 21 stress-related genes were summarized according to the annotation of the scaffold genes. Our previous work revealed the molecular basis of response to high-pH stress by analyzing L. vannamei transcriptome data (Huang et al. 2018); and we identified 6 candidate high-pH response genes based on RNA- and RAD-Seq results. The expression patterns of the 6 candidate genes were demonstrated to be significantly influenced by the high-pH environment, with 5 of the 6 candidate high-pH response genes showing results consistent with those of the RNA-Seq, indicating the important roles of those genes in response to high-pH stress and validating the locations of the HP QTLs for L. vannamei in this study.
In conclusion, a “semidirectional cross” method was used to generate the L. vannamei full-sib family in the present study. High-pH tolerance and growth trait-related QTLs were detected in one genetic map by ddRAD-Seq, and candidate genes associated with the high-pH tolerance and growth traits were discovered by alignment them with the L. vannamei genomic scaffolds and analysis of high-pH transcriptome data. We herein provide baseline data and the first report of shrimp breeding to induce high-pH tolerance traits. Our results will be beneficial for further MAS work and might be useful for promoting genomic research on the shrimp L. vannamei.
References
Abdelrahman ElHady M, Alcivar-Warren A, Allen S, Al-Tobasei R, Bao L, Beck B, Blackburn H, Bosworth B, Buchanan J, Chappell J, Daniels W, Dong S, Dunham R, Durland E, Elaswad A, Gomez-Chiarri M, Gosh K, Guo X, Hackett P, Hanson T, Hedgecock D, Howard T, Holland L, Jackson M, Jin Y, Khalil K, Kocher T, Leeds T, Li N, Lindsey L, Liu S, Liu Z, Martin K, Novriadi R, Odin R, Palti Y, Peatman E, Proestou D, Qin G, Reading B, Rexroad C, Roberts S, Salem M, Severin A, Shi H, Shoemaker C, Stiles S, Tan S, Tang KF, Thongda W, Tiersch T, Tomasso J, Prabowo WT, Vallejo R, van der Steen H, Vo K, Waldbieser G, Wang H, Wang X, Xiang J, Yang Y, Yant R, Yuan Z, Zeng Q, Zhou T (2017) Aquaculture genomics, genetics and breeding in the United States: current status, challenges, and priorities for future research. BMC Genomics 18:191
Adelman K, Marr MT, Werner J, Saunders A, Ni Z, Andrulis ED, Lis JT (2005) Efficient release from promoter-proximal stall sites requires transcript cleavage factor TFIIS. Mol Cell 17:103–112
Alcivar-Warren A, Meehan-Meola D, Park SW, Xu Z, Delaney M, Zuniga G (2007) ShrimpMap: a low-density, microsatellite-based linkage map of the pacific whiteleg shrimp, Litopenaeus vannamei: identification of sex-linked markers in linkage group. J Shellfish Res 26:1259–1277
Andriantahina F, Liu XL, Huang H (2013) Genetic map construction and quantitative trait locus (QTL) detection of growth-related traits in Litopenaeus vannamei for selective breeding applications. PLoS One 8:e75206
Baird NA, Etter PD, Atwood TS, Currey MC, Shiver AL, Lewis ZA, Selker EU, Cresko WA, Johnson EA (2008) Rapid SNP discovery and genetic mapping using sequenced RAD markers. PLoS One 3:e3376
Berthier-Schaad Y, Kao WH, Coresh J, Zhang L, Ingersoll RG, Stephens R, Smith MW (2007) Reliability of high-throughput genotyping of whole genome amplified DNA in SNP genotyping studies. Electrophoresis 28:2812–2817
Bolger AM, Lohse M, Usadel B (2014) Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30:2114–2120
Bourgeois YX, Lhuillier E, Cézard T, Bertrand JA, Delahaie B, Cornuault J, Duval T, Bouchez O, Milá B, Thébaud C (2013) Mass production of SNP markers in a nonmodel passerine bird through RAD sequencing and contig mapping to the zebra finch genome. Mol Ecol Resour 13:899–907
Blagoev B, Ong SE, Kratchmarova I, Mann M (2004) Temporal analysis of phosphotyrosine-dependent signaling networks by quantitative proteomics. Nat Biotechnol 22:1139–1145
Catchen J, Hohenlohe PA, Bassham S, Amores A, Cresko WA (2013) Stacks: an analysis tool set for population genomics. Mol Ecol 22:3124–3140
Carney JP, Maser RS, Olivares H, Davis EM, Le Beau M, Yates JR 3rd, Hays L, Morgan WF, Petrini JH (1998) The hMre11/hRad50 protein complex and Nijmegen breakage syndrome: linkage of double-strand break repair to the cellular DNA damage response. Cell 93:477–486
Choi SH, Chung AR, Kang W, Park JY, Lee MS, Hwang SW, Kim DY, Kim SU, Ahn SH, Kim S, Han KH (2014) Silencing of hypoxia-inducible factor-1β induces anti-tumor effects in hepatoma cell lines under tumor hypoxia. PLoS One 9:e103304
Chuang SM, Madura K (2005) Saccharomyces cerevisiae Ub-conjugating enzyme Ubc4 binds the proteasome in the presence of translationally damaged proteins. Genetics 171:1477–1484
Cross TG, Scheel-Toellner D, Henriquez NV, Deacon E, Salmon M, Lord JM (2000) Serine/threonine protein kinases and apoptosis. Exp Cell Res 256:34–41
Dai X, Wang T, Gonzalez G, Wang Y (2018) Identification of YTH domain-containing proteins as the readers for N1-methyladenosine in RNA. Anal Chem 90:6380–6384
Das S, Upadhyaya HD, Bajaj D, Kujur A, Badoni S, Laxmi KV, Tripathi S, Gowda CL, Sharma S, Singh S, Tyagi AK, Parida SK (2015) Deploying QTL-seq for rapid delineation of a potential candidate gene underlying major trait-associated QTL in chickpea. DNA Res 22:193–203
de Vries MS, Webb SJ, Tu J, Cory E, Morgan V, Sah RL, Deheyn DD, Taylor JR (2016) Stress physiology and weapon integrity of intertidal mantis shrimp under future ocean conditions. Sci Rep 6:38637
Deng CX, Brodie SG (2000) Roles of BRCA1 and its interacting proteins. Bioessays 22:728–737
Du ZQ, Ciobanu DC, Onteru SK, Gorbach D, Mileham AJ, Jaramillo G, Rothschild MF (2010) A gene-based SNP linkage map for pacific white shrimp, Litopenaeus vannamei. Anim Genet 41:286–294
FAO (2012) Fisheries and Aquaculture Department: the state of world fisheries and aquaculture. Food and Agriculture Organization of the United Nations, Rome, Italy, p 37
Fehsenfeld S, Kiko R, Appelhans Y, Towle D, Zimmer M, Melzner F (2011) Effects of elevated seawater pCO2 on gene expression patterns in the gills of the green crab, Carcinus maenas. BMC Genomics 12:488
Fu B, Liu H, Yu X, Tong J (2016) A high-density genetic map and growth related QTL mapping in bighead carp (Hypophthalmichthys nobilis). Sci Rep 6:28679
Geng X, Liu S, Yao J, Bao L, Zhang J, Li C, Wang R, Sha J, Zeng P, Zhi D, Liu Z (2016) A genome-wide association study identifies multiple regions associated with head size in catfish. G3 (Bethesda) 6:3389–3398
Goto Y, Hattori A, Ishii Y, Mizutani S, Tsujimoto M (2006) Enzymatic properties of human aminopeptidase a. regulation of its enzymatic activity by calcium and angiotensin IV. J Biol Chem 281:23503–23513
Groisman R, Polanowska J, Kuraoka I, Sawada J, Saijo M, Drapkin R, Kisselev AF, Tanaka K, Nakatani Y (2003) The ubiquitin ligase activity in the DDB2 and CSA complexes is differentially regulated by the COP9 signalosome in response to DNA damage. Cell 113:357–367
Horne I, Haritos VS, Oakeshott JG (2009) Comparative and functional genomics of lipases in holometabolous insects. Insect Biochem Mol Biol 39:547–567
Huang W, Ren C, Li H, Huo D, Wang Y, Jiang X, Tian Y, Luo P, Chen T, Hu C (2017) Transcriptomic analyses on muscle tissues of Litopenaeus vannamei provide the first profile insight into the response to low temperature stress. PLoS One 12:e0178604
Huang W, Li H, Cheng C, Ren C, Chen T, Jiang X, Cheng K, Luo P, Hu C (2018) Analysis of the transcriptome data in Litopenaeus vannamei reveals the immune basis and predicts the hub regulation-genes in response to high-pH stress. PLoS One 13:e0207771
Ichikawa Y, Bayeva M, Ghanefar M, Potini V, Sun L, Mutharasan RK, Wu R, Khechaduri A, Jairaj Naik T, Ardehali H (2012) Disruption of ATP-binding cassette B8 in mice leads to cardiomyopathy through a decrease in mitochondrial iron export. Proc Natl Acad Sci U S A 109:4152–4157
Imprialou M, Petretto E, Bottolo L (2017) Expression QTLs mapping and analysis: a Bayesian perspective. Methods Mol Biol 1488:189–215
Jansen RC, Stam P (1994) High resolution of quantitative traits into multiple loci via interval mapping. Genetics 136:1447–1455
Kang HS, Beak JY, Kim YS, Petrovich RM, Collins JB, Grissom SF, Jetten AM (2006) NABP1, a novel RORgamma-regulated gene encoding a single-stranded nucleic-acid-binding protein. Biochem J 397:89–99
Kong S, Ke Q, Chen L, Zhou Z, Pu F, Zhao J, Bai H, Peng W, Xu P (2019) Constructing a high-density genetic linkage map for large yellow croaker (Larimichthys crocea) and mapping resistance trait against ciliate parasite Cryptocaryon irritans. Mar Biotechnol 21:262–275
Kujur A, Upadhyaya HD, Shree T, Bajaj D, Das S, Saxena MS, Badoni S, Kumar V, Tripathi S, Gowda CL (2015) Ultra-high density intraspecific genetic linkage maps accelerate identification of functionally relevant molecular tags governing important agronomic traits in chickpea. Sci Rep 5:9468
Li C, Chen J (2008) The immune response of white shrimp Litopenaeus vannamei and its susceptibility to Vibrio alginolyticus under low and high pH stress. Fish Shellfish Immun 25:701–709
Li E, Chen L, Zeng C, Chen X, Yu N, Lai Q, Qin JG (2007) Growth, body composition, respiration and ambient ammonia nitrogen tolerance of the juvenile white shrimp, Litopenaeus vannamei, at different salinities. Aquaculture 265:385–390
Li J, Pu L, Han M, Zhu M, Zhang R, Xiang Y (2014) Soil salinization research in China: advances and prospects. J Geogr Sci 24:943–960
Li HL, Gu XH, Li BJ, Chen CH, Lin HR, Xia JH (2017) Genome-wide QTL analysis identified significant associations between hypoxia tolerance and mutations in the GPR132 and ABCG4 genes in Nile Tilapia. Mar Biotechnol 19:441–453
Li BJ, Zhu ZX, Gu XH, Lin HR, Xia JH (2019) QTL mapping for red blotches in Malaysia red tilapia (Oreochromis spp.). Mar Biotechnol 21:384–395
Liang L, Ren B, Chang Y, Tang R, Zhang L (2013) Inland brackish (saline-alkaline) water resources and fisheries utilization in China. Chin Fish Econ 31:138–145 (In China)
Lien S, Gidskehaug L, Moen T, Hayes BJ, Berg PR, Davidson WS, Omholt SW, Kent MP (2011) A dense SNP-based linkage map for Atlantic salmon (Salmo salar) reveals extended chromosome homeologies and striking differences in sex-specific recombination patterns. BMC Genomics 12:615
Lin X, Taguchi A, Park S, Kushner JA, Li F, Li Y, White MF (2004) Dysregulation of insulin receptor substrate 2 in beta cells and brain causes obesity and diabetes. J Clin Invest 114:908–916
Linley AJ, Mathieu MG, Miles AK, Rees RC, McArdle SE, Regad T (2012) The helicase HAGE expressed by malignant melanoma-initiating cells is required for tumor cell proliferation in vivo. J Biol Chem 287:13633–13643
Liu C, Wang J, Zhang Y, Liu L (2008) Effects of salinity and Na+/K+ in percolating water from saline-alkali soil on the growth of Litopenaeus vannamei. Chin J Appl Ecol 19:1337–1342 (In China)
Liu Y, Fang H, Lai Q, Liang L (2016) The current state and development strategy for China’s saline-alkaline fisheries. Eng Sci CAE 18:74–78 (In China)
Liu H, Fu B, Me P, Feng X, Yu X, Tong J (2017) A high-density genetic linkage map and QTL fine mapping for body weight in crucian carp (Carassius auratus) using 2b-rad sequencing. G3 (Bethesda) 7:2473–2487
Livak K, Schmittgen T (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408
Louro B, Kuh H, Tine M, de Koning DJ, Batargias C, Volckaert FAM, Reinhardt R, Canario AVM, Deborah M (2016) Power characterization and refinement of growth related quantitative trait loci in European sea bass (Dicentrarchus labrax) using a comparative approach. Aquaculture 455:8–21
Lu X, Luan S, Hu LY, Mao Y, Tao Y, Zhong SP, Kong J (2016) High-resolution genetic linkage mapping, high-temperature tolerance and growth-related quantitative trait locus (QTL) identification in Marsupenaeus japonicas. Mol Gen Genomics 291:1391–1405
Luan Z, Pan L, Dong S (2003) A study of the culture technology of shrimp in low-lying soline-alkali soil along the yellow river. Chin J Oceanol Limn 3:71–77 (In China)
MacFarlane AW 4th, Yamazaki T, Fang M, Sigal LJ, Kurosaki T, Campbell KS (2008) Enhanced NK-cell development and function in BCAP-deficient mice. Blood 112:131–140
Malkowska M, Kokoszynska K, Rychlewski L, Wyrwicz L (2013) Structural bioinformatics of the general transcription factor TFIID. Biochimie 95:680–691
Márquez J, Olaso E (2014) Role of discoidin domain receptor 2 in wound healing. Histol Histopathol 29:1355–1364
Miller MR, Dunham JP, Amores A, Cresko WA, Johnson EA (2007) Rapid and cost-effective polymorphism identification and genotyping using restriction site associated. DNA (RAD) Markers 17:240–248
Ouellette LA, Reid RW, Blanchard SG, Brouwer CR (2018) LinkageMapView—rendering high-resolution linkage and QTL maps. Bioinformatics 34:306–307
Peterson BK, Weber JN, Kay EH, Fisher HS, Hoekstra HE (2012) Double digest RADseq: an inexpensive method for de novo SNP discovery and genotyping in model and non-model species. PLoS One 7:e37135
Piepho HP (2001) A quick method for computing approximate thresholds for quantitative trait loci detection. Genetics 157:425–432
Roggatz C, Lorch M, Hardege J, Benoit D (2016) Ocean acidification affects marine chemical communication by changing structure and function of peptide signaling molecules. Glob Change Biol 22:3914–3926
Rowe HC, Renaut S, Guggisberg A (2011) RAD in the realm of next generation sequencing technologies. Mol Ecol 20:3499–3502
Seo M, Peeters AJ, Koiwai H, Oritani T, Marion-Poll A, Zeevaart JA, Koornneef M, Kamiya Y, Koshiba T (2000) The Arabidopsis aldehyde oxidase 3 (AAO3) gene product catalyzes the final step in abscisic acid biosynthesis in leaves. Proc Natl Acad Sci U S A 97:12908–12913
Separovic D, Hanada K, Maitah MY, Nagy B, Hang I, Tainsky MA, Kraniak JM, Bielawski J (2007) Sphingomyelin synthase 1 suppresses ceramide production and apoptosis post-photodamage. Biochem Biophys Res Commun 358:196–202
Shao C, Niu Y, Rastas P, Liu Y, Xie Z, Li H, Wang L, Jiang Y, Tai S, Tian Y, Sakamoto T, Chen S (2015) Genome-wide SNP identification for the construction of a high-resolution genetic map of Japanese flounder (Paralichthys olivaceus): applications to QTL mapping of Vibrio anguillarum disease resistance and comparative genomic analysis. DNA Res 22:161–170
Stam P (1993) Construction of integrated genetic linkage maps by means of a new computer package: JoinMap. Plant J 3:739–744
Stölting KN, Nipper R, Lindtke D, Caseys C, Waeber S, Castiglione S, Lexer C (2013) Genomic scan for single nucleotide polymorphisms reveals patterns of divergence and gene flow between ecologically divergent species. Mol Ecol 22:842–855
Soñanez-Organis JG, Peregrino-Uriarte AB, Gómez-Jiménez S, López-Zavala A, Forman HJ, Yepiz-Plascencia G (2009) Molecular characterization of hypoxia inducible factor-1 (HIF-1) from the white shrimp Litopenaeus vannamei and tissuespecific expression under hypoxia. Comp Biochem Physiol C Toxicol Pharmacol 150:395–405
Sun X, Liu D, Zhang X, Li W, Liu H, Hong W, Jiang C, Guan N, Ma C, Zeng H, Xu C, Song J, Huang L, Wang C, Shi J, Wang R, Zheng X, Lu C, Wang X, Zheng H (2013) SLAF-seq: an efficient method of large-scale denovo SNP discovery and genotyping using high-throughput sequencing. PLoS One 8:e58700
Sun C, Niu Y, Ye X, Dong J, Hu W, Zeng Q, Chen Z, Tian Y, Zhang J, Lu M (2017) Construction of a high-density linkage map and mapping of sex determination and growth-related loci in the mandarin fish (Siniperca chuatsi). BMC Genomics 18:446
Tong JG, Sun XW (2015) Genetic and genomic analyses for economically important traits and their applications in molecular breeding of cultured fish. Science China-Life Sci 58:178–186
Toulany M, Rodemann HP (2015) Phosphatidylinositol 3-kinase/Akt signaling as a key mediator of tumor cell responsiveness to radiation. Semin Cancer Biol 35:180–190
van Daal A, Elgin SC (1992) A histone variant, H2AvD, is essential in Drosophila melanogaster. Mol Biol Cell 3:593–602
Van Ooijen JW (2011) Multipoint maximum likelihood mapping in a full-sib family of an out breeding species. Genet Res 93:343–349
van Wijk SJ, Timmers HT (2010) The family of ubiquitin-conjugating enzymes (E2s): deciding between life and death of proteins. FASEB J 24:981–993.
Wan SM, Liu H, Zhao BW, Nie CH, Wang WM, Gao ZX (2017) Construction of a high-density linkage map and fine mapping of QTLs for growth and gonad related traits in blunt snout bream. Sci Rep 7:46509
Wang PY, Weng J, Anderson RG (2005) OSBP is a cholesterol-regulated scaffolding protein in control of ERK 1/2 activation. Science 307:1472–1476
Wang WN, Zhou J, Wang P, Tian TT, Zheng Y, Liu Y, Mai WJ, Wang AL (2009) Oxidative stress, DNA damage and antioxidant enzyme gene expression in the Pacific white shrimp, Litopenaeus vannamei when exposed to acute pH stress. Comp Biochem Phys C 150:428–435
Wang S, Meyer E, McKay JK, Matz MV (2012) 2b-RAD: a simple and flexible method for genome-wide genotyping. Nat Methods 9:808–810
Wang J, Luo MC, Chen Z, You FM, Wei Y, Zheng Y, Dvorak J (2013a) Aegilops tauschii single nucleotide polymorphisms shed light on the origins of wheat genome genetic diversity and pinpoint the geographic origin of hexaploid wheat. New Phytol 198:925–937
Wang X, Li E, Xiong Z, Chen K, Yu N, Du Z, Chen L (2013b) Low salinity decreases the tolerance to two pesticides, beta-cypermethrin and acephate, of whiteleg shrimp, Litopenaeus vannamei. J Aquac Res Development 4:190
Wang S, Feng Q, Zhou Y, Mao X, Chen Y, Xu H (2017) Dynamic changes in water and salinity in saline-alkali soils after simulated irrigation and leaching. PLoS One 12:e0187536
Wang X, Fu B, Yu X, Qu C, Zhang Q, Tong J (2018) Fine mapping of growth-related quantitative trait loci in Yellow River carp (Cyprinus carpio haematoperus). Aquaculture 484:277–285
Watt MJ, Spriet LL (2010) Triacylglycerol lipases and metabolic control: implications for health and disease. Am J Physiol Endocrinol Metab 299:E162–E168
Wu F, Wang T, Cui S, Xie Z, Dupont S, Zeng J, Gu H, Kong H, Hu M, Lu W, Wang Y (2017) Effects of seawater pH and temperature on foraging behavior of the Japanese stone crab Charybdis japonica. Mar Pollut Bull 120:99–108
Xu J, Zhao Z, Zhang X, Zheng X, Li J, Jiang Y, Kuang Y, Zhang Y, Feng J, Li C, Yu J, Li Q, Zhu Y, Liu Y, Xu P, Sun X (2014) Development and evaluation of the first high-throughput SNP array for common carp (Cyprinus carpio). BMC Genomics 15:307
Xue M (2018) Experiment and analysis of pond ecological farming technique in salt and alkaline soil of the yellow river beach. Shaanxi Water Resources 1:88–96 (In China)
Yu Y, Zhang X, Yuan J, Li F, Chen X, Zhao Y, Huang L, Zheng H, Xiang J (2015) Genome survey and high-density genetic map construction provide genomic and genetic resources for the Pacific White Shrimp Litopenaeus vannamei. Sci Rep 5:15612
Yu Y, Wang Q, Zhang Q, Luo Z, Wang Y, Zhang X, Huang H, Xiang J, Li F (2019) Genome scan for genomic regions and genes associated with growth trait in pacific white shrimp Litopeneaus vannamei. Mar Biotechnol 21:374–383
Yue GH (2014) Recent advances of genome mapping and marker-assisted selection in aquaculture. Fish Fish 15:376–396
Zhang D (2016) Technology for healthily breeding Penaeus vannamei boone in saline-alkali land of our country’s northern region. Shanxi Hydrotechnics 4:127–128 (In China)
Zhang G, Zhang X, Ye H, Jiang S, Yu H, Li J, Shi Q, Chen G, Zhou Z, Luo J, You X (2018) Construction of high-density genetic linkage maps and QTL mapping in the golden pompano. Aquaculture 482:90–95
Zhang X, Yuan J, Sun Y, Li S, Gao Y, Yu Y, Liu C, Wang Q, Lv X, Zhang X, Ma KY, Wang X, Lin W, Wang L, Zhu X, Zhang C, Zhang J, Jin S, Yu K, Kong J, Xu P, Chen J, Zhang H, Sorgeloos P, Sagi A, Alcivar-Warren A, Liu Z, Wang L, Ruan J, Chu KH, Liu B, Li F, Xiang J (2019) Penaeid shrimp genome provides insights into benthic adaptation and frequent molting. Nat Commun 10:356
Zimmerman SW, Yi YJ, Sutovsky M, van Leeuwen FW, Conant G, Sutovsky P (2014) Identification and characterization of RING-finger ubiquitin ligase UBR7 in mammalian spermatozoa. Cell Tissue Res 356:261–278
Acknowledgments
We thank the anonymous reviewers for comments on the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No. 31602135), Institution of South China Sea Ecology and Environmental Engineering, Chinese Academy of Sciences (No. ISEE2018PY03), the Science & Technology Promoting Projects for Oceanic & Fishery in Guangdong Province (SDYY-2018-01), and the Guangdong Province Program (2017B030314052, 2018A030313857, 2016A030310112, 2015A030310120, 2015B020231007 and A2015230).
Author information
Authors and Affiliations
Contributions
WH and CH contributed to project conception. Experiment and data analysis was conducted by WH, CC, JL, XZ, CR, XJ, TC, KC, and HL. The manuscript was prepared by WH. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Statement of Human and Animal Rights
Shrimp care and experiments were carried out according to the Care and Use of Agricultural Animals in Agricultural Research and Teaching and approved by the Science and Technology Bureau of China. Approval from the Department of Wildlife Administration was not required for the experiments conducted in this paper. All experiments in this paper were performed with permits obtained from the Government of the People’s Republic of China and endorsed by the Animal Experimentation Ethics Committee of Chinese Academy of Sciences.
Conflict of Interest
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Supplementary Table 1
(XLSX 12 kb)
Supplementary Table 2
(XLSX 9 kb)
Supplementary Table 3
(XLSX 9 kb)
Supplementary Table 4
(XLSX 777 kb)
Supplementary Table 5
(XLSX 775 kb)
Supplementary Fig. 1
Propagation characteristics of the shrimp L. vannamei. a Fertile female Pacific white shrimp, with a surgically removed eyestalk, which was usually used for stimulus-induced maturation of the shrimp, in the box, with arrows indicating the mature gonad of the female shrimp. b Fertile male Pacific white shrimp, with the spermatophore position of the male shrimp while packed with white spermatia shown in the framed area. c The thelycum position of the female shrimp, indicated by the arrow, with white spermatia adhering to the thelycum of the mated female shrimp. d The spermatophore position is framed, and the mated male parent is empty (without packed spermatium) in this position. Bars = 1 cm. (PDF 148 kb)
Rights and permissions
About this article
Cite this article
Huang, W., Cheng, C., Liu, J. et al. Fine Mapping of the High-pH Tolerance and Growth Trait-Related Quantitative Trait Loci (QTLs) and Identification of the Candidate Genes in Pacific White Shrimp (Litopenaeus vannamei). Mar Biotechnol 22, 1–18 (2020). https://doi.org/10.1007/s10126-019-09932-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10126-019-09932-8