Abstract
Oocyte maturation and hydration are regulated by a complex interplay of various hormones and local factors. We have investigated the morphological changes of follicles and serum steroid levels during the HCG (human choionic gonadotophin)-induced oocyte maturation in the orange-spotted grouper. For the first time, a large-scale transcriptomic analysis of follicles during the maturation has been conducted in a fish species which produce pelagic oocytes. Eight cDNA libraries of follicle samples, from full-grown immature follicles to mature follicles, were constructed. A total of 402,530,284 high-quality clean reads were obtained after filtering, 79.66% of which perfectly mapped to the orange-spotted grouper genome. Real-time PCR results of 12 representative genes related to oocyte maturation and hydration verified the reliability of the RNA-seq data. A large number of genes related to oocyte maturation and hydration were identified in the transcriptome dataset. And the transcriptomic analysis revealed the dynamic changes of the steroid synthesis pathway and the pathway of hydration during oocyte maturation. The present study will facilitate future study on the oocyte maturation and hydration in the orange-spotted grouper and other marine pelagic egg spawner.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oocyte maturation is critical to fertility in animals, and therefore the regulation of oocyte maturation is a hot topic in reproductive biology. In teleosts, it has been well established that GtHs (gonadotropic hormones) primarily take control of oocyte development. Oocytes complete their growth under the regulation of FSH (follicle-stimulating hormone), due to the accumulation of yolk proteins in the cytoplasm. Full-grown immature follicles are arrested at the first meiotic prophase and are ready for the next phase of oogenesis, which is oocyte maturation. LH (luteinizing hormone) is released to stimulate MIH (maturation-inducing hormone) synthesis in the follicular cells through LHR (luteinizing hormone receptor). Then, MIH binds to mPR (membrane progestin receptor) located on the oocyte surface, triggering the formation of MPF (maturation-promoting factor). And the resumption of meiosis starts along with several biological process such as GVBD (breakdown of the germinal vesicle), chromosome condensation, and formation of the first polar body. Finally, the oocyte breaks through the follicular cells and becomes an ovule (Yamashita et al. 1995; Nagahama and Yoshikuni 2008; Li and Cheng 2018). There are also other endocrine or paracrine factors derived from follicular cells or oocytes that are involved in oocyte development (Clelland and Peng 2009).
According to their habitat, fish are classified into two groups: freshwater and saltwater fish. They have evolved notable features to adapt to the environment, including specific egg phenotypes. In order to increase embryo survival and dispersal, most extant marine teleosts spawn pelagic oocytes, while freshwater teleosts tend to spawn benthic oocytes (Lubzens et al. 2010). These two types of oocytes exhibit differences during maturation. For example, full-grown immature follicles of freshwater teleosts can be easily induced to mature in vitro by treating them with HCG (a LH analogue) or DHP (17α, 20β-dihydroxy-4-pregnen-3-one) (Jalabert 1976; Selman et al. 1994). In contrasts, inducing the oocyte maturation of marine fish under the same conditions is not as easy. The MIH of most freshwater teleosts is DHP, while 20β-S (17α,20β,21-trihydroxy-4-pregnen-3-one) is the MIH in Atlantic croaker (Micropogonias undulatus) (Trant et al. 1986; Trant and Thomas 1989). In some marine teleosts, DHP and 20β-S can be both detected during the oocyte maturation (King et al. 1995). Post-vitellogenic follicles from marine fish cannot be induced to maturation without priming them with HCG in vitro (Patiño and Thomas 1990b). In black sea bass and the southern flounder, even if follicles are primed with HCG, they do not undergo maturation in response to both DHP and 20β-S, or just be induced in a low maturation rate, respectively (Cerdá et al. 1996; Picha et al. 2012). Furthermore, oocyte hydration during maturation is observed in marine fish species, which is a unique and interesting phenomenon. Hydration involves several biological activities in the oocyte, such as yolk proteolysis, ion transport, as well as water uptake (Lubzens et al. 2010). Oocyte hydration and maturation are closely related and cannot be taken apart. Thus, the regulation of oocyte maturation in marine teleosts seems to be much more complex than that in freshwater teleosts.
The regulation of oocyte maturation and hydration in teleost has attracted attention for a long time. However, the mechanism underlying this physiological phenomenon remains largely unclear. In this study, we used orange-spotted grouper (Epinephelus coioides, a popular commercial marine species in east and southeast Asia) as an experiment model; we performed large-scale transcriptomic analysis for the isolated follicles at different stages of oocyte maturation to get an overview of the transcriptome dynamics and gene regulatory mechanism during the process of oocyte maturation and hydration.
Materials and Methods
Experimental Animals and Follicle Samples Collection
The gravid female orange-spotted groupers were obtained from the Guangdong Daya Bay Fishery Development Center (Huizhou, Guangdong, China). The reproductive season of this grouper is from April to October. Fishes were first injected with HCG (Ningbo Sansheng Pharmaceutical Co., China) of a 300 IU/kg dosage in their dorsal muscle to induce the oocyte maturation in vivo. And then they were euthanized in different interval after injection. As the orange-spotted grouper is a partial synchronous spawner, we checked the biggest follicles at different time points by biopsy. Follicles were isolated carefully with fine forceps and placed in a Leibovitz L-15 medium (Procell Life Science & Technology Co., China). The diameters of follicles were measured under a dissecting microscope. According to the diameters and characteristics of oocyte morphology of orange-spotted grouper, the oocyte maturation process was divided into eight stages: O450-470, O490-510, O520-560, O560-590, O590-610, O630-680, O695-735, and O770-830. At each stage, three fish were sampled, and about 50 healthy follicles at the same stage were selected for total RNA extraction. Three replicates (150 follicles in total) were obtained for each stage. All animal experiments were conducted in accordance with the guidelines and approval of the respective Animal Research and Ethics Committees of the Sun Yat-Sen University.
RNA Extraction
Total RNA was extracted from samples homogenized in TRIzol reagent (Thermo Fisher Scientific, USA). Two hundred-microliter chloroform was added to each homogenate and vortex for 15 s. After incubating at room temperature for 10 min, the samples were centrifuged at 12,000g for 15 min. Then, the supernatant was transferred to a new tube containing 500 μl isopropanol, mixed gently, and left for 10 min. After centrifuged for 10 min at 12,000g, samples were rinsing with 1 ml 75% ethanol and air-drying for 5 min. The RNA pellets were dissolved in 20 μl nuclease-free H2O and stored in a − 80 °C freezer. RNA integrity was verified by electrophoresis on 1% agarose gel and quantified with ND-2000 (NanoDrop Technologies, DE, USA).
Library Construction and Sequencing
Equal amounts of RNAs of three replicates from the same stages were pooled for transcriptome sequencing and one cDNA library prepared for each biological group. The mRNA was enriched by Oligo (dT) beads. Then, the enriched mRNA was fragmented into short fragments using fragmentation buffer and reverse transcripted into cDNA with random primers. Second-strand cDNA was synthesized by DNA polymerase I, RNase H, dNTP, and buffer. Then, the cDNA fragments were purified with QiaQuick PCR extraction kit, end repaired, poly(A) added, and ligated to Illumina sequencing adapters. The ligation products were size selected by agarose gel electrophoresis, PCR amplified, and sequenced using Illumina HiSeq™ 2500.
Mapping of the Reads to Reference Genome and Transcripts Assembly
Clean reads were obtained by removing reads containing adapters, reads containing more than 10% of N (unknown nucleotides), and low-quality reads containing more than 50% of low-quality (Q value ≤ 20) bases from raw data. Meanwhile, short reads alignment tool Bowtie2 (Langmead and Salzberg 2012) was used for mapping reads to rRNA database. The rRNA mapped reads will be removed. The rRNA removed reads, HQ (high quality) clean reads, of each sample were mapped to reference genome of the orange-spotted grouper (unpublished) by TopHat2 (version2.0.3.12) (Kim et al. 2013), respectively. The reconstruction of transcripts was carried out with software Cufflinks (Trapnell et al. 2012). The program RABT (reference annotation-based transcripts) was preferred. Cufflinks constructed faux reads according to reference to make up for the influence of low-coverage sequencing. During the last step of assembly, all of the reassembles fragments were aligned with reference genes and then similar fragments were removed. Then we used Cuffmerge to merge transcripts from different replicas of a group into a comprehensive set of transcripts, and then merge the transcripts from multiple groups into a finally comprehensive set of transcripts for further downstream differential expression analysis.
Gene Expression and Enrichment Analysis
The gene expression level was normalized by using FPKM (Fragments Per Kilobase of transcript per Million mapped reads) method. To identify DEGs (differentially expressed genes) across sample groups, the edgeR package (http://www.rproject.org/) was used. We identified genes with a |log2 (fold change)| ≥ 1 and a FDR (false discovery rate) < 0.05 in a comparison as significant DEGs. DEGs were then subjected to enrichment analysis of GO functions and KEGG (Kyoto encyclopedia of genes and genomes database) pathways. The calculated p value of both enrichment analyses was gone through FDR correction, taking FDR ≤ 0.05 as a threshold. GO terms or pathways meeting this condition were defined as significantly enriched in DEGs.
Validation of DEGs from Transcriptome with Quantitative Real-Time PCR
Twelve representative genes identified from the transcriptome were examined by qPCR (quantitative real-time PCR) to verify their expression profiles. One-microgram total RNA was reverse transcribed using a ReverTra Ace qPCR RT Kit (Toyobo, Japan) according to manufacturer’s instructions. The qPCR reactions were performed on a Roche LightCycler 480 real-time PCR system (Roche, Switzerland) using the LightCycler 480 SYBR Green I Master (Roche, Switzerland) in a 10-μl reaction volume. The reaction conditions were as follows: pre-denaturation at 95 °C for 10 min, followed by 40 amplification cycles of 95 °C for 10 s, 58 °C for 20 s, and 72 °C for 20 s. The qPCRs used to detect all the target genes and β-actin reference gene were performed with three biological replicates and their specificities of amplifications were confirmed by melting curve analysis. Most primers used for qPCR had also been verified in previous studies (Chen et al. 2011; Wang et al. 2017a, b; Peng et al. 2018). The relative expression level of target genes was normalized to that of β-actin and calculated with the 2−ΔΔCT method (Livak and Schmittgen 2001). Sequences of gene-specific primers are shown (Supplementary file 2: Table. S1).
Enzyme-Linked Immunosorbent Assay
Blood samples of fishes were collected from caudal vein at 0, 10, 20, 24, and 32 h after HCG injection. Serum samples were then collected after centrifugation (6000g, 10 min) of bloods and stored at − 20 °C until used for measuring the levels of estradiol, testosterone, progesterone, and DHP. The enzyme-linked immunosorbent assays were carried out using the EIA Assay kits (Cayman, USA) for each object following the manufacturer’s instructions.
Results
Morphology of Follicles at Different Developmental Stages After HCG Injection In Vivo
The morphology of follicles during maturation is shown in Fig. 1. The diameter of the full-grown follicles in gravid fish without HCG injection was 450 to 470 μm. At 10 h after HCG injection, the diameter of most follicles in the ovaries increased to 490–510 μm. At 20 h after injection, follicles reached a size of 520–560 μm in diameter. At 24 h after injection, GVBD process was initiated in vivo. There were four types of follicles in the ovaries with diameters of 560–590 μm, 590–610 μm, 630–680 μm, and 695–735 μm. Along with yolk proteolysis and water uptake, follicles gradually became bigger and more transparent. Finally, about 32 h after injection, a large number of follicles became completely transparent with an oil drop in the cytoplasm, and completed their maturation and hydration, reaching a terminal diameter of 770–830 μm.
Levels of Steroid Hormones in the Serum During Oocyte Maturation
As show in Fig. 2, progesterone, the precursor of steroid biosynthesis, remained in a stable level of about 250 pg/mL during oocyte maturation, which slightly decreased at 24 h. DHP remained at a low concentration at 0, 10, and 20 h, although there was a slight concentration increase at 10 h. A dramatic increase in DHP was observed at 24 h, which corresponds to the onset of the GVBD process. Estradiol levels increased gradually, but had a slight decrease at 32 h. Testosterone remained at a steady low level during the whole maturation process.
Steroid hormones levels during oocyte maturation. Serum samples were collected at different time points for measuring progesterone, DHP, estradiol, and testosterone levels. Data are mean ± SEM (n = 3). Data from all time points are compared to 0 h. One-way analysis of variance was used to estimate statistical significance (P < 0.05, asterisks)
Transcriptome Sequencing and Assembly
Eight cDNA libraries (designated as O450-470, O490-510, O520-560, O560-590, O590-610, O630-680, O695-735, and O770-830) were constructed. A total of 417,285,594 raw reads and 408,624,736 clean reads were obtained after filtering (Supplementary file 2: Table S2). All clean reads were mapped to an rRNA database. The mapped reads were removed producing the remaining total 402,530,284 HQ clean reads for alignment with the orange-spotted genome (Supplementary file 2: Table S3). In total, 80.69% of HQ clean reads mapped to the genome, with 79.66% unique mapped reads (Table 1).
DEGs and Enrichment Analysis Between Follicles at Different Developmental Stages During Oocyte Maturation
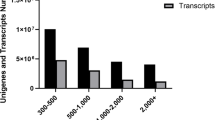
Comparisons of gene expression between eight developmental stage follicles were performed: O450-470 vs. O490-510, O490-510 vs. O520-560, O520-560 vs. O560-590, O560-590 vs. O590-610, O590-610 vs. O630-680, O630-680 vs. O695-735, and O695-735 vs. O770-830. From each pair of comparisons, 2696, 5206, 2527, 1734, 1496, 850, and 7184 DEGs were identified (Supplementary file 3: Table S1–7). The numbers of upregulated and downregulated DEGs are described in Fig. 3, and in Supplementary file 1 (Fig. S1).
GO (gene ontology) analysis showed that DEGs were mostly enriched in cellular process (GO:0009987), single-organism process (GO:0044699), metabolic process (GO:0008152), and biological regulation (GO:0065007) at the biological process level. In terms of cellular components, cell (GO:0005623), cell part (GO:0044464), membrane (GO:0016020), and membrane part (GO:0044425) ranked among the top four categories. As for molecular function, binding (GO:0005488) and catalytic activity (GO:0003824) were the top two categories (Supplementary file 4: Table S1–7).
KEGG pathway analysis showed that the GnRH signaling pathway (ko04912), gap junction (ko04540), MAPK signaling pathway (ko04010), and calcium signaling pathway (ko04020) were frequently observed at the early stages of oocyte maturation. As maturation progressed, DEGs were enriched in steroid hormone biosynthesis (ko00140) (Fig. 4, Supplementary file 5: Table S1–7). Other pathways like ECM-receptor interaction (ko04512), focal adhesion (ko04510), and AGE-RAGE signaling pathway in diabetic complications (ko04933) were also frequent observed during oocyte maturation (Fig. 4, Supplementary file 5: Table S1–7).
Expression of Genes Related to Oocyte Maturation
Seventeen genes related to oocyte maturation were identified from transcriptome data. As shown in Fig. 5, among these genes, fshr (follicle-stimulating hormone receptor), lhr1, cyp11a1 (cytochrome P450 11a1), cyp17a2 (cytochrome P450 17a2), cyp19a1a (cytochrome P450 19a1a), and 17β-hsd (17β-hydroxysteroid dehydrogenase) had low expression during oocyte maturation. The other 11 genes had a relatively high expression with the highest FPKM ranking from 14.68 to 425.24. LH/HCG inducing oocyte maturation is manly mediated by the gonadotropin receptors (FSHR and LHR). Although the expression of fshr and lhr1 was low, their expression in O490-510 and O560-590 changed significantly, compared with that in O450-470. Interestingly, lhr2 (a novel LH receptor in Epinephelus coioides) increased from O520-560 and remained at a high level until O695-735. Steroid hormone biosynthesis is critical in oocyte maturation. Star (steroidogenic acute regulatory protein) expression was upregulated after HCG injection until O590-610. Then, its expression decreased gradually until the oocytes mature completely. Similar expression patterns were also observed in cyp17a1 (cytochrome P450 17a1), cyp17a2, and cyp21a2 (cytochrome P450 21a2). The genes 3β-hsd (3β-hydroxysteroid dehydrogenase/Δ5–Δ4 isomerase) and 20β-hsd (20β-hydroxysteroid dehydrogenase) expressed stably at early stages of maturation, but their expression decreased gradually during later stages. The progestin receptor, mprα, remained at a relatively stable level, with a slight increase in O520-560. However, the expression level of mprβ and mprγ was nearly undetectable. Interestingly, npr (nuclear progestin receptor) expression was dramatically upregulated from O520-560 to O695-735, and then the expression declined at the final maturation stage. Three ER (estrogen receptor) genes (erα, erβ1, and erβ2) exhibited similar expression patterns. Their expression increased at the early stages and decreased later.
Expression of Genes Related to Oocyte Hydration
Along with oocyte maturation, hydration took place as the intra-oocyte osmotic pressure increased. FAAs (free amino acids) were cleaved from the yolk protein, and inorganic ions were pumped into the oocyte, which contributed to most of the osmotic pressure. Cathepsins played an important role in yolk proteolysis, resulting in the production of FAAs and PO4−. As shown in Fig. 6, catb, catd, and catl (cathepsin B, D and L) were expressed at high levels during the oocyte hydration. By contrast, catf (cathepsin F) remained at a low level with FPKM of around 20 during maturation. Na+/K+-ATPase was one of the most important ion channels during oocyte hydration. The expression nktaα1 and nktaβ1 (Na+,K+-ATPase subunit α1 and β1) was dramatically upregulated. In addition, the expression of CICP (cicp2 and cicp4, chloride intracellular channel protein) and NKCC (Na-K-Cl co-transporter) genes was also significantly upregulated during hydration. Water uptake was mediated by Aquaporin 1. aqp1a (aquaporin 1a) expression increased dramatically in O520-560, and then remained at a relatively stable level until oocytes completely matured. By contrast, aqp1b (aquaporin 1b) was highly expressed in O450-470 and O490-510, and decreased subsequently.
Quantitative Real-Time PCR Confirmation of the Data Inferred from the Transcriptome
Quantitative real-time PCR was performed to verify the expression pattern of 12 genes related to oocyte maturation or hydration. These genes were lhr1, lhr2, star, 20β-hsd, cyp21a2, mprα, npr, erα, erβ1, erβ2, aqp1a, and aqp1b. The qPCR results were basically consistent with their transcriptome expression data (Fig. 7, Supplementary file 1: Fig. S2), indicating that the transcriptome data and the associated methodologies of analysis were reliable.
Quantitative real-time PCR validation of the expression of the 12 most representative transcripts identified from transcriptomic data. Data are plotted as the mean ± SEM (n = 3). One-way analysis of variance (P < 0.05) was used to estimate the statistical significance between each pair of groups. Bars sharing the same letter(s) are not significantly different (P > 0.05)
Discussion
In the present study, we have performed a transcriptomic analysis of the follicles during oocyte maturation and hydration. To our best knowledge, this study is the first large-scale transcriptomic analysis of oocyte maturation and hydration using follicles at different stages of maturation, as most studies in teleosts focused on ovaries in different conditions (Tingaud-Sequeira et al. 2009; Chapman et al. 2014; Ravi et al. 2014; Xu et al. 2016; Jia et al. 2018). These data provide valuable information to identify critical genes and regulatory signaling pathways during the maturation of pelagic oocytes in the orange-spotted grouper.
Genes Related to Oocyte Maturation
In teleosts, the oocyte maturation and ovulation are triggered by the LH/LHR signaling system. Recently, two lhrs were identified and characterized in several fish species, such as the European eel (Anguilla anguilla), the Japanese eel (Anguilla japonica), and orange-spotted grouper (Maugars and Dufour 2015; Peng et al. 2018). However, the function of these two lhrs in oocyte maturation is unknown. Our study showed that two lhr were upregulated during oocyte maturation. Interestingly, the expression of lhr2 increased much more dramatically than that of lhr1 during the maturation process. Our previous study indicated that both LHRs could be activated by HCG, but LHR2 showed higher potency to HCG activation compared with LHR1 in orange-spotted grouper (Peng et al. 2018). These data suggested that LHR1 and LHR2 may work in cooperation during oocyte maturation, and that LHR2 may play a more important role in HCG-induced oocyte maturation in the orange-spotted grouper.
Along with LH signal transduction through LHR, many enzymes are regulated in follicles in response to steroid synthesis, which results in the change of steroid levels in the serum. Estradiol and MIHs take control of oocyte growth and maturation, respectively. There is a dramatic shift in steroidogenic pathway from estradiol to MIHs before oocyte maturation (Nagahama and Yoshikuni 2008). From the very beginning of steroid synthesis, cholesterol is transferred into the mitochondria by StAR, which is a rate-limiting factor of steroidogenesis (Wang et al. 2017b). The expression of star increased gradually, ensuring enough cholesterol for subsequent steroid synthesis. The expression pattern of star is strongly correlated with lhr, which is similar to the European sea bass (Rocha et al. 2009). Then, the steroidogenic pathway was initiated with the synthesis of the steroid precursor pregnenolone by P450ssc (cholesterol side chain cleavage cytochrome P450, encoded by cyp11a1), which was expressed at the highest levels at O450-470 and O490-510. High expression of cyp11a1 in the early stages during oocyte maturation produced enough steroid precursors for later steroid synthesis. Pregnenolone was then converted to progesterone by 3β-HSD. This enzyme expression was upregulated in follicles ranging from 490 to 610 μm, remained stable during the later maturation process, and decreased when the oocytes matured completely. A similar expression pattern of 3β-hsd was also observed in rainbow trout during maturation and ovulation (Sakai et al. 1993). High expression of 3β-hsd is important for the conversion of Δ5-steroids to Δ4-steroids. In addition to pregnenolone, other Δ5-steroids, like 17α-hydroxypregnenolone and dehydroepiandrosterone, also need to be converted into Δ4-steroids by 3β-HSD. P450c17 (cytochrome P450 17) contains both 17α-hydroxylase and 17,20-lyase activities. Two forms of the enzyme (P450c17a1 and P450c17a2), encoded by cyp17a1 and cyp17a2, respectively, are found in teleosts. P450c17a1 has both hydroxylase and lyase activity, while P450c17a2 lacks lyase activity (Clelland and Peng 2009). According to our data, cyp17a1 expression increased greatly from O560-590 to O590-610, corresponding to the onset of GVBD. However, cyp17a2 was expressed at a low level during oocyte maturation. The high expression of cyp17a1 suggests that this enzyme may be responsible for acting upon progesterone primarily in grouper to produce 17α-hydroxyprogesterone, which is a key precursor for estradiol and DHP. Although serum estradiol levels showed a slight increase at 24 h after HCG injection, the expression of key enzymes (17β-HSD and CYP19A1a) responsible for the biosynthesis of estradiol was low during the whole maturation process. The enzyme 20β-HSD is a rate-limiting enzyme for MIH synthesis. Its expression remained at a sufficiently high level to promote MIH synthesis before maturation finished. In many teleosts, DHP is the MIH to induce final oocyte maturation. However, some marine fish species like the Atlantic croaker (Micropogonias undulatus) primarily respond to 20β-S (Trant and Thomas 1989). The MIH in the orange-spotted grouper is still unclear. According to our data, DHP levels increased dramatically before GVBD process, suggesting that DHP may be the potential MIH in grouper. In this study, we did not examine the 20β-S levels due to the lack of detection reagents. However, evidence indicated that 20β-S may also be a possible MIH in grouper. cyp21a2 expression increased sharply before the onset of GVBD. This gene encoded steroid 21-hydroxylase, which can convert the 17α-hydroxyprogesterone to 17α,21-dihydroxyprogesterone (11-deoxycortisol); 11-deoxycortisol can be converted to 20β-S by 20β-HSD catalysis. Due to the high expression of cyp21a2, we hypothesized that DHP may be converted to 20β-S through the catalysis of steroid 21-hydroxylase (Supplementary file 1: Fig. S3), but this hypothesis needs to be tested in a further study. Here, it is interesting to note that serum estradiol levels showed a slight increase after HCG injection. The expression of key enzymes (17β-HSD and CYP19A1a) responsible for the biosynthesis of estradiol was also upregulated during the maturation process. However, recent studies found that estrogen is an inhibitory factor in oocyte maturation in the mouse and zebrafish (Pang and Thomas 2010; Tarumi et al. 2014). Further functional studies on the roles of estrogen in oocyte maturation in the orange-spotted grouper will be interesting.
MIH triggers the resumption of meiosis by binding to the progestin receptor. mPRs and nPR are able to mediate non-genomic progestin signaling. mPRs, especially mPRα, are considered to mediate the DHP induction of oocyte maturation. nPR is more crucial in mediating ovulation in teleosts (Patiño and Thomas 1990a; Zhu et al. 2003; Tubbs et al. 2010; Hanna and Zhu 2011; Tokumoto et al. 2012; Tang et al. 2016). In this study, a slight increase of mprα expression was observed in O520-560. The mRNA expression of mprβ and mprγ was barely detected during HCG-induced oocyte maturation. From their expression patterns, the roles of mPRs in progestin induction of oocyte maturation were not clear in this species, and require further study. Interestingly, npr expression increased dramatically before the onset of GVBD, suggesting that nPR may play an important role in final oocyte maturation in the orange-spotted grouper.
In addition, other pathways like ECM-receptor interaction, focal adhesion, and AGE-RAGE signaling pathway in diabetic complications were also significantly enriched during the oocyte maturation. To our best knowledge, at least for now, there is little information about these enriched pathways involving in the regulation of oocyte maturation in vertebrates. As we all know, HCG administration can induce oocyte maturation and ovulation. The gene expression changes are not only for promoting the oocyte maturation but also for preparation of ovulation or early development of embryo. For example, fibronectin and collagen in ECM-receptor interaction pathway are related to extracellular-matrix remodeling and ovulation, while integrin are cell adhesion molecules that are thought to be involved in sperm-oocyte interaction (Kaji and Kudo 2002; Viana et al. 2018). Therefore, the physiological roles of these enriched pathways await further evaluation.
Genes Related to Oocyte Hydration
`Oocyte hydration involves yolk proteolysis, ion transport, and water uptake. Yolk acidification is the first step of yolk proteolysis through activation of cathepsins; it requires the cooperation of two channels, V-ATPase (Vacuolar-type H+-ATPase) and Cl− channel (Cerdà 2009; Sullivan and Yilmaz 2018). The expression of all the V-ATPase subunit genes did not change significantly during oocyte maturation (Supplementary file 2: Table S4), as the disassembly and reassembly of V-ATPase did not require new protein synthesis, but needed an intact microtubular network (Holliday et al. 2000). For the Cl− intracellular channel, expression of the related genes cicp2 and cicp4 was upregulated. After yolk acidification, cathepsins were activated, as evidenced by the steady increase in the expression of catb, catd, and catl during maturation in grouper. Cathepsin B is the major protease involved in yolk processing during oocyte maturation and hydration in fish (Matsubara et al. 2003; Raldúa et al. 2005). High expression of catb may mediate the activation of cathepsin D (Carnevali et al. 2006). In vitro experiments demonstrated that cathepsin L isolated from sea bream ovaries participated in the second proteolysis of yolk (Carnevali et al. 2008). Cathepsin F was upregulated during the final stage of oocyte maturation in Fundulus heteroclitus (Fabra and Cerdà 2004). However, there was no significant change in expression of catf in grouper, suggesting that this cathepsin may not participate in second proteolysis of yolk in grouper. Then, the activated cathepsins cleave yolk proteins into small peptides or FAAs. The increasing concentration of FAAs enhances the intracellular osmotic pressure for water influx into the oocyte.
The accumulation of ions also contributes to osmotic pressure in the oocyte. Na+,K+-ATPase is an important transporter that pumps K+ into the cytoplasm. K+ influx promotes yolk crystal disassembly and yolk sphere fusion (Selman et al. 2001). nktaα1 and nktaβ1, the genes of the two subunits of Na+,K+-ATPase, were highly expressed during the oocyte maturation. This result is consistent with the role of Na+,K+-ATPase in oocyte hydration in other teleost (LaFleur and Thomas 1991). NKCC acts as an aid for the active transport of sodium, potassium, and chloride into cells (Haas and Forbush 1998), and its expression was also significantly upregulated after HCG injection. These data suggested that these two transporters were activated and participated in hydration in orange-spotted grouper.
Along the accumulation of osmotic pressure, aquaporins (responsible for water uptake) are also expressed. There are two subtypes of AQP1 (AQP1a and AQP1b) in teleosts. AQP1b is specifically expressed in the ovary and was considered as the main aquaporin protein in water uptake during hydration (Fabra et al. 2005; Fabra et al. 2006; Zapater et al. 2011). In the orange-spotted grouper, aqp1b was highly expressed in O450-470 and O490-510, and its mRNA levels decreased afterwards. This result indicated that AQP1b may not be regulated by HCG at the level of mRNA transcription. The decrease in aqp1b mRNA level during hydration may be associated with the regulation in a post-transcription level (Fabra et al. 2006). Interestingly, aqp1a expression was hardly detected in O450-470 and O490-510, but its expression increased from O520-560 until O560-590. Unlike AQP1b requiring an intra-cellular transportation, AQP1a is located on the oocyte surface immediately after translation (Zapater et al. 2013). It can be hypothesized that AQP1a could mediate the rapid water uptake in cooperation with AQP1b during oocyte hydration in orange-spotted grouper.
Conclusion
In summary, we have investigated the morphological changes of follicles and serum steroid levels during the HCG-induced oocyte maturation in the orange-spotted grouper. For the first time, a large-scale transcriptomic analysis of follicles during the maturation has been conducted in a fish species which produce pelagic oocytes. Our results suggest that the novel functional LHR2 may mediate LH signaling in cooperation with LHR1, and may play a more important role in HCG-induced oocyte maturation in vivo. Both DHP and 20βs could be the potential MIH of Epinephelus coioides. The sharp increase of npr during oocyte maturation suggested that it may play a role not only in ovulation but also in maturation. For oocyte hydration, cathepsins, ion pumps, and ion channels were activated and drove water uptake through both AQP1 subtypes. This analysis will provide a valuable insight in understanding the mechanism underlying the oocyte maturation and hydration in grouper and other marine pelagic egg spawner.
Data Availability
Raw sequencing data is available through the NCBI Sequence Read Archive under BioProject Accession number PRJNA517188.
References
Carnevali O, Cionna C, Tosti L, Lubzens E, Maradonna F (2006) Role of cathepsins in ovarian follicle growth and maturation. Gen Comp Endocrinol 146:195–203
Carnevali O, Cionna C, Tosti L, Cerda J, Gioacchini G (2008) Changes in cathepsin gene expression and relative enzymatic activity during gilthead sea bream oogenesis. Mol Reprod Dev 75:97–104
Cerdà J (2009) Molecular pathways during marine fish egg hydration: the role of aquaporins. J Fish Biol 75:2175–2196
Cerdá J, Selman K, Wallace RA (1996) Observations on oocyte maturation and hydration in vitro in the black sea bass, Centropristis striata (Serranidae). Aquat Living Resour 9:325–335
Chapman RW, Reading BJ, Sullivan CV (2014) Ovary transcriptome profiling via artificial intelligence reveals a transcriptomic fingerprint predicting egg quality in striped bass, Morone saxatilis. PLoS One 9:1–10
Chen H, Zhang Y, Li S, Lin M, Shi Y, Sang Q, Liu M, Zhang H, Lu D, Meng Z, Liu X, Lin H (2011) Molecular cloning, characterization and expression profiles of three estrogen receptors in protogynous hermaphroditic orange-spotted grouper (Epinephelus coioides). Gen Comp Endocrinol 172:371–381
Clelland E, Peng C (2009) Endocrine/paracrine control of zebrafish ovarian development. Mol Cell Endocrinol 312:42–52
Fabra M, Cerdà J (2004) Ovarian cysteine proteinases in the teleost Fundulus heteroclitus: molecular cloning and gene expression during vitellogenesis and oocyte maturation. Mol Reprod Dev 67:282–294
Fabra M, Raldúa D, Power DM, Deen PMT, Cerdà J (2005) Marine fish egg hydration is aquaporin-mediated. Science 307:545–545
Fabra M, Raldúa D, Bozzo MG, Deen PMT, Lubzens E, Cerdà J (2006) Yolk proteolysis and aquaporin-1o play essential roles to regulate fish oocyte hydration during meiosis resumption. Dev Biol 295:250–262
Haas M, Forbush B (1998) The Na-K-cl cotransporters. J Bioenerg Biomembr 30:161–172
Hanna RN, Zhu Y (2011) Controls of meiotic signaling by membrane or nuclear progestin receptor in zebrafish follicle-enclosed oocytes. Mol Cell Endocrinol 337:80–88
Holliday LS, Lu M, Lee BS, Nelson RD, Solivan S, Zhang L, Gluck SL (2000) The amino-terminal domain of the B subunit of vacuolar H+-ATPase contains a filamentous actin binding site. J Biol Chem 275:32331–32337
Jalabert B (1976) In vitro oocyte maturation and ovulaion in rainbow trout (Salmo gairdneri), northern pike (Esox lucius), and goldfish (Carassius auratus). J Fish Res Board Can 33:974–988
Jia Y, Nan P, Zhang W, Wang F, Zhang R, Liang T, Ji X, Du Q, Chang Z (2018) Transcriptome analysis of three critical periods of ovarian development in Yellow River carp (Cyprinus carpio). Theriogenology 105:15–26
Kaji K, Kudo A (2002) The mechanism of sperm– oocyte fusion in mammals. Reproduction 127:423–429
Kim D, Pertea G, Trapnell C, Pimentel H, Kelley R, Salzberg SL (2013) TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol 14:R36
King VW, Berlinsky DL, Sullivan CV (1995) Involvement of gonadal steroids in final oocyte maturation of white perch (Morone americana) and white bass (M. chrysops): in vivo and in vitro studies. Fish Physiol Biochem 14:489–500
LaFleur GJ, Thomas P (1991) Evidence for a role of Na+,K+-ATPase in the hydration of Atlantic croaker and spotted seatrout oocytes during final maturation. J Exp Zool 258:126–136
Langmead B, Salzberg SL (2012) Fast gapped-read alignment with Bowtie 2. Nat Methods 9:357–359
Li JZ, Cheng CHK (2018) Evolution of gonadotropin signaling on gonad development: insights from gene knockout studies in zebrafish. Biol Reprod 99:686–694
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 25:402–408
Lubzens E, Young G, Bobe J, Cerdà J (2010) Oogenesis in teleosts: how fish eggs are formed. Gen Comp Endocrinol 165:367–389
Matsubara T, Nagae M, Ohkubo N, Andoh T, Sawaguchi S, Hiramatsu N, Sullivan CV, Hara A (2003) Multiple vitellogenins and their unique roles in marine teleosts. Fish Physiol Biochem 28:295–299
Maugars G, Dufour S (2015) Demonstration of the coexistence of duplicated LH receptors in teleosts, and their origin in ancestral actinopterygians. PLoS One 10:1–29
Nagahama Y, Yoshikuni M (2008) Regulation of oocyte maturation in fish. Develop Growth Differ 50:S195–S219
Pang Y, Thomas P (2010) Role of G protein-coupled estrogen receptor 1, GPER, in inhibition of oocyte maturation by endogenous estrogens in zebrafish. Dev Biol 342:194–206
Patiño R, Thomas P (1990a) Characterization of membrane receptor activity for 17α,20β,21-trihydroxy-4-pregnen-3-one in ovaries of spotted seatrout (Cynoscion nebulosus). Gen Comp Endocrinol 78:204–217
Patiño R, Thomas P (1990b) Effects of gonadotropin on ovarian intrafollicular processes during the development of oocyte maturational competence in a teleost, the Atlantic croaker: evidence for two distinct stages of gonadotropin control of final oocyte maturation. Biol Reprod 43:818–827
Peng C, Xiao L, Chen H, Han Y, Huang M, Zhao M, Li S, Liu Y, Yang Y, Zhang H, Zhang Y, Lin H (2018) Cloning, expression and functional characterization of a novel luteinizing hormone receptor in the orange-spotted grouper, Epinephelus coioides. Gen Comp Endocrinol 267:90–97
Picha ME, Shi B, Thomas P (2012) Dual role of IGF-II in oocyte maturation in southern flounder Paralichthys lethostigma: up-regulation of mPRα and resumption of meiosis. Gen Comp Endocrinol 177:220–230
Raldúa D, Fabra M, Bozzo MG, Weber E, Cerdà J (2005) Cathepsin B-mediated yolk protein degradation during killifish oocyte maturation is blocked by an H+-ATPase inhibitor: effects on the hydration mechanism. Am J Physiol Regul Integr Comp Physiol 290:R456–R466
Ravi P, Jiang J, Liew WC, Orbán L (2014) Small-scale transcriptomics reveals differences among gonadal stages in Asian seabass (Lates calcarifer). Reprod Biol Endocrinol 12:1–14
Rocha A, Zanuy S, Carrillo M, Gómez A (2009) Seasonal changes in gonadal expression of gonadotropin receptors, steroidogenic acute regulatory protein and steroidogenic enzymes in the European sea bass. Gen Comp Endocrinol 162:265–275
Sakai N, Tanaka M, Takahashi M, Adachi S, Nagahama Y (1993) Isolation and expression of rainbow trout (Oncorhynchus mykiss) ovarian cDNA encoding 3β-hydroxysteroid dehydrogenase/Δ5-4-isomerase. Fish Physiol Biochem 11:273–279
Selman K, Petrino TR, Wallace RA (1994) Experimental conditions for oocyte maturation in the zebrafish, Brachydanio rerio. J Exp Zool 269:538–550
Selman K, Wallace RA, Cerdà J (2001) Bafilomycin A1 inhibits proteolytic cleavage and hydration but not yolk crystal disassembly or meiosis during maturation of sea bass oocytes. J Exp Zool 290:265–278
Sullivan CV, Yilmaz O (2018) Vitellogenesis and yolk proteins, fish. In: Skinner MK (ed) Encyclopedia of reproduction, 2nd edn. Elsevier
Tang H, Liu Y, Li J, Yin Y, Li G, Chen Y, Li S, Zhang Y, Lin H, Liu X, Cheng HK (2016) Gene knockout of nuclear progesterone receptor provides insights into the regulation of ovulation by LH signaling in zebrafish. Sci Rep 6:28545
Tarumi W, Itoh MT, Suzuki N (2014) Effects of 5α-dihydrotestosterone and 17β-estradiol on the mouse ovarian follicle development and oocyte maturation. PLoS One 9:e99423
Tingaud-Sequeira A, Chauvigné F, Lozano J, Agulleiro MJ, Asensio E, Cerdà J (2009) New insights into molecular pathways associated with flatfish ovarian development and atresia revealed by transcriptional analysis. BMC Genomics 10:434
Tokumoto T, Tokumoto M, Oshima T, Shimizuguchi K, Fukuda T, Sugita E, Suzuki M, Sakae Y, Yi A, Nakayama R, Roy SR, Rahman SM, Pang Y, Dong J, Thomas P (2012) Characterization of multiple membrane progestin receptor (mPR) subtypes from the goldfish ovary and their roles in the induction of oocyte maturation. Gen Comp Endocrinol 177:168–176
Trant JM, Thomas P (1989) Isolation of a novel maturation-inducing steroid produced in vitro by ovaries of Atlantic croaker. Gen Comp Endocrinol 75:397–404
Trant JM, Thomas P, Shackleton C (1986) Identification of 17α, 20β, 21-trihydroxy-4-pregnen-3-one as the major ovarian steroid produced by the teleost Micropogonias undulatus during final oocyte maturation. Steroids 47:89–99
Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, Pimentel H, Salzberg SL, Rinn JL, Pachter L (2012) Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protoc 7:562–578
Tubbs C, Pace M, Thomas P (2010) Expression and gonadotropin regulation of membrane progestin receptor alpha in Atlantic croaker (Micropogonias undulatus) gonads: role in gamete maturation. Gen Comp Endocrinol 165:144–154
Viana IKS, Gonçalves LAB, Ferreira MAP, Mendes YA, Rocha RM (2018) Oocyte growth, follicular complex formation and extracellular-matrix remodeling in ovarian maturation of the imperial zebra pleco fish Hypancistrus zebra. Sci Rep 8:13760
Wang Q, Liu Y, Peng C, Wang X, Xiao L, Wang D, Tang Z, Li S, Zhang H, Zhang Y, Lin H (2017a) Molecular regulation of sex change induced by methyltestosterone -feeding and methyltestosterone -feeding withdrawal in the protogynous orange-spotted grouper. Biol Reprod 97:324–333
Wang Q, Qi X, Tang H, Guo Y, Li S, Li G, Yang X, Zhang H, Liu X, Lin H (2017b) Molecular identification of StAR and 3βHSD1 and characterization in response to GnIH stimulation in protogynous hermaphroditic grouper (Epinephelus coioides). Comp Biochem Physiol B Biochem Mol Biol 206:26–34
Xu G, Du F, Li Y, Nie Z, Xu P (2016) Integrated application of transcriptomics and metabolomics yields insights into population-asynchronous ovary development in Coilia nasus. Sci Rep 6:31835
Yamashita M, Kajiura H, Tanaka T, Onoe S, Nagahama Y (1995) Molecular mechanisms of the activation of maturation-promoting factor during goldfish oocyte maturation. Dev Biol 168:62–75
Zapater C, Chauvigné F, Norberg B, Finn RN, Cerdà J (2011) Dual neofunctionalization of a rapidly evolving aquaporin-1 paralog resulted in constrained and relaxed traits controlling channel function during meiosis resumption in teleosts. Mol Biol Evol 28:3151–3169
Zapater C, Chauvigné F, Tingaud-Sequeira A, Finn RN, Cerdà J (2013) Primary oocyte transcriptional activation of aqp1ab by the nuclear progestin receptor determines the pelagic egg phenotype of marine teleosts. Dev Biol 377:345–362
Zhu Y, Rice CD, Pang Y, Pace M, Thomas P (2003) Cloning, expression, and characterization of a membrane progestin receptor and evidence it is an intermediary in meiotic maturation of fish oocytes. Proc Natl Acad Sci U S A 100:2231–2236
Acknowledgements
We are grateful to Guangdong Daya Bay Fishery Development Center (Huizhou, Guangdong, China) for providing mature female orange-spotted groupers for this study, and Mr. Zeshu Yu for his assistance in collecting samples.
Funding
This research was supported by the National Natural Science Foundation of China (No. 31672631, No. 31572596), and Guangzhou Science and Technology Program (201804020013) and the open fund of Guangdong South China Sea Key Laboratory of Aquaculture for Aquatic Economic Animals (KFKT2019ZD07).
Author information
Authors and Affiliations
Contributions
HRL, SSL, and YZ conceived and designed the study; LT performed the experiments and bioinformatics analysis; JXC, ZFY, LT, MZ, and SSL worked for fish breeding and sample collecting. LT and SSL wrote the paper. All authors read the manuscript and approved the final version.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tang, L., Chen, J., Ye, Z. et al. Transcriptomic Analysis Revealed the Regulatory Mechanisms of Oocyte Maturation and Hydration in Orange-Spotted Grouper (Epinephelus coioides). Mar Biotechnol 21, 537–549 (2019). https://doi.org/10.1007/s10126-019-09902-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10126-019-09902-0