Abstract
Agarase is a promising biocatalyst for several industrial applications. Agarase production was evaluated by the marine fungus Dendryphiella arenaria utilizing Palisada perforata as a basal substrate in semi-solid state fermentation. Seaweed biomass, glucose, and sucrose were the most significant parameters affecting agarase production, and their levels were further optimized using Box-Behnken design. The maximum agarase activity was 7.69 U/mL. Agarase showed a degree of thermostability with half-life of 99 min at 40 °C, and declining to 44.72 min at 80 °C. Thermodynamics suggested an important process of protein aggregation during thermal inactivation. Additionally, the enzymatic saccharification of the seaweed biomass using crude agarase was optimized with respect to biomass particle size, solid/liquid ratio, and enzyme loadings. The amount of biosugars obtained after optimization was 26.15 ± 1.43 mg/g. To the best of our knowledge, this is the first report on optimization of agarase in D. arenaria.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Red macroalgae (Rhodophyta) have been considered as a sustainable renewable resource for the production of bioenergy and bio-based chemicals. The main advantages of the macroalgal biomass are high content and easily depolymerized polysaccharides in comparison to terrestrial plants without the need for land, freshwater, or fertilizer for growth, and thus exhibited no competition with the food resources (Yun et al. 2016).
Among red macroalgae, the genus Gracilaria, Poryphra, Kappaphycus, and Gelidium are the most commonly used on biotechnological level. On the contrary, Palisada is a less exploited genus in the Rhodophyceae, despite it being distributed worldwide and found in large quantities in Egypt (Issa et al. 2014). Palisada perforata is quite similar to the other Rhodophyceae in the composition of the cell wall polysaccharides. Usov and Elashvili (1991) investigated the composition of the polysaccharides from different Laurencia complex species (including Palisada spp.) and found to be composed of sulfated and/or methylated polymers having an agarose backbone. Agarose is a linear polysaccharide composed of D-galactose and 3,6-anhydro-L-galactose linked by alternative α-1,3 and β-1,4 linkage groups (Yun et al. 2016).
Agarases are enzymes that catalyze the hydrolysis of agar or agarose. Generally, there are two distinct types of agarases, α-agarase (E.C. 3.2.1.158) and β-agarase (E.C. 3.2.1.81), depending on which glycosidic bond is cleaved. Oligosaccharides obtained by agarases have received a great attention due to their various biological activities such as antioxidant (Wang et al. 2004), skin moistening and whitening effect (Kobayashi et al. 1997), and antitumor activity (Fernández et al. 1989). They have been used for the enzymatic saccharification of biomass for the production of biofuel (Kim et al. 2017). Besides that, agarases have been used to isolate protoplasts from the red seaweeds and to recover DNA from agarose gel (Fu and Kim 2010).
Extracellular agarases have been reported from several bacteria, such as Pseudomonas sp., Bacillus sp., Vibrio sp., Agarivorans sp., Cytophaga sp., Thalassomonas sp., Pseudoalteromonas sp., and Acinetobacter sp. (Fu and Kim 2010). However, studies on fungal agarases are scarce. Dendryphiella arenaria is an obligate marine fungus, which is generally found associated with marine macroalgae (Abdel-Gawad et al. 2014) and was reported as a potential source of agarase (Gomaa et al. 2015). More recently, algal associated fungi have attracted attention in the field of biotechnology (Abdel-Gawad et al. 2017; Hifney et al. 2017). The objective of this current study is to evaluate the importance of different nutritional supplements on agarase production by the filamentous fungus D. arenaria in semi-solid state fermentation using P. perforata as a basal substrate. Plackett-Burman and Box-Behnken designs were employed for identifying and optimizing the critical variables for maximum agarase production. Thermostability of crude agarase extract was determined at various temperatures. In addition, the crude agarase was applied in the enzymatic saccharification of red algal biomass. To the best of our knowledge, this is the first report on fungal agarase optimization-mediated saccharification of the red algal biomass.
Materials and Methods
Seaweed Material
Naturally growing P. perforata (Bory) K.W. Nam (Rhodophyta) was collected during summer from the intertidal zone of Hurghada, Egypt (27° 12′ N, 33° 50′ E). The macroalgal biomass was air-dried and milled in a home blender.
Microorganism and Fermentation Conditions
D. arenaria Nicot was isolated by thallus-surface sterilization method using 70% ethanol (Gomaa et al. 2015) from the red alga P. perforata collected from the Red Sea, Egypt. D. arenaria was cultivated on potato dextrose agar (PDA) plates for 9 days at 28 °C. Fungal spores were collected in sterile natural seawater and counted using a hemocytometer to give a final inoculum concentration of about 1 × 107 propagules mL−1.
The fermentation was carried out in 40-mL glass bottles with 10 mL of natural seawater (either diluted 75% v/v or undiluted, salinity 42 PSU) supplemented with the following nutrients (0.0–0.05% w/v): glucose, sucrose, NaNO3, urea, yeast extract, and peptone, and K2HPO4 (0.0–0.1% w/v). The pH values of the culture media were adjusted to pH of either 6 or 8 before sterilization. All the culture media were sterilized separately by autoclaving (121 °C, 20 min) and then oven sterilized (120 °C, 90 min) seaweed biomass (particle size, 600 μm) was added at a concentration of 2.5 or 5% w/v. The culture media were inoculated by 100 μL of the fungal inoculum and incubated at temperature 25 or 35 °C for 3 days at 120 rpm.
Screening of the Main Factors Influencing Agarase Production
Plackett-Burman (PB) experimental design was evaluated for the screening of the most critical factors for enhancing agarase production in a natural medium. Two natural components (seaweed biomass and natural seawater), two culture conditions (temperature and pH), and seven additional nutritional parameters (glucose, sucrose, NaNO3, urea, yeast extract, peptone, and K2HPO4) were examined for 12 combinations of experiments at two levels: low level and high level coded (− 1 and + 1), respectively (Table 1). All experiments were carried out in duplicate and the average agarase activity (U/mL) was assigned as response. PB design is based on the first order model:
Where Y is agarase activity (response or dependent variable), β 0 is the model intercept, β i is the linear coefficient, and X i is the level of the independent variable.
Box-Behnken Experimental Design
Box-Behnken design was developed to determine the optimum level of selected variables (seaweed biomass (2.5–7.5% w/v), glucose (0.1–0.25% w/v), and sucrose (0.1–0.25% w/v)) that were selected from the PB design, and to investigate their interactions on agarase production. The three variables were tested at three levels: high, medium, low levels coded as + 1, 0, and − 1, respectively (Table 2). A total of 17 experiments including 5 replicates at the center point were carried out and agarase activity was assigned as response. Box-Behnken experimental design (BBD) is based on a second order polynomial model:
Where Y is the predicted response; X i and X j are the independent variables; β o is the intercept (regression coefficient of the model); β i , β ii , and β ij are the linear, quadratic, and interaction coefficients, respectively.
The optimum conditions for agarase production developed by BBD were evaluated for its validity. Thus, experiments were performed under the optimized conditions and the experimental values were compared with the predicted results.
Determination of Agarase Activity
The fermentation broth was separated by centrifugation (6000 rpm, 10 min), and used for the determination of agarase activity. Agarase activity was determined by measurement of reducing sugar production using 3,5 dinitrosalicylic acid (DNS) method (Miller 1959). A hundred microliters of the crude enzyme solution was mixed with 900 μL of substrate (agarose, 0.1% w/v, dissolved in 50 mM phosphate buffer, pH 7.0). The mixture was incubated at 40 °C for 60 min, and then the reaction was stopped by adding dinitrosalicylic acid reagent. The mixture was heated at 90 °C for 10 min, and then the tubes were cooled down and the developed color was measured at 540 nm using UV-Vis spectrophotometer. The absorbance values were calculated using D-galactose as standard. One unit of agarase activity (1 U) was defined as the amount of enzyme that released 1 μmol of reducing sugars per minute under the conditions of the proposed assay.
Thermal Stability of Agarase
The thermal stability of crude agarase from D. arenaria was measured by incubating 100 μL of the crude enzyme, in the absence of substrate, at temperature from 40 to 80 °C for 30 and 60 min. At the end of incubation, the tubes were cooled down in an ice bath, and the residual enzyme activity was measured (according to section Determination of Agarase Activity).
The values of residual enzyme activity at each temperature were used to calculate the thermal deactivation rate constant (Kd, min−1) by linear regression of the data of ln (% residual activity) vs. time, according to the Arrhenius kinetic model (Marín et al. 2003; de Castro et al. 2015),
Where A is the remaining activity after heat treatment, A0 is the initial enzyme activity, and t (min) is time of thermal treatment.
The exposure time required to reach a value of 50% residual activity (half-life time, t1/2) was determined from the relationship:
The D-value (the decimal reduction time) or the time required to maintain 10% residual activity was calculated as:
The Z-value (the temperature required to reduce the D-value by one logarithmic cycle) was calculated from the slope of the plot between log (D) vs. temperature (°C) (Pal and Khanum 2011) using the equation:
The activation energy of denaturation (Ed) was determined by the plot of ln (kd) vs. reciprocal of the absolute temperature (K) using the following equation:
Where R (gas constant) = 8.3145 J/mol/K.
The enthalpy of inactivation (ΔH°, kJ/mol), Gibbs free energy (ΔG°, kJ/mol), and variation in entropy (ΔS°, J/mol/K) were calculated from the following equations:
Where h is the Planck constant (11.04 × 10−36 J min), and KB is the Boltzmann constant (1.3806 × 10−23 J/K).
Enzymatic Saccharification of the Macroalgal Biomass
The crude agarase produced by the culture of D. arenaria at optimized conditions was evaluated for enzymatic saccharification of P. perforata biomass without any chemical pretreatment. For this, 100 mg dry weight of the macroalgal sample, sieved to different particle sizes (1, 0.6, 0.2, and < 0.15 mm using mesh no. 18, 30, 70, and 100, respectively), was mixed with 2 mL phosphate buffer (pH 7, 0.1 M) and 100 μL of crude agarase. The mixture was agitated (100 rpm) at temperature 40 °C for 24 h. Samples were centrifuged (6000 rpm, 10 min) and the supernatant was used for reducing sugar quantification by DNS method. Similarly, the effects of different solid/liquid (S/L) ratios (1:20, 1:30, 1:40, and 1:50 g/mL) and different enzyme loadings (1:1, 2:1, 3:1, 4:1, 5:1, and 6:1 mL/g) were also determined using algal biomass of particle size < 0.15 mm.
Statistical Analysis
The statistical analysis was carried out using Design Expert 7.0.0 statistical software (Stat-Ease Inc., Minneapolis, USA). The experimental results were analyzed by multiple regression analysis and analysis of variance (ANOVA). The values of enzymatic saccharification were expressed as mean ± SD. Multiple comparisons of means were performed by the Tukey’s B test. Values of p < 0.05 were considered significant for all the tests.
Results and Discussion
Evaluation of Variables Affecting on Agarase Production
The PB experimental design is a powerful method for investigating and evaluating the important variables that influence the response. The data in Table 1 indicate that there is a wide variation in the agarase activity (2.26 to 6.23 U/mL) in the 12 experiments, reflecting that medium optimization is important to obtain a high agarase production. The relationship between agarase production and the 11 independent variables was determined by multiple regression analysis. The data revealed that pH (D) and peptone (K) are insignificant variables with no effect on agarase production. Thus, instead of analyzing the maximum model factors, backward regression of insignificant factors was applied (at alpha = 0.1) to eliminate pH and peptone from the model analysis. Table S1 represents the results of ANOVA analysis of PB design. The values of the effect (Table S1) indicate the change in the response as the factor changes from its low (− 1) level to its high (+ 1) level. Seaweed biomass (A), temperature (C), glucose (E), sucrose (F), NaNO3 (G), yeast extract (J), and K2HPO4 (K) showed significant positive effect on agarase production, while seawater concentration (B) and urea (H) showed significant negative effect.
Pareto chart (Fig. 1) illustrates the order of significance of the different factors on agarase production. It showed that glucose and sucrose supplementation to the seaweed natural medium is important for agarase production using P. perforata biomass. Additionally, seawater and K2HPO4 concentrations were the least significant factors influencing agarase production.
The first order polynomial equation (in terms of coded factors) for agarase production was derived as a function of the significant independent variables and neglecting the insignificant factors:
Box-Behnken Experimental Design
The results of PB design suggested that the concentrations of seaweed biomass, glucose, and sucrose are the most critical factors affecting agarase production. These variables were selected for further optimization using BBD (Table 2), while other less significant variables were used in all the experiments at their (− 1) level and (+ 1) level, for the negatively influencing variables and the positively influencing variables, respectively. The results of BBD design for agarase production are listed in Table 2.
The empirical relationship between agarase activity and the three independent factors is represented by the following second order polynomial equation:
The fitness and adequacy of the developed model were checked by determination coefficient (R 2). The high R 2 value (98.51%, Table S2) indicates that agarase model is adequate and that only 1.5% of the total variation is not explained by the model. The adjusted-R 2 (95.83%) is in reasonable agreement with the predicted-R 2 (78.03%, Table S2), and reflected a high correlation between the experimental and predicted results (Abdel-Gawad et al. 2017). Adequate precision of 19.39 is high and indicates adequate model discrimination. The relatively low value of coefficient of variation (9.79%) reflects a good accuracy of the experimental values (Fawzy et al. 2017). In addition, the lack of fit is not significant (p ˃ 0.05, Table S2). Thus, the proposed model could predict agarase production according to the tested parameters.
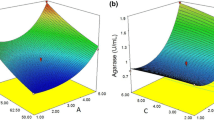
The significance, adequacy, and goodness of fit for the agarase model were explained by Pareto analysis of variance (ANOVA), and the results are listed in Table S2. The developed model appeared to be highly significant for the experimental results at very low probability value (p = 0.0005). To investigate the main and interactive effects between the independent variables on agarase production, ANOVA results were analyzed and the relationship was visualized using three-dimensional response surface graphs. ANOVA analysis revealed that concentration of seaweed biomass (A) is the high statistically significant factor influencing agarase production (p value = 0.0001, Table S2). Among the two tested additional carbon sources, increasing sucrose concentration (F) is more important than increasing glucose concentration (E), as indicated by the lower p value (0.0002) for sucrose in comparison to glucose (p value = 0.0136). Moreover, both glucose and sucrose were important nutritional factors, and since sucrose is a disaccharide made up of glucose and fructose, the fermentation media could be developed using just sucrose as an additional carbon source. This result was confirmed by the high statistically significant interaction between seaweed biomass (A) and sucrose (F) (p value = 0.0005). The interaction between either seaweed biomass and glucose or sucrose and glucose was not significant (p ˃ 0.05) (Table S2). In this context, glucose supplementation to the fermentation media induced agarase production by Micrococcus sp. GNUM-08124 (Choi et al. 2011).
Figure 2 depicts the 3-D response surface plots showing the influence of seaweed biomass (A), glucose (E), and sucrose on agarase activity. There was an upsurge in the agarase production with the increase in the concentration of seaweed biomass from 2.5 to 7.5% (w/v). At low concentration of seaweed biomass (2.5% w/v), no obvious variation in agarase production was observed with the addition of sucrose (Fig. 2b). In contrast, at high seaweed biomass levels, a clear escalating trend was observed with increasing sucrose concentration (Fig. 2b).
Under unoptimized conditions, agarase production was about 2.25 U/mL (with no addition of nutrients to the fermentation media), which increased to 7.63 U/mL (at experiment no. 8, Table 2). This corresponds to the increase of more than 3-fold in agarase production. This finding could be observed because seaweed biomass and natural seawater provide essential nutrients for the growth of microorganisms (Gomaa et al. 2015).
Validation of the Experimental Model
An optimum condition to maximize agarase production was defined using the numerical optimization and Derringer’s desirability function in the Design expert software. This function searches for the most desirable combination of factor levels to reach a specific goal for the response (Hifney et al. 2016). Applying the desirability method, the predicted optimum levels for various parameters were as follows: seaweed biomass (7.5% w/v), glucose (0.08% w/v), and sucrose (0.25% w/v) give 7.76 U/mL of agarase activity. Triplicate experiments were carried out under the aforementioned conditions and the agarase activity was found to be 7.69 ± 0.4 U/mL. The experimental results are very close to the data obtained from optimization analysis using desirability functions, which indicates the adequacy of the developed quadratic models. Thus, Box-Behnken design in corporate with desirability functions could be effectively used to optimize agarase production from the marine fungus D. arenaria. The composition of the final optimized medium was as follows: seaweed biomass (7.5% w/v), glucose (0.08% w/v), and sucrose (0.25% w/v), NaNO3 (0.05% w/v), yeast extract (0.05% w/v), and K2HPO4 (0.05% w/v).
Reports on agarase activity from filamentous fungi are limited. However, the values obtained through optimization are higher than reported previously (Gomaa et al. 2015). Additionally, studies on bacterial agarases in the fermentation medium recorded agarase production of 0.45, 1.8, and 2.5 U/mL, by Acinetobacter sp. AG LSL-1, Alteromonas sp. SY37-12, and Agarivorans sp. HZ105, respectively (Lakshmikanth et al. 2006; Wang et al. 2006; Hu et al. 2009).
Thermal Inactivation of Agarase
The thermal stability of crude agarase was determined by preincubating the enzyme at temperatures ranged from 40 to 80 °C, and calculating the residual enzyme activities. Agarase retained more than 75% of its original activity after exposure to temperature 40 and 50 °C for 30 min, while increasing exposure time (60 min) decreased enzyme activity to 65.74, and 55.46% at temperature 40 and 50 °C, respectively (Table 3). In the current study, agarase from D. arenaria was more thermostable at temperature 40 and 50 °C. Additionally, more than one third of enzyme activity still remains after incubation at 80 °C for 60 min. Some bacterial agarases were reported to have short range of thermal stability (Gupta et al. 2013; Cui et al. 2014). Exo-β-agarase from the algal endophytic Pseudomonas sp. lost 50% of its activity after incubation for 1 h at 35 °C (Gupta et al. 2013). Similarly, exo-β-agarases produced by Pseudoalteromonas sp. AG52 and Agarivorans sp. LQ48 reached 50% residual activity after incubation at 40 °C (Oh et al. 2010; Long et al. 2010). Thus, the fungal agarase produced in the current study appear to be more thermostable than at least some of the previously reported bacterial agarases.
A half-life (t1/2) of an enzyme is a reliable estimation of its thermostability, where a longer t1/2 is an indication that an enzyme can withstand a certain temperature for a longer period. Agarase exhibited a half-life of 99.02 min at 40 °C, and declining to 44.72 min at 80 °C. The D-values (time required for 90% reduction of agarase activity) are higher at temperature 40 and 50 °C, and showed a remarkable decrease at temperature 60 to 80 °C (Table 3). Higher values of t1/2 and D-values at temperatures 40 and 50 °C are important and desirable for industrial application. The plot of log D-values vs. temperature (graph not shown) was used to calculate Z-value. The Z-value for agarase was found to be 111.11 °C. Generally, low Z-values mean that an enzyme is more sensitive to increase in temperature, while high Z-values mean more sensitivity to the duration of heat treatment (Marín et al. 2003). Therefore, agarase from D. arenaria is more sensitive to the duration of thermal treatment rather than increase in temperature.
The value of activation energy (Ed) of thermal denaturation was 19.41 kJ/mol (Table 3), which is considered low and indicates that low energy is required to initiate thermal denaturation of the treated agarase. The Ed value is directly related to another thermodynamic parameter, the change in enthalpy (ΔH°). The values of ΔH° were also low, which clearly indicate that low energy is required for thermal unfolding of agarase, as well as thermal denaturation is easier at higher temperatures. Additionally, positive ΔH° values indicated the endothermic nature of the inactivation reaction. On the other hand, Gibbs free energy (ΔG°) is a more reliable measure of enzyme thermostability. A smaller ΔG° values indicate a lower stability of an enzyme (Marangoni 2003). ΔG° was 100.36 kJ/mol at 40 °C with small decrease with increase in temperature (Table 3), indicating a degree of thermostability of agarase. The denaturation of an enzyme is accompanied by increasing the extent of disordering of the active site or the enzyme structure, and thus results in an increase in entropy (ΔS°) (Marín et al. 2003; Han et al. 2015). The values of ΔS° are negative and indicated that the native form of agarase is more in ordered state (Table 3). In addition, it indicates that there is an important process of protein aggregation instead of unfolding during thermal inactivation (Marín et al. 2003; Pal and Khanum 2011). Generally, when ΔH° is positive and ΔS° is negative, the process is not spontaneous at any temperature. This result is similar to many fungal enzymes (Pal and Khanum 2011; de Castro et al. 2015; Han et al. 2015).
The agarase produced in the current study provide promising perspectives of application in the processes that require temperatures around 40 to 50 °C. For instance, in the simultaneous saccharification and fermentation technologies for bioethanol production, the optimum conditions for microbial fermentation are between 30 and 37 °C, and the reaction of enzyme may occur at 40 to 50 °C (Ishola et al. 2013). In addition, the production of oligosaccharides from agar, agarose, requires high activity and stability at temperatures higher than the gelling temperature of these polysaccharides (around 34–40 °C) (Cui et al. 2014).
Enzymatic Saccharification of the Red Algal Biomass
The enzymatic saccharification of P. perforata biomass using semi-solid state-derived agarase was optimized with respect to algal particle size (mm), solid/liquid (S/L ratio) (g/mL), and enzyme loadings (mL/g). The data in Fig. 3a demonstrates that decreasing the particle size of the macroalgal biomass improved the enzymatic saccharification efficiency of the macroalgal biomass. In general, the reduction of the particle size was the first step in the pretreatment of the raw material. Smaller particle size may increase the susceptibility of the macroalgal biomass to enzymatic degradation through decreasing the degree of polymerization of the algal polysaccharides. Particle size of < 0.15 mm was chosen to determine the optimum S/L ratio. Figure 3b depicts the effect of different buffer volumes at the same solid loadings on the enzymatic saccharification of the macroalgal biomass. The release of reducing sugars was significantly enhanced at S/L ratio of 1:40 and 1:50 g/mL. The high amount of the saccharification buffer reduced the product concentration and subsequently prevented enzymatic inhibition. A S/L ratio of 1:50 g/mL was considered optimum, and used for further optimization of the enzyme loadings. Figure 3c shows the enzymatic hydrolysis of the algal biomass under different dose of the crude agarase in the range of 1:1 to 6:1 mL enzyme/g substrate. Increasing the enzymatic loading to 5:1 or 6:1 mL/g significantly enhanced the enzymatic production of reducing sugars.
The maximum amount of fermentable sugars obtained after optimization was 26.15 ± 1.43 mg/g substrate, using algal particle size < 0.15 mm, S/L ratio 1:50 g/mL, and agarase loading 5:1 mL/g. Previous reports used chemical pretreatment (dilute HCl or acetic acid) for the production of reducing sugars from the red algal biomass or agarose (Kim et al. 2012; Kim et al. 2017). Thus, the amount of reducing sugars in the current study is considered lower than the values reported previously (Yun et al. 2016) using recombinant agarase in combination with acid pretreatment. Generally, acid pretreatment increases the cost of the whole process and can display undesirable effects such as the formation of hydroxymethylfulfural, levulinic acid, and formic acid (Park et al. 2012), which act as inhibitors for the microbial fermentation (Yun et al. 2016). Besides that, an additional neutralization step is required and causes a further cost for the use of alkali.
Conclusion
Agarase produced by the algal associated fungus D. arenaria was enhanced by the addition of nutritional supplements (glucose and sucrose) to a basal natural medium composed of P. perforata and seawater in semi-solid state fermentation. The use of the red algal biomass for the production of agarase contributed to decreasing the cost of fermentation media as well as adding economic value to macroalgae biomass. The crude agarase could be used for the valorization of the macroalgal biomass for the production of fermentable sugars, which could be subsequently used for the production of fermentative chemicals such as bioethanol.
References
Abdel-Gawad KM, Hifney AF, Issa AA, Gomaa M (2014) Spatio-temporal, environmental factors, and host identity shape culturable-epibiotic fungi of seaweeds in the Red Sea, Egypt. Hydrobiologia 740:37–49
Abdel-Gawad KM, Hifney AF, Fawzy MA, Gomaa M (2017) Technology optimization of chitosan production from Aspergillus niger biomass and its functional activities. Food Hydrocoll 63:593–601
Choi HJ, Hong JB, Park JJ, Chi W-J, Kim M-C, Chang Y-K, Hong S-K (2011) Production of agarase from a novel Micrococcus sp. GNUM-08124 strain isolated from the East Sea of Korea. Biotechnol Bioprocess Eng 16:81–88
Cui F, Dong S, Shi X, Zhao X, Zhang X-H (2014) Overexpression and characterization of a novel thermostable β-agarase YM01-3, from marine bacterium Catenovulum agarivorans YM01T. Mar Drugs 12:2731–2747
de Castro RJS, Ohara A, Nishide TG, Albernaz JRM, Soares MH, Sato HH (2015) A new approach for proteases production by Aspergillus niger based on the kinetic and thermodynamic parameters of the enzymes obtained. Biocatal Agric Biotechnol 4:199–207
Fawzy MA, Gomaa M, Hifney AF, Abdel-Gawad KM (2017) Optimization of alginate alkaline extraction technology from Sargassum latifolium and its potential antioxidant and emulsifying properties. Carbohydr Polym 157:1903–1912
Fernández LE, Valiente OG, Mainardi V, Bello JL, Vélez H, Rosado A (1989) Isolation and characterization of an antitumor active agar-type polysaccharide of Gracilaria dominguensis. Carbohydr Res 190:77–83
Fu XT, Kim SM (2010) Agarase: review of major sources, categories, purification method, enzyme characteristics and applications. Mar Drugs 8:200–218
Gomaa M, Hifney AF, Fawzy MA, Issa AA, Abdel-Gawad KM (2015) Biodegradation of Palisada perforata (Rhodophyceae) and Sargassum sp. (Phaeophyceae) biomass by crude enzyme preparations from algicolous fungi. J Appl Phycol 27:2395–2404
Gupta V, Trivedi N, Kumar M, Reddy C, Jha B (2013) Purification and characterization of exo-β-agarase from an endophytic marine bacterium and its catalytic potential in bioconversion of red algal cell wall polysaccharides into galactans. Biomass Bioenergy 49:290–298
Han W, Lam WC, Melikoglu M, Wong MT, Leung HT, Ng CL, Yan P, Yeung SY, Lin CSK (2015) Kinetic analysis of a crude enzyme extract produced via solid state fermentation of bakery waste. ACS Sustain Chem Eng 3:2043–2048
Hifney AF, Fawzy MA, Abdel-Gawad KM, Issa AA, Gomaa M (2017) In vitro comparative evaluation of antioxidant activity of hydrophobic and hydrophilic extracts from Algicolous fungi. J Aquat Food Prod Technol 26:124–131
Hifney AF, Fawzy MA, Abdel-Gawad KM, Gomaa M (2016) Industrial optimization of fucoidan extraction from Sargassum sp. and its potential antioxidant and emulsifying activities. Food Hydrocoll 54:77–88
Hu Z, Lin B-K, Xu Y, Zhong M, Liu G-M (2009) Production and purification of agarase from a marine agarolytic bacterium Agarivorans sp. HZ105. J Appl Microbiol 106:181–190
Ishola MM, Jahandideh A, Haidarian B, Brandberg T, Taherzadeh MJ (2013) Simultaneous saccharification, filtration and fermentation (SSFF): a novel method for bioethanol production from lignocellulosic biomass. Bioresour Technol 133:68–73
Issa AA, Hifney AF, Abdel-Gawad KM, Gomaa M (2014) Spatio temporal and environmental factors influencing macroalgal β diversity in the Red Sea, Egypt. Bot Mar 57:99–110
Kim HT, Lee S, Kim KH, Choi I-G (2012) The complete enzymatic saccharification of agarose and its application to simultaneous saccharification and fermentation of agarose for ethanol production. Bioresour Technol 107:301–306
Kim SW, Kim Y-W, Hong C-H, Lyo I-W, Lim H-D, Kim G-J, Shin H-J (2017) Recombinant agarase increases the production of reducing sugars from HCl-treated Gracilaria verrucosa, a red algae. Algal Res. https://doi.org/10.1016/j.algal.2017.01.008
Kobayashi R, Takisada M, Suzuki T, Kirimura K, Usami S (1997) Neoagarobiose as a novel moisturizer with whitening effect. Biosci Biotechnol Biochem 61:162–163
Lakshmikanth M, Manohar S, Patnakar J, Vaishampayan P, Shouche Y, Lalitha J (2006) Optimization of culture conditions for the production of extracellular agarases from newly isolated Pseudomonas aeruginosa AG LSL-11. World J Microbiol Biotechnol 22:531–537
Long M, Yu Z, Xu X (2010) A novel β-agarase with high pH stability from marine Agarivorans sp. LQ48. Mar Biotechnol 12:62–69
Marangoni AG (2003) Enzyme kinetics: a modern approach. Wiley-Interscience, Hoboken
Marín E, Sanchez L, Perez M, Puyol P, Calvo M (2003) Effect of heat treatment on bovine lactoperoxidase activity in skim milk: kinetic and thermodynamic analysis. J Food Sci 68:89–93
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Oh C, Nikapitiya C, Lee Y, Whang I, Kang D-H, Heo S-J, Choi Y-U, Lee J (2010) Molecular cloning, characterization and enzymatic properties of a novel βeta-agarase from a marine isolate Psudoalteromonas sp. AG52. Braz J Microbiol 41:876–889
Pal A, Khanum F (2011) Covalent immobilization of xylanase on glutaraldehyde activated alginate beads using response surface methodology: characterization of immobilized enzyme. Process Biochem 46:1315–1322
Park J-H, Hong J-Y, Jang HC, Oh SG, Kim S-H, Yoon J-J, Kim YJ (2012) Use of Gelidium amansii as a promising resource for bioethanol: a practical approach for continuous dilute-acid hydrolysis and fermentation. Bioresour Technol 108:83–88
Usov AI, Elashvili MY (1991) Polysaccharides of algae. 44. Investigation of sulfated Galactan from Laurencia nipponica Yamada (Rhodophyta, Rhodomelaceae) using partial reductive hydrolysis. Bot Mar 34:553–560
Wang J, Jiang X, Mou H, Guan H (2004) Anti-oxidation of agar oligosaccharides produced by agarase from a marine bacterium. J Appl Phycol 16:333–340
Wang J, Mou H, Jiang X, Guan H (2006) Characterization of a novel β-agarase from marine Alteromonas sp. SY37–12 and its degrading products. Appl Microbiol Biotechnol 71:833–839
Yun EJ, Kim HT, Cho KM, Yu S, Kim S, Choi I-G, Kim KH (2016) Pretreatment and saccharification of red macroalgae to produce fermentable sugars. Bioresour Technol 199:311–318
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Electronic Supplementary Material
ESM 1
(PDF 365 kb)
Rights and permissions
About this article
Cite this article
Gomaa, M., Hifney, A.F., Fawzy, M.A. et al. Statistical Optimization of Culture Variables for Enhancing Agarase Production by Dendryphiella arenaria Utilizing Palisada perforata (Rhodophyta) and Enzymatic Saccharification of the Macroalgal Biomass. Mar Biotechnol 19, 592–600 (2017). https://doi.org/10.1007/s10126-017-9778-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10126-017-9778-0