Abstract
The rearing environment of first-feeding turbot larvae, usually with high larvae densities and organic matter concentrations, may promote the growth of opportunistic pathogenic Vibrionaceae bacteria, compromising the survival of the larvae. The aim of this study was to assess the effectiveness of the biofilm-forming probiotic Phaeobacter 27-4 strain grown on a ceramic biofilter (probiofilter) in preventing Vibrio anguillarum infections in turbot larvae. In seawater with added microalgae and maintained under turbot larvae rearing conditions, the probiofilter reduced the total Vibrionaceae count and the concentration of V. anguillarum, which was undetectable after 144 h by real-time PCR. The probiofilter also improved the survival of larvae challenged with V. anguillarum, showing an accumulated mortality similar to that of uninfected larvae (35–40 %) and significantly (p < 0.05) lower than that of infected larvae with no probiofilter (76 %) due to a decrease in the pathogen concentration and in total Vibrionaceae. Furthermore, the probiofilter improved seawater quality by decreasing turbidity. Phaeobacter 27-4 released from the probiofilters was able to survive in the seawater for at least 11 days. The bacterial diversity in the larvae, analysed by denaturing gradient gel electrophoresis, was low, as in the live prey (rotifers), and remained unchanged in the presence of V. anguillarum or the probiofilter; however, the probiofilter reduced the bacterial carrying capacity of the seawater in the tanks. Phaeobacter-grown biofilters can constantly inoculate probiotics into rearing tanks and are therefore potentially useful for bacterial control in both open and recirculating industrial units.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rearing first-feeding turbot larvae on rotifers and Artemia promotes the growth of opportunistic bacteria (Skjermo et al. 1997) because of the high density of larvae and the high levels of bacteria (Olafsen 2001). This leads to reduced growth and survival of larvae (Salvesen et al. 1999). Various Vibrionaceae species are considered to be potentially opportunistic pathogens. Some species, such as Vibrio anguillarum, have been found in live prey (Thomson et al. 2005; Verdonck et al. 1997) and seawater (Reid et al. 2009; Sandlund and Bergh 2008; Thomson et al. 2005), resulting in infectious diseases in fish farms (Zhang and Austin 2000).
The survival of fish larvae can be improved by the use of probiotics (Planas et al. 2006; Vine et al. 2006), defined by FAO/WHO as “live microorganisms which, when administered in adequate amounts, confer health benefit on the host”. Their use has increased in aquaculture as an alternative to antibiotics and disinfectants, which can lead to antibiotic-resistant bacteria and loss of a stable microbial population (Cabello 2006; Skjermo and Vadstein 1993). The intestinal microbial community of aquatic organisms is closely linked to the microbiota in their environment (seawater). Accordingly, Verschuere et al. (2000) redefined probiotics in aquaculture as “a live microbial adjunct which has a beneficial effect on the host by modifying the host-associated or ambient microbial community, by ensuring improved use of the feed or enhancing its nutritional value, by enhancing the host response towards disease, or by improving the quality of its ambient environment”, extending the concept to proteobacteria species that have a beneficial effect on the host by modifying the microbial community of seawater. This broadened definition thus includes microorganisms that improve seawater quality (Dalmin et al. 2001) or act as biocontrols to reduce the levels of pathogenic (Kennedy et al. 1998) and total bacteria (Makridis et al. 2001) in seawater.
Some α-proteobacteria of the Roseobacter clade, such as Phaeobacter gallaeciensis, Phaeobacter inhibens and Ruegeria mobilis, can reduce growth and kill the fish pathogen V. anguillarum by producing the antibiotic tropodithietic acid (TDA; Brinkhoff et al. 2004; Bruhn et al. 2005; D’Alvise et al. 2010; Porsby et al. 2008). This antibiotic does not induce resistance or tolerance in the target bacteria, thus avoiding the problem of resistance associated with classical antibiotics (Porsby et al. 2011). TDA-producing Roseobacter bacteria are thus promising fish probiotics. They are distributed globally (Buchan et al. 2005; Gram et al. 2010; Wagner-Döbler and Biebl 2006) and have been found in turbot hatcheries with widely different water sources (e.g. Spain and Denmark) and aquaculture systems (e.g. classical flow-through and recirculating systems; Hjelm et al. 2004a; Michaud et al. 2009; Porsby et al. 2008). The strain Phaeobacter 27-4, isolated from a turbot farm (Hjelm et al. 2004a, b), was shown to have a probiotic effect in vivo when bioencapsulated in rotifers used to feed turbot larvae challenged with V. anguillarum (Planas et al. 2006). This probiotic strain does not, however, permanently colonize turbot larvae (Planas et al. 2006) or rotifers (Pintado et al. 2010), and repeated additions are required to maintain effective levels in the rearing system. Furthermore, it has a short residence time in the seawater of rearing tanks (Pintado et al. 2010).
Roseobacter bacteria can rapidly colonize a variety of inorganic and organic marine surfaces (Dang and Lovell 2002; Mayali et al. 2008; Rao et al. 2006) and Phaeobacter 27-4 can colonize inert surfaces (Bruhn et al. 2006). In a previous study (Prol-García et al. 2012), we demonstrated that Phaeobacter 27-4 immobilized on ceramic biofilters, can antagonize the Vibrionaceae pathogens V. anguillarum and V. splendidus and remain in seawater with added microalgae rearing tanks for at least 11 days under turbot larval rearing conditions, which covers the period during which larvae are fed on rotifers and which is considered critical for larval survival. On the basis of those results, we hypothesized that biofilters colonized with this probiotic bacterium could be used to deliver and maintain the probiont in turbot larvae rearing systems, preventing Vibrio infections and therefore increasing larval survival.
The purpose of this study was to assess the effectiveness of Phaeobacter 27-4 grown on ceramic biofilters in preventing V. anguillarum infections in turbot larvae. The antagonism of matured Phaeobacter 27-4 ceramic biofilters (hereafter referred to as probiofilters) against V. anguillarum 90-11-287 was studied under larval rearing conditions with various nutrient levels, and the effect of the probiofilter in vivo was investigated at pilot scale on turbot larvae challenged with V. anguillarum from infected rotifers.
Materials and methods
Bacterial strains
The fish pathogen V. anguillarum 90-11-287 (Skov et al. 1995) and the fish probiotic Phaeobacter strain 27-4 (Hjelm et al. 2004a) were kindly provided by Professor Lone Gram (DTU Systems Biology, Denmark). All the strains were kept at −80 °C in Marine Broth (MB, Difco 2219) with glycerol (final concentration, 15 %) and routinely cultured on MB, as previously described (Prol et al. 2009).
Turbot larvae
Turbot larvae were kindly provided 1 day after hatching by the Insuíña Pescanova (Mougás, Galicia, Spain) and transported to the Instituto de Investigacións Mariñas (Vigo, Spain). After arrival, the larvae were acclimatized by raising the room-controlled temperature progressively during the following 3 days from 15 °C to 18 °C.
Preparation and maturation of the probiofilter
Ceramic biofilter cylinders (Bio Max, Hagen, Germany) with a volume of 2.53 cm3 per unit were introduced into a Phaeobacter 27-4 suspension (107 CFU·ml−1) in MB and cultured under stagnant conditions for 4 days at 20 °C. The probiofilters were prepared with 100 Phaeobacter-bearing ceramic cylinders and matured for 10 days in seawater with added microalgae (2·105 Isochrysis galbana cells·ml−1) under turbot larval rearing conditions, at 18 °C and under constant aeration (> 90 % oxygen saturation) and illumination. Daylight was provided from fluorescent lamps and the light intensity at the seawater surface was 305 μW·s−1·m−2.
Trials of the probiofilter against V. anguillarum in seawater with added microalgae in vitro
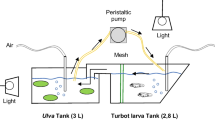
The activity of the probiofilter against V. anguillarum was tested in tanks containing 2.5 l of seawater with added microalgae and with or without 5 ml of MB (final concentration, 0.2 %) to mimic the concentrations of organic matter present in rearing tanks from live feed, faeces and larval debris and favour the growth of Vibrionaceae bacteria (Planas et al. 2006). Tanks with 2.5-l seawater with added microalgae were either (a) left untreated (control), (b) inoculated with 25 μl of a 24-h culture of V. anguillarum (104–105 CFU·ml−1; Va), (c) fitted with the probiofilter (BPh) or (d) treated with V. anguillarum and fitted with the probiofilter (VaBPh). Probiofilters were prepared and matured as described previously. Probiotic-grown cylinders were washed twice with 5 l of autoclaved seawater and distributed into a plastic mesh bag containing ten cylinders each, which were introduced into tanks BPh and VaBPh. The control and Va tanks were fitted with biofilters made from ten autoclaved cylinders. The four treatments were performed with and without addition of 0.2 % MB. Each condition was run in duplicate.
The samples of seawater were taken at various times between 0 and 144 h for microbiological and real-time PCR analyses. Samples were taken from the probiofilters at the beginning (0 h) and end of the experiment (144 h).
Trials of the probiofilter in turbot larvae in vivo
Turbot larvae (35 larvae·l−1) were cultured in 5-l tanks filled with 4 l of seawater with added microalgae (2·105 Isochrysis galbana cells·ml−1). Water was moderately aerated (>90 % oxygen saturation) and room-controlled temperature adjusted to 18 °C. Larvae were maintained in the dark until day 3, the light intensity at the water surface was adjusted to 3.5 μE·s−1·m−2. The larvae were fed rotifers (Brachionus plicatilis) maintained with baker’s yeast (Saccharomyces cerevisiae) daily from day 3 until day 10 after hatching. The rotifers (200 rotifers·ml−1) were previously enriched with I. galbana (2·106 cells·ml−1) for 24 h in 25-l tanks containing 10 l of aerated seawater (>90 % oxygen saturation) at 23 °C and daylight provided by fluorescent lamps. The density of rotifers in the rearing tanks was adjusted daily (3–5 rotifers·ml−1), and the seawater was partially (30–40 %) renewed every 2 days with the addition of 0.25 l of I. galbana culture (2·107 cells·ml−1).
To ascertain the probiotic effect of the probiofilter, turbot larvae were challenged with V. anguillarum in an infection model based on bioencapsulation of the pathogen in B. plicatilis, described by Planas et al. (2005). Briefly, 1 million rotifers enriched with I. galbana were filtered through a 30-μm nylon mesh and resuspended in 1 l of 1-μm-filtered seawater. Then, 200 ml of a 24-h culture of V. anguillarum (109 CFU·ml−1) were incorporated into the rotifer cultures, and the volume was adjusted to 5 l with 1-μm-filtered seawater. After 3 h, the rotifers were filtered through a 30-μm nylon mesh, washed and added to the larvae. The challenged larvae were fed rotifers loaded with V. anguillarum on days 4, 6 and 8 post-hatching.
Matured probiofilters with 16 cylinders were introduced in a 250-μm nylon mesh, to avoid entry of the turbot larvae. As in the in vitro experiment, four conditions were studied: (a) control: larvae were fed rotifers enriched with I. galbana; (b) Va: larvae were fed on alternate days with I. galbana-enriched rotifers loaded with V. anguillarum without the probiofilter; (c) BPh: larvae were fed rotifers enriched with I. galbana in the presence of the probiofilter and (d) VaBPh: larvae were fed on alternate days with I. galbana-enriched rotifers loaded with V. anguillarum in the presence of the probiofilter. Biofilters made from 16 autoclaved cylinders were introduced into the control and Va tanks. Each condition was run in duplicate.
The bottoms of the tanks were siphoned daily to remove and count dead larvae. The experiment was run until day 10 post-hatching, covering the critical period, with higher mortality in turbot larval rearing (Planas et al. 2006). The significance of the probiotic effect was ascertained by comparing the accumulated mortality in the control and the experimental groups with the t test for related samples (5 % level of significance). Differences in accumulated mortalities at day 10 post-hatching were analysed between all the different treatments using one-way analysis of variance (ANOVA) and Tukey HDS Test.
Sampling of larvae, rotifers, seawater and probiofilters
Samples of larvae, rotifers and seawater were taken on days 3–10 after larval hatching for culture of total bacteria and total Vibrionaceae and to extract bacterial DNA. Ten larvae were separated with a 250-μm nylon mesh and 400 rotifers with a 30-μm mesh. Larvae were anaesthetized with 3-aminobenzoic acid ethyl ester 0.2 mg·ml–1 (Sigma), and larvae and rotifers were washed with sterile seawater and homogenized. Samples from the probiofilters were taken at the beginning (day 3 post-hatching) and end (day 10 post-hatching) of the experiment.
One ceramic cylinder was taken under sterile conditions and washed twice with 50 ml of autoclaved seawater with gentle agitation to eliminate non-adhered bacteria. Then, the supports were homogenized in a sterile mortar with 10 ml of autoclaved seawater, and the homogenate was transferred to a sterile tube.
Microbiological methods
The 10-fold serial dilutions were prepared in autoclaved seawater from samples of 50-μm-filtered seawater, from bacterial suspensions obtained from homogenized cylinders and from homogenized samples of rotifers and larvae. The total number of bacteria was counted by plating 100 μl of each dilution on Marine Agar (MA, Difco 2219) and incubating for 120 h at 20 °C in the dark.
When indicated, MA plates with 30–300 colonies were replicated on thiosulfate-citrate-bile salts-sucrose (TCBS) agar and incubated at 20 °C in the dark for 24–48 h for total Vibrionaceae counts.
Bacterial DNA extraction
For DNA extraction, 10,000 rotifers and 10 larvae were treated as described by Prol et al. (2009). Seawater samples (50 ml) were filtered over a 30-μm nylon mesh and centrifuged for 10 min at 5,000×g at 20 °C. The resulting pellet was washed once with 1.5 ml autoclaved seawater. DNA was extracted from 1-ml samples of larvae or rotifers homogenates, seawater, and the solutions obtained by washing and homogenizing cylinders, with the phenol:chloroform:isoamyl alcohol protocol described by Prol et al. (2009). All DNA samples were frozen at −20 °C until analysed.
Real-time PCR
Phaeobacter 27-4 and V. anguillarum 90-11-287 were specifically quantified by real-time PCR (Prol et al. 2009) to avoid interference from other bacteria present in the tanks. DNA extracted from duplicate samples was quantified by real-time PCR, also in duplicate, with the appropriate standard curves and the primers VA (5′-CATACGCAGCCAAAAATCAA-3′; 5′-GCACTGTCCGTCATGCTATC-3′) and tdbR (5′-GCGCTTCTCAAGCACCTAAC-3′; 5′-ACGGTGTCCCTTACCTTCCT-3′). These primers were designed from within genes encoding for virulence or antagonism and have been shown to be specific for V. anguillarum and Phaeobacter 27-4 (Prol et al. 2009). The standard curves were prepared in the presence of the appropriate background organism (larvae, rotifers or microalgae): 100 ng of DNA diluted 10-fold (larvae and rotifers) or 100-fold (seawater) were mixed with the appropriate primers (final concentration, 4.3 μmol/l) and Power SYBR® Green master mix (Applied Biosystems) containing AmpliTaq Gold® DNA polymerase, the double-stranded DNA-binding dye Power SYBR® Green and the reference dye ROX®. The real-time PCR program was run on a 7500 Fast Real-Time PCR System (Applied Biosystems). PCR was amplified as described by Bruhn et al. (2006), with some modifications (Prol et al. 2009). Dissociation curves were used to test the specificity of the PCR products. No-template DNA controls and samples of the appropriate background matrix with no added bacteria were used as negative controls in each run.
Denaturing gradient gel electrophoresis (DGGE)
Purified DNA was amplified with the primers gc338f (5′-CGCCCGCCGCGCGCG GCGGGCGGGGCGGGGGCACGGGGGGACTCCTACGGGAGGCAGCAG-3′) and 518r (5′-ATTACCGCGGCTGCTGG-3′) spanning the V3 region of 16S rDNA (Muyzer et al. 1993). Amplification was performed in a GeneAmp 2700 PCR System (Applied Biosystems) thermal cycler, with 100 ng of DNA mixed with each primer (0.25 μmol/l), deoxynucleotide triphosphate mix (0.2 mmol/l), MgCl2 (1.5 mmol/l), PCR buffer for Taq polymerase (1X), Taq polymerase (0.05 U⋅μl−1) and bovine serum albumin (0.4 mmol/l) to a final volume of 50 μl.
PCR was performed as described by Muyzer et al. (1993). The amplification products were analysed by electrophoresis in 2 % agarose gel and quantified with Hyperladder IV (Bio-Rad) and Quantity One software (Bio-Rad).
DGGE was used to analyse 500 ng of the PCR products in a Bio-Rad DCode by the procedure described by Muyzer et al. (1993). Samples were loaded onto 8 % (w/v) polyacrylamide gels in 1X tris-acetate-EDTA. All parallel electrophoreses were performed at 60 °C on gels containing a 30–60 % gradient urea-formamide (100 % corresponded to 7 mol/l urea and 40 % [v/v] formamide) increasing in the direction of electrophoresis. The gels were run at 20 V for 10 min, followed by 3 h at 200 V, stained by bathing for 30 min in a 0.5 % (v/v) ethidium bromide solution and rinsed for 30 min in distilled water.
Analysis of DGGE profiles
The DGGE profiles were subsequently processed with the Quantity One v4.4.1 software package (Bio-Rad), and range-weighted richness (Rr), dynamics and functional organization were calculated (Marzorati et al. 2008).
Rr relates the number of bands present in the DGGE profile to the percentage of denaturing gradient required to describe the total diversity of the sample analysed. In the challenge trial with turbot larvae, Rr was calculated each time larvae and seawater were analysed (days 3–10 after hatching of larvae) to determine the influence of introduced strains on the carrying capacity of the system.
The dynamics of a microbial community can be interpreted as the average number of species that come to significant dominance (above the detection limit of the technique) in a given habitat during a defined interval. Dynamics was calculated by moving-window analysis, in which a matrix of similarities for the relative intensities of the densiometric curves of the band patterns was calculated from Pearson product–moment correlation coefficients with Statistica v9 software and used for moving-window analysis by plotting the correlation between two dates.
Functional organization is the result of the actions of the microorganisms that are best fitted to ongoing environmental–microbiological interactions. In order to represent the structure of the bacterial community in turbot larvae graphically, Pareto–Lorenz evenness curves were set up (Marzorati et al. 2008).
A matrix was constructed from the DGGE profiles of the rotifer, larval and seawater samples taken during the challenge trial, on the basis of the presence or absence of individual bands and the relative percentage contribution of each band to the total intensity of each sample. This matrix was used to calculate the distance matrix from normalized Euclidean distances (root mean square differences) with Statistica v9 software. Finally, a dendrogram of the different samples was obtained by the unweighted-pair group method with average linkages and Statistica.
Results
Trials in vitro of the probiofilter against V. anguillarum in seawater with added microalgae
The capacity of the probiofilter to antagonize V. anguillarum was tested under normal non-axenic turbot larval rearing conditions in the absence or presence of nutrients (MB).
V. anguillarum and Phaeobacter 27-4
Introduction of the probiofilter inhibited V. anguillarum in both the presence and the absence of MB. Inhibition started at 6 h in tanks without MB and at 24 h in those with MB. In Va tanks, the levels of V. anguillarum were stable (104–105 CFU·ml−1) for 3 days and decreased by one or three log units afterwards (Fig. 1). The presence of MB initially promoted the growth of V. anguillarum with a larger decrease subsequently, probably due to nutrient depletion.
Effect of the probiofilter on survival of Vibrio anguillarum 90-11-287 in green seawater tanks maintained under turbot rearing conditions. Levels of V. anguillarum in presence (filled circle) and absence (circle) of the probiofilter and of Phaeobacter bacteria transferred from the probiofilter to the tank seawater (diamond) are shown. Data are means and standard deviations of the two replicate tanks for each condition, and each assay value is the average of a technical duplicate. MB– without addition of MB, MB+ with addition of 0.2 % MB
In tanks with the probiofilter (VaBPh), the level of V. anguillarum decreased progressively and was undetectable by real-time PCR at 144 h (Fig. 1). The slope of disappearance of V. anguillarum indicated that the level was 0 between 96 and 144 h, when the concentration in the seawater of the tanks without probiofilters was about 104 bacteria·ml−1. V. anguillarum was not detected in any of the probiofilters or autoclaved filters.The Phaeobacter 27-4 concentration on the probiofilters introduced into the BPh and VaBPh tanks (0 h) was (2.11 ± 0.12)·105 bacteria·cm−3. Phaeobacter was released from the probiofilters into seawater during the first 24 h, reaching a maximum concentration of 105 bacteria·ml−1 in the presence of MB and 103 bacteria·ml−1 in the absence of MB (Fig. 1). After 72 h, the concentration of Phaeobacter in seawater was 102 bacteria·ml−1 in the absence of MB and 103 bacteria·ml−1 in the presence of MB. In the probiofilters, the concentration at the end of the experiment was (1.84 ± 0.31)·106 bacteria·cm−3 without MB and (5.73 ± 0.14)·106 bacteria·cm−3 in the presence of MB.
Total bacteria, total Vibrionaceae and turbidity
The total bacteria concentrations in seawater, obtained from MA plate counts, were similar in all tanks, ranging from (1.22 ± 0.20)·104 CFU·ml−1 at the beginning to (1.24 ± 0.26)·105 CFU·ml−1 at 144 h. The total bacteria count in the probiofilters at the end of the trial was (8.35 ± 0.36)·106 in the absence and (3.20 ± 0.14)·107 CFU·cm−3 in the presence of MB.
The total Vibrionaceae count in Va tanks was highest (>50 %) at the beginning of the experiment, in both the presence and the absence of MB. In the BPh tanks, Vibrionaceae were detected only at 24 h in the presence of MB (2 % of total bacteria). The Va and VaBPh tanks had similar concentrations of Vibrionaceae (104–105 CFU·ml−1) in seawater at the beginning of the experiment, independently of added MB. The presence of a probiofilter (VaBPh) promoted a decrease of Vibrionaceae numbers below the detection limit in seawater after 24 h, whereas in the Va tanks Vibrionaceae remained detectable until 72 h. In samples taken from the probiofilters in the BPh and VaBPh tanks at the end of the trial (144 h), the Vibrionaceae concentration was (2.49 ± 0.43)·102 CFU·cm−3 in the absence of MB and 99.8 ± 0.35 CFU·cm−3 with MB. In the control and Va tanks, the Vibrionaceae count attained (1.85 ± 0.08)·104 CFU·cm−3 with MB and (4.34 ± 0.26)·103 CFU·cm−3 without MB.
The turbidity (OD600) of the seawater of tanks containing probiofilters was lower than that in the control and Va tanks, independently of added MB (Table 1).
Probiotic effect of the probiofilter on turbot larvae in vivo
Larvae mortality
The probiofilters significantly reduced mortality due to V. anguillarum (p < 0.05; Fig. 2a, Table 2). The accumulated mortality in 10-day-old larvae infected with V. anguillarum was 76 ± 20 %, whereas that in infected larvae with a probiofilter was similar to that of controls (35–40 %). The accumulated mortality at the end of the trial (day 10 post-hatching) was significantly different (ANOVA, p = 0.01; Tukey HSP, p < 0.05) between Control and Va and between VaBPh and Va and not significantly different (ANOVA, p = 0.01; Tukey HSP, p > 0.05) between Control and Bph, Control and VaBPh, BPh and VaBPh (Table 2). The differences between Va and BPh were also non-significant (ANOVA, p = 0.01; Tukey HSP, p > 0.05); however, this was mainly due to the high deviations between replicates of Va treatment.
Probiotic effect of the probiofilter on turbot larvae challenged with Vibrio anguillarum 90-11-287. A Percentage accumulated mortality (triangle control, larvae fed rotifers; diamond BPh, larvae fed rotifers in presence of probiofilter; filled circle Va, larvae fed rotifers loaded with V. anguillarum, without probiofilter; filled diamond VaBPh, larvae fed rotifers loaded with V. anguillarum in presence of probiofilter). B Levels of V. anguillarum in infected larvae treated (filled diamond VaBPh) or not (filled circle Va) with the probiofilter. C Levels of Phaeobacter 27-4 in tank seawater with turbot larvae challenged (filled diamond VaBPh) or not (diamond BPh) with V. anguillarum. D Levels of V. anguillarum in seawater of Va (filled circle) and VaBPh (filled diamond) tanks. Data are means and standard deviations of two independent assays, and each assay value is the average of a technical duplicate
The initial concentration of Phaeobacter 27-4 in the probiofilter was (4.26 ± 1.10)·106 bacteria·cm−3, which was maintained until the end of the trial. The concentrations of V. anguillarum in rotifers used to infect turbot larvae on days 4, 6 and 8 post-hatching were (1.85 ± 0.06)·102, (8.20 ± 0.09)·102 and (5.61 ± 0.00)·103 bacteria·rotifer−1, respectively, showing variability in the incorporation of the pathogen by the different batches of rotifers. Phaeobacter and V. anguillarum were not detected by real-time PCR in uninfected rotifers used to feed turbot larvae on days 3, 5, 7 and 9. Phaeobacter was not detected in larvae in BPh and VaBPh tanks, whereas V. anguillarum was detected in all larvae treated with the pathogen (Fig. 2b). The concentrations of V. anguillarum in 5-day-old larvae were almost the same in the Va and VaBPh tanks (40 bacteria·10 larvae−1). In larvae exposed to Phaeobacter V. anguillarum concentration decreased, whereas the level increased to 102 bacteria·10 larvae−1 at day 9 in the Va tanks. In both cases, V. anguillarum was undetectable in larvae at the end of the experiment (day 10).
Phaeobacter was found in seawater in tanks BPh and VaBPh (103 bacteria·ml−1) 24 h after the probiofilters were introduced (day 4 post-hatching; Fig. 2c), but the concentration had decreased by day 8 and remained at 102 bacteria·ml−1 until the end of the trial (day 10).
Phaeobacter and V. anguillarum were not detected in samples of rotifers taken from any of the tanks on day 10.
The concentration of V. anguillarum in seawater from Va and VaBPh tanks increased to a maximum of 103–104 bacteria·ml−1 2 days after first feeding (day 4 post-hatching; Fig. 2d) and decreased thereafter, stabilizing at 102 bacteria·ml−1 from day 7 in Va tanks and becoming undetectable or very low from day 8 in VaBPh tanks (Fig. 2d).
Total bacteria, total Vibrionaceae and turbidity
Similar total bacteria (MA) and total Vibrionaceae (TCBS) counts were detected in larvae and Vibrionaceae represented >40 % of total bacteria in all tanks by the end of the trial (data not shown).
The total bacteria concentration in seawater was similar (105–106 CFU·ml−1) in all tanks at all times (Fig. 3). Va tanks had the highest total concentration of Vibrionaceae (105–106 CFU·ml−1), whereas the control, BPh and VaBPh tanks had 105 and 104 CFU·ml−1 on days 7 and 9, respectively. Vibrionaceae were not detected in BPh or VaBPh tanks from day 9 or in control tanks from day 10.
Total Vibrionaceae counts from larvae (A) and seawater (B). triangle control, larvae fed rotifers; diamond BPh, larvae fed rotifers in presence of probiofilter; filled circle Va, larvae fed rotifers loaded with V. anguillarum, without probiofilter; filled diamond VaBPh, larvae fed rotifers loaded with V. anguillarum in presence of probiofilter. Data are means and standard deviations of the two replicate tanks for each condition, and each assay value is the average of a technical duplicate
In the probiofilters, the concentration of total bacteria at the end of the trial (day 10) was around 107 CFU·cm−3 in all tanks. The total Vibrionaceae concentration in the probiofilters was 1 log unit lower ((2.84 ± 1.77)·103 CFU·cm−3) than in the control and Va tanks ((1.95 ± 2.56)·104 CFU·cm−3). V. anguillarum was not detected in probiofilters or autoclaved cylinders from any tank at the end of the trial.
The presence of the probiofilters reduced the turbidity (OD600), from 0.055 ± 0.024 in the control and 0.075 ± 0.024 in Va tanks to 0.033 ± 0.013 in BPh and 0.023 ± 0.004 in VaBPh tanks.
Modification of bacterial communities
DGGE profiles
Larvae from all tanks had similar DGGE profiles, and no bands with migration corresponding to V. anguillarum or Phaeobacter 27-4 were detected (Supp. Fig 1A). The larval profiles clustered as a function of time and grouped with the profiles of rotifers enriched with microalgae (Fig. 4).
Euclidean-distance dendrogram generated from the DGGE profiles of samples of turbot larvae, seawater, and rotifer taken from control, BPh, Va, and VaBPh tanks on different days (D) and from rotifers enriched with I. galbana (Rot Ig) and rotifers loaded with V. anguillarum (Rot Va) used as feed. The dendrogram was determined by the unweighted-pair group method with average linkages
The profiles of uninfected I. galbana-enriched rotifers were similar (Supp. Fig 1B) and formed a cluster (Fig. 4). Differences observed in the profiles of the rotifers loaded with V. anguillarum used to infect the larvae indicate variation in the bioencapsulation of the pathogen, in accordance with the results obtained with real-time PCR quantification of V. anguillarum. The profiles of the rotifers loaded with V. anguillarum showed a single predominant band (band Va) with a migration pattern similar to that of V. anguillarum (band C of the marker) on days 6 and 8, resulting in separation of these profiles from those of other rotifers in the dendrogram (Fig. 4). On day 4, the Va band was less predominant in the profile of infected rotifers, which clustered with that of uninfected I. galbana-enriched rotifers. The profiles of rotifers obtained from the tanks on day 10 post-hatching clustered with those of samples of seawater taken at the same time (Fig. 4), indicating that the seawater bacterial community of rearing tanks influences the microbiota of rotifers.
The DGGE profiles of seawater from all the tanks (Supp. Fig 1C) were similar. Seawater from BPh and VaBPh tanks had a band with a similar migration pattern to that of Phaeobacter 27-4 from day 4 (band Ph). The profiles for seawater clustered independently from those for larvae and uninfected rotifers (Fig. 4). Three main clusters could be distinguished within the seawater profiles: tanks with V. anguillarum (Va and VaBPh), tanks with the probiofilter and without the pathogen (BPh) and control tanks. A separate group clustered samples of seawater and rotifers taken from the tanks at the end of the experiment (day 10 post-hatching).
Richness, dynamics and functional organization
Richness, dynamics and functional organization were calculated to determine the influence of V. anguillarum and Phaeobacter 27-4 on larvae and on seawater bacterial communities. Rr was always <10 in larvae in all tanks (Table 3), indicating that the bacterial diversity in turbot larvae was low. The Rr values were higher in seawater than in larvae, with some differences according to condition. The seawater in control tanks had values of 9.80–12.80 throughout the trial. Va tanks showed higher Rr values than control tanks, with values of 12.80–16.20 up to day 10, when the lowest value was achieved (7.35). The presence of the probiofilter (BPh and VaBPh) decreased the microbial diversity of seawater (3.75–9.80) from day 6 or 7. In BPh tanks, medium Rr values were recorded at the end of the experiment (day 10).
The changes in bacterial microbiota (every 2 days) were established by moving-window analysis in larvae and seawater (Fig. 5). In all cases, the largest changes occurred after day 7. Larvae in control tanks showed a shift of 62 % within the first 48 h (day 5) and a second shift of 75–100 % on days 9 and 10. Less pronounced changes were observed in larvae in BPh tanks, with a 28 % change on day 5 and a 51 % change on day 10. In tanks treated with V. anguillarum (Va and VaBPh), similar shifts were observed at 57–83 % on day 9 and 65–77 % on day 10.
Major changes in seawater were seen on days 9 and 10 in control and Va tanks and from day 7 in tanks with a probiofilter (BPh and VaBPh). As for larvae, community shifts were less pronounced with BPh, as demonstrated by shorter fluctuations in the moving-window analysis plot.
The average values for these rates of change, expressed as [∆ t (2 days)], showed that the total community changed more rapidly in larvae of control tanks (53.67 ± 26.50 %) and in seawater in VaBPh tanks (31.67 ± 45.46 %) than in larvae (30–33 %) and seawater (4–28 %) in the other tanks. The lowest value was observed in Va tanks (4.33 ± 1.53 %).
In order to represent the structure of the bacterial community in larvae and seawater graphically, Pareto–Lorenz evenness curves were constructed from DGGE profiles (Supp. Fig 2). For all tanks, 20 % of the bands present in the DGGE profiles of larvae corresponded to 43–53 % (average, 49 %) of the cumulative band intensities and to 59–69 % (average, 64 %) in seawater.
Discussion
We demonstrated in this study that (a) immobilization of Phaeobacter 27-4 on ceramic biofilters guarantees its presence in rearing systems for at least 10 days without continual additions and (b) biofilters colonized by this probiotic Phaeobacter strain increase the survival of turbot larvae challenged with the fish pathogen V. anguillarum.
In small trials conducted under larval rearing conditions and various nutrient levels, the probiofilter decreased the concentration of V. anguillarum previously inoculated into seawater with added microalgae until it disappeared, as well as total Vibrionaceae. In the absence of Phaeobacter, an increase in the level of nutrients in the rearing tank, mimicked by the addition of MB, increased the concentration of total Vibrionaceae in both seawater and autoclaved biofilters. This indicates an effect of the probiofilter against these bacteria and shows that it can act as a control agent in rearing tanks, even at high nutrient levels. Similarly, in a challenge trial with turbot larvae, addition of MB to the seawater of rearing tanks increased the concentration of total Vibrionaceae, leading to higher larval mortality (Planas et al. 2006). Michaud et al. (2009) detected both Roseobacter and potentially pathogenic Vibrionaceae associated mainly with biofilters in a recirculating aquaculture system and suggested that the indigenous microbiota of the system could control the development of pathogenic organisms in fish rearing systems. We demonstrate that introduction of a probiotic strain immobilized on biofilters not only controls potentially pathogenic Vibrionaceae in seawater but also limits the colonization of biofilters by these bacteria. This result is in accordance with the hypothesis of D’Alvise et al. (2010) that antagonistic Roseobacter biofilms might reduce the presence of opportunistic pathogens in small-scale systems by preventing their establishment and proliferation.
Turbidity has been negatively correlated with growth and survival in red tilapia fry (Ardjosoediro and Rammarine 2002), and 1-year-old turbots appear to feed better at low turbidity levels (Mallekh et al. 1998). The quality of seawater in rearing systems is usually improved by the use of biofilters, which maintain nitrifying or slow-growing (K strategist) bacteria (Crab et al. 2007; Michaud et al. 2006; Salvesen et al. 1999; Skjermo et al. 1997). This resulted in enhanced survival of halibut yolk-sac larvae (Skjermo et al. 1997) and growth of turbot larvae (Salvesen et al. 1999). Our study demonstrates that the probiofilter also contributes to seawater conditioning in rearing tanks by diminishing its turbidity.
Addition of the probiofilter significantly reduced mortality due to V. anguillarum, especially from day 8 post-hatching, and reduced the number of pathogens in seawater. Phaeobacter 27-4 was not detected in turbot larval samples, confirming its incapacity to colonize turbot larvae (Planas et al. 2006), perhaps because Roseobacter bacteria are not part of the normal microbiota of turbot larvae. In a year-round study in two turbot rearing plants in Galicia (north-west Spain), Phaeobacter were found to predominate among isolates antagonistic to Vibrionaceae fish pathogens. Most antagonistic Roseobacter were isolated from the walls of turbot larvae rearing tanks, and only a few were detected in the seawater (Hjelm et al. 2004a). None were isolated directly from larvae.
In our experiments, Phaeobacter 27-4 was released from the probiofilter into seawater, reaching levels similar to those observed when it is bioencapsulated in rotifers and delivered to turbot larvae (Planas et al. 2006). In both cases, the probiotic effect might also have been from the seawater, although it would be limited by the continuous presence of the probiotic in the rearing tanks, as it cannot be detected 72 h after it is inoculated at a concentration of 107 CFU·ml−1 into seawater with added microalgae (Pintado et al. 2010). In the present study, the probiofilter guaranteed a constant supply of the probiont to seawater throughout the experiment. Thus, the use of probiotic biofilters simplifies the procedure to a single application, obviating repeated culture and bioencapsulation of the probiotic. The contribution of probiotic bacteria immobilized in biofilters and released from the biofilter into seawater to the antagonistic effect observed could not be elucidated in this study, and studies should be conducted on the expression of genes for production of the antagonistic compound.
In the challenge trial with turbot larvae, the levels of V. anguillarum in seawater were reduced by introduction of the probiofilter into the rearing tanks. This finding contrasts with that of Planas et al. (2006), who did not observe a drop in V. anguillarum concentration. They therefore attributed the probiotic effect to reduced pathogenesis or to an antagonistic effect at specific sites. This discrepancy in V. anguillarum counts might be due to a difference in the technique used to identify the introduced strains. In the present study, real-time PCR (Prol et al. 2009) was used to quantify the introduced strains specifically, whereas Planas et al. (2006) identified both the pathogen and the probiotic visually on MA and TCBS plates, which might have led to bias in identification and quantification of the target strains. Nevertheless, the possibility that Phaeobacter 27-4 has different mechanisms of action, depending on the delivery procedure (addition to seawater, bioencapsulated in live prey or immobilized in biofilters) must not be discarded.
High total numbers of Vibrionaceae in seawater, live prey or facility surfaces constitute a potential threat to farmed organisms. V. anguillarum is typically found in live prey and can colonize rotifers and be released to seawater (Prol-García et al. 2010). Turbot larval rearing conditions enhance the proliferation and maintenance of pathogenic bacteria in rotifers and/or seawater, promoting re-infection of the larvae (Olsson et al. 1998; Sugita et al. 2008). V. anguillarum does not colonize the gut of turbot larvae but was detected in the epidermis (Planas et al. 2005). We observed release of V. anguillarum from rotifers to seawater, which might favour preferential infection of the epidermis (Prol-García et al. 2010). Therefore, controlling potential pathogenic bacteria, such as V. anguillarum, in the seawater of rearing tanks may be the most suitable means of preventing bacterial infections of larvae. Salvesen et al. (1999) produced matured seawater by passing filtered seawater through a biofilter and demonstrated that it can select for non-opportunistic bacteria, with beneficial effects on turbot larvae. Our study shows that the introduction of Phaeobacter biofilters into rearing tanks is a good alternative to spontaneous bacterial colonization (Salvesen et al. 1999), preventing larval infection by reducing total Vibrionaceae in seawater and biofilters.
No bands with migration patterns similar to those of Phaeobacter 27-4 and V. anguillarum were detected in turbot larvae by DGGE. The absence of a band corresponding to Phaeobacter 27-4 might be due to the inability of this strain to colonize turbot larvae, as mentioned above, even when introduced via rotifers (Planas et al. 2006). The absence of a migration pattern similar to V. anguillarum might be due to the detection limit of DGGE, as the concentration was low (1–10 CFU·larvae−1 in real-time PCR analysis). Although DGGE may not be the best technique for detecting introduced bacteria (done by real-time PCR in this study), it is useful for monitoring changes caused by the introduction of probiotic and pathogenic strains into larval rearing systems (Pintado et al. 2010; Prol-García et al. 2010; Qi et al. 2009).
Incorporation of Phaeobacter 27-4 or V. anguillarum did not significantly displace or modify the bacterial microbiota of larvae or rotifers. In samples of rotifers taken from the tanks at the end of the experiment, the microbiota was more similar to that of seawater than that of uninfected rotifers enriched with microalgae, confirming an exchange of bacteria between rotifers and seawater (Prol-García et al. 2010). Marine fish larvae accumulate bacteria by drinking seawater for osmoregulation (Reitan et al. 1998) and by feeding on live prey (Fjellheim et al. 2007; Skjermo and Vadstein 1993; Verschuere et al. 1997). In this study, the changes observed in the bacterial community of the turbot larvae reflected the influence of the surrounding seawater and rotifers. The microbiota of the larvae was influenced more by the bacteria in the rotifers than by those in the seawater (Fig. 4). Several studies have demonstrated that the intestinal microbiota of first-feeding turbot larvae depends more on the bacterial community of live prey than on the microbiota in the seawater (Blanch et al. 1997; Munro et al. 1993; Reitan et al. 1998). In contrast, the presence of the probiofilter led to a decrease in the carrying capacity of the seawater in the rearing tanks, suggesting a direct effect of the probiofilter on seawater bacterial communities.
Under all four conditions (control, BPh, Va and VaBPh), 20 % of the DGGE profiles corresponded to an average of 49 % of the cumulative intensity of the bands for turbot larvae and 64 % of those for seawater over time. Therefore, the fittest species dominated and were present in large numbers, whereas the remaining 80 % was present in smaller numbers. This internal structure of the highly dynamic larvae bacterial community suggests that only a small group of species plays a numerically dominant role at a given moment and that this dominance is even more pronounced in seawater than in larvae.
Conclusions
This is the first report of use of a probiotic bacterium cultured on biofilters in rearing turbot in vivo. Phaeobacter grown on ceramic biofilters and matured for 10 days guaranteed the permanence of these probiotic bacteria in the rearing tanks for at least 8 days, maintaining constant transfer of the probiotic to seawater. The biofilters increased the survival of turbot larvae challenged with the fish pathogen V. anguillarum by diminishing its levels, mainly in seawater, and by improving seawater quality.
Use of probiotic strains such as Phaeobacter 27-4 in biofilters increases the residence time of the probiont in larval rearing systems as compared with repeated addition (e.g. directly to the seawater or bioencapsulated in rotifers), making this method applicable to industrial hatcheries. The probiofilters provide a constant inoculum of the probiotic to the system, making it useful for controlling pathogens and opportunistic bacteria in both open and recirculating systems. Competition between the introduced probiotics and nitrifying bacteria for oxygen, nutrients and space inside the biofilms might, however, reduce the nitrification rates in biofilters (Michaud et al. 2006). Therefore, further research is required to ascertain the impact of introduced probiotic bacteria on the global microbiota of rearing systems.
References
Ardjosoediro I, Rammarine IW (2002) The influence of turbidity on growth, feed conversion and survivorship of the Jamaica red tilapia strain. Aquaculture 212:159–165
Blanch AR, Alsina M, Simon M, Jofre J (1997) Determination of bacteria associated with reared turbot (Scophthalmus maximus) larvae. J Appl Microbiol 82:729–734
Brinkhoff T, Bach G, Heidorn T, Liang L, Schlingloff A, Simon M (2004) Antibiotic production by a Roseobacter clade-affiliated species from the German Wadden Sea and its antagonistic effects on indigenous isolates. Appl Environ Microbiol 70:2560–2565
Bruhn JB, Nielsen KF, Hjelm M, Hansen M, Bresciani J, Schulz S, Gram L (2005) Ecology, inhibitory activity and morphogenesis of a marine antagonistic bacterium belonging to the Roseobacter clade. Appl Environ Microbiol 71:7263–7270
Bruhn JB, Haagensen JAJ, Bagge-Ravn D, Gram L (2006) Culture conditions of Roseobacter strain 27-4 affect its attachment and biofilm formation as quantified by real-time PCR. Appl Environ Microbiol 72:3011–3015
Buchan A, Gonzalez JM, Moran MA (2005) Overview of the marine Roseobacter lineage. Appl Environ Microbiol 71:5665–5677
Cabello FC (2006) Heavy use of prophylactic antibiotics in aquaculture: a growing problem for human and animal health and for the environment. Environ Microbiol 8:1137–1144
Crab R, Avnimelech Y, Defoirdt T, Bossier P, Verstraete W (2007) Nitrogen removal techniques in aquaculture for a sustainable production. Aquaculture 270:1–14
D’Alvise PW, Melchiorsen J, Porsby CH, Nielsen KF, Gram L (2010) Inactivation of Vibrio anguillarum by attached and placktonic Roseobacter cells. Appl Environ Microbiol 76:2366–2370
Dalmin G, Kathiresan K, Purushotaman A (2001) Effect of probiotics on bacterial population and health status of shrimp in culture pond ecosystem. J Exp Biol 39:939–942
Dang HY, Lovell CR (2002) Seasonal dynamics of particle-associated and free-living marine Proteobacteria in a salt march tidal creek as determined using fluorescence in situ hybridization. Environ Microbiol 4:287–295
Fjellheim AJ, Playfoot KJ, Skjermo J, Vadstein O (2007) Vibrionaceae dominates the microflora antagonistic towards Listonella anguillarum in the intestine of cultured Atlantic cod (Gadus morhua L.) larvae. Aquaculture 269:98–106
Gram L, Melchiorsen J, Bruhn JB (2010) Antibacterial activity of marine culturable bacteria collected from a global sampling of ocean surface waters and surface swabs of marine organisms. Mar Biotechnol 12:439–451
Hjelm M, Bergh Ø, Riaza A, Nielsen J, Melchiorsen J, Jensen S, Duncan H, Ahrens P, Birkbeck H, Gram L (2004a) Selection and identification of autochthonous potential probiotic bacteria from turbot larvae (Scolphthalmus maximus) rearing units. Syst Appl Microbiol 27:360–371
Hjelm M, Riaza A, Formoso F, Melchiorsen J, Gram L (2004b) Seasonal incidence of autochthonous antagonistic Roseobacter spp. and Vibrionaceae strains in a turbot larva (Scolphthalmus maximus) rearing system. Appl Environ Microbiol 70:7288–7294
Kennedy SB, Tucker JW, Neidig CL, Vermeer GK, Cooper VR, Jarrell JL, Sennett DG (1998) Bacterial management strategies for stock enhancement of warm water marine fish: a case study with common snook (Centropomus undecimalis). Bull Mar Sci 62:573–588
Makridis P, Bergh Ø, Skjermo J, Vadstein O (2001) Addition of bacteria bioencapsulted in Artemia metanauplii to a rearing system for halibut larvae. Aquac Int 9:225–235
Mallekh R, Lagardere JP, Anras MLB, Lafaye JY (1998) Variability in appetite of turbot, Scophthalmus maximus under intensive rearing conditions: the role of environmental factors. Aquaculture 165:123–138
Marzorati M, Wittebolle L, Boon N, Daffonchio D, Verstraete W (2008) How to get more out of molecular fingerprints: practical tools for microbial ecology. Environ Microbiol 10:1571–1581
Mayali X, Franks PJS, Azam F (2008) Cultivation and ecosystem role of a marine Roseobacter clade-affiliated cluster bacterium. Appl Environ Microbiol 74:2595–2603
Michaud L, Blancheton JP, Bruni V, Piedrahita R (2006) Effect of particulate organic carbon on heterotrophic bacterial populations and nitrification efficiency in biological filters. Aquac Int 34:224–233
Michaud L, Giudice AL, Troussellier M, Smedile F, Bruni V, Blancheton JP (2009) Phylogenetic characterization of the heterotrophic bacterial communities inhabiting a marine recirculating aquaculture system. J Appl Microbiol 107:1935–1946
Munro PD, Barbour A, Birkbeck TH (1993) Bacterial flora of rotifers Brachionus plicatilis; evidence for a major location on the external surface and methods for reducing the rotifer bacterial load. In: Reinertsen H, Dahle LA, Jørgensen L, Tyinnereim K (eds) Fish farming technology. A.A. Balkema, Rotterdam, pp 93–100
Muyzer G, de Waal EC, Uitterlinden AG (1993) Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl Environ Microbiol 59:695–700
Olafsen JA (2001) Interactions between fish larvae and bacteria in marine aquaculture. Aquaculture 200:223–247
Olsson JC, Jöborn A, Westerdahl A, Blomberg L, Kjelleberg S, Conway PL (1998) Survival, persistence and proliferation of Vibrio anguillarum in juvenile turbot, Scopththalmus maximus (L.), intestine and faeces. J Fish Dis 21:1–9
Pintado J, Pérez-Lorenzo M, Luna-González A, Sotelo CG, Prol MJ, Planas M (2010) Monitoring of the bioencapsulation of a probiotic Phaeobacter strain in the rotifers Brachionus plicatilis using denaturing gradient gel electrophoresis. Aquaculture 302:182–194
Planas M, Pérez-Lorenzo M, Vázquez A, Pintado J (2005) A model for experimental infections with Vibrio (Listonella) anguillarum in first feeding turbot (Scophthalmus maximus) larvae under hatchery conditions. Aquaculture 250:232–243
Planas M, Pérez-Lorenzo M, Hjelm M, Gram L, Fiksdal IU, Bergh Ø, Pintado J (2006) Probiotic effect in vivo of Roseobacter strain 27-4 against Vibrio (Listonella) anguillarum infections in turbot (Scophthalmus maximus L.) larvae. Aquaculture 255:323–333
Porsby CH, Nielsen KF, Gram L (2008) Phaeobacter and Ruegeria species of the Roseobacter clade colonize separate niches in a Danish turbot (Scophthalmus maximus)-rearing farm and antagonize Vibrio anguillarum under different growth conditions. Appl Environ Microbiol 74:7356–7364
Porsby CH, Webber MA, Nielsen KF, Piddock LJV, Gram L (2011) Resistance and tolerance to tropodithietic acid, an antimicrobial in aquaculture, is hard to select. Antimicrob Agents Chemother 55:1332–1337
Prol MJ, Bruhn JB, Pintado J, Gram L (2009) Real-time PCR detection and quantification of fish probiotic Phaeobacter strain 27-4 and fish pathogenic Vibrio in microalgae, rotifer, Artemia and first feeding turbot (Psetta maxima) larvae. J Appl Microbiol 106:1292–1303
Prol-García MJ, Planas M, Pintado J (2010) Different colonization and residence time of Listonella anguillarum and Vibrio splendidus in the rotifer Brachionus plicatilis determined by real-time PCR and DGGE. Aquaculture 302:26–35
Prol-García MJ, Gómez M, Sánchez L, Pintado J (2012) Phaeobacter grown in biofilters: a new strategy for the control of Vibrionaceae in aquaculture. Aquac Res. doi:10.1111/are.12046
Qi Z, Dierckens K, Defoirdt T, Sorgeloos P, Boon N, Bao Z, Bossier P (2009) Effects of feeding regime and probionts on the diverting microbial communities in rotifer Brachionus culture. Aquac Int 17:303–315
Rao D, Webb JS, Kjelleberg S (2006) Microbial colonization and competition on the marine alga Ulva australis. Appl Environ Microbiol 72:5547–5555
Reid HI, Treasurer JW, Adam B, Birkbeck TH (2009) Analysis of bacterial populations in the gut of developing cod larvae and identification of Vibrio logei, Vibrio anguillarum and V. splendidus as pathogens of cod larvae. Aquaculture 288:36–43
Reitan KI, Natvik CM, Vadstein O (1998) Drinking rate, uptake of bacteria and microalgae in turbot larvae. J Fish Biol 53:1145–1154
Salvesen I, Skjermo J, Vadstein O (1999) Growth of turbot (Scophthalmus maximus L.) during first feeding in relation to the proportion of r/K-strategists in the bacterial community of the rearing water. Aquaculture 175:337–350
Sandlund N, Bergh Ø (2008) Screening and characterisation of potentially pathogenic bacteria associated with Atlantic cod Gadus morhua larvae: bath challenge trials using a multidish system. Dis Aquat Org 81:203–217
Skjermo J, Vadstein O (1993) Characterization of the bacterial flora of mass cultivated Brachionus plicatilis. Hydrobiologia 255(256):185–191
Skjermo J, Salvesen I, Oie G, Olsen Y, Vadstein O (1997) Microbially matured water: A technique for selection of a non-opportunistic bacterial flora in water that may improve performance of marine larvae. Aquac Int 5:13–28
Skov MN, Pedersen K, Larsen JL (1995) Comparison of pulsed-field gel electrophoresis, ribotyping, and plasmid profiling for typing of Vibrio anguillarum serovar O1. Appl Environ Microbiol 61:1540–1545
Sugita H, Mizuki H, Shiro I (2008) Prevalence of a fish pathogen, Listonella anguillarum, in the intestinal tract of fish collected off the coast of Japan. Aquac Res 39:103–105
Thomson R, Macpherson HL, Riaza A, Birkbeck TH (2005) Vibrio splendidus biotype 1 as a cause of mortalities in hatchery-reared larval turbot, Scophthalmus maximus (L.). J Appl Microbiol 99:243–250
Verdonck L, Grisez L, Sweetman E, Minkoff G, Sorgeloos P, Ollevier F, Swings J (1997) Vibrios associated with routine production of Brachionus plicatilis. Aquaculture 149:203–214
Verschuere L, Dhont J, Sorgeloos P, Verstraete W (1997) Monitoring Biolog patterns and r/K-strategists in the intensive culture of Artemia juveniles. J Appl Microbiol 83:603–612
Verschuere L, Rombaut G, Sorgeloos P, Verstraete W (2000) Probiotic bacteria as biological control agents in aquaculture. Microbiol Mol Biol Rev 64:655–671
Vine NG, Leukes WD, Kaiser H (2006) Probiotics in marine larviculture. FEMS Microbiol Rev 30:404–427
Wagner-Döbler I, Biebl H (2006) Environmental biology of the marine Roseobacter lineage. Annu Rev Microbiol 60:255–280
Zhang XH, Austin B (2000) Pathogenicity of Vibrio harveyi to salmonids. J Fish Dis 23:93–102
Acknowledgments
We thank Professor Lone Gram for kindly providing the strains used in this study. We are grateful to Dr Miquel Planas for critical revision of the manuscript and to Alexandro Chamorro for technical assistance. We also thank Elisabeth Heseltine for editing the manuscript.
María J. Prol-García obtained a grant from the I3P Program of CSIC, co-financed by European Social Fund. Research funding was also provided by INIA (ACU03-003) and by the I3 Program of the Spanish Ministry of Education and Science (CSIC-PIE 2006 7 01067).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary figure 1
PCR-DGGE profile of 16S rDNA fragments of A) turbot larvae from control, BPh, Va and VaBPh tanks; B) rotifer samples from tanks on day 10 post-hatching (rotifer LD10), of rotifers enriched with I. galbana (rotifers Ig) and of rotifers loaded with V. anguillarum (rotifers Va); and C) seawater samples taken from control, BPh, Va and VaBPh tanks. M: marker (A: Kordia algicida, B: Tenacibaculum discolor, C: Vibrio anguillarum 90-11-287, D: Phaeobacter 27-4, E: Ruegeria mobilis, F: Flexibacter sp). Va: band with a migration pattern similar to V. anguillarum, Ph: band with a migration pattern similar to Phaeobacter 27-4. (DOCX 6544 kb)
Supplementary figure 2
Functional organization based on Pareto–Lorenz distribution curves obtained from DGGE profiles on days 3 (×), 5 (◊), 7 (Δ), 9 (○) and 10 (□) after larval hatching of larvae and seawater from control, BPh, Va and VaBPh tanks. The dashed vertical line at the 0.2x axis is plotted to evaluate the range of the Pareto values. (DOCX 701 kb)
Rights and permissions
About this article
Cite this article
Prol-García, M.J., Pintado, J. Effectiveness of Probiotic Phaeobacter Bacteria Grown in Biofilters Against Vibrio anguillarum Infections in the Rearing of Turbot (Psetta maxima) Larvae. Mar Biotechnol 15, 726–738 (2013). https://doi.org/10.1007/s10126-013-9521-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10126-013-9521-4