Abstract
In this study, we screened eight terpenes isolated from the organic extract of Sphaerococcus coronopifolius for their antifouling activity in order to find possible new sources of non-toxic or less toxic bioactive antifoulants. The anti-settlement activity (EC50) and the degree of toxicity (LC50) of S. coronopifolius metabolites was evaluated using larvae of the cirriped crustacean Amphibalanus (Balanus) amphitrite (cyprids and nauplii) as model organism. For five of eight tested metabolites EC50 was lower than 5 mg/L. The most promising results were observed for bromosphaerol (3), which expressed an EC50 value of 0.23 mg/L, in combination with low toxicity levels (LC50 > 100 mg/L). The therapeutic ratio—an index used to estimate whether settlement inhibition is due to toxicity or other mechanisms—is also calculated and discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Marine fouling affects a wide range of human activities in the aquatic environment and, especially in shipping, it is associated with economic loss due to speed reduction and higher costs both for fuel and for hull maintenance. Technological problems related to biofouling and methods for protecting surfaces have been widely discussed in the past few years (Omae 2003; Yebra et al. 2004; Chambers et al. 2006). The majority of vessels are protected by antifouling paints containing biocides (Almeida et al. 2007); the key property of a good antifouling biocide with respect to the environment is to be effective in preventing fouling, without causing persisting adverse environmental effects. The effects and behaviour of biocides used in antifouling paints have been extensively studied and the related data are available (see reviews: Thomas 2001; Thomas and Brooks 2010). Currently, a wide range of chemicals are used as antifouling biocides, governed by different regulations, depending on the legislation in each country. Copper, copper pyrithione, zinc pyrithione, TPBT, diuron, SeaNine 211, Irgarol 1051, chlorothalonil, cuprous thyocianate, Ziram, Zineb, naphthenic acid copper salts, tolyfluanid, Econea and capsaicin are just a few of the antifoulants in use. Nowadays, marine antifouling technology is at a crossroads: in September 2008, IMO banned the use of self-polishing tributyltin coatings, while there is an increasing opposition to the use of copper.
Silicone-based fouling release technologies are providing an alternative solution, however they are relatively expensive and can be easily damaged (Swain et al. 2007).
Preventing the settlement of fouling organisms in a non-toxic manner is the ideal solution; it is therefore necessary to investigate new avenues, which may be inspired by a biomimetic approach, studying structures and functions of biological systems as models for the design of antifouling solutions. Much effort has been directed to identify natural chemistries that may act as antifoulants (Pawlik 1992; Abarzua et al. 1999; Rittschof 2001; de Nys and Steinberg 2002; Burgess et al. 2003; Ralston and Swain 2009). In the marine environment, a number of organisms are equipped with natural physical or chemical defence mechanisms against fouling (Engel et al. 2002; Hellio et al. 2002; Paul and Puglisi 2004). Several marine metabolites show significant levels of antibacterial, antimacrofouling, antifungal, and antiprotozoan properties, and have good potentials to be developed as antifouling paints (Hellio et al. 2000; Da Gama et al. 2002; Steinberg and de Nys 2002; Ali et al. 2002; Kubanek et al. 2003; Faimali et al. 2003; Fusetani 2004; Hellio et al. 2005).
There are many reports on marine organisms that show their ability to resist epibiosis (Fusetani 2004), including several species of algae that contain a diverse spectrum of chemical entities with antifouling activity. Algae seem to be chemically protected by surface-bound or continuously released water-soluble compounds that can deter invertebrate larvae from settling. Algal metabolites can affect the development and grazing behaviour of some settling organisms, pointing to the existence of chemical antifouling mechanisms. Isolation of these bioactive secondary macroalgae metabolites might lead to the development of new eco-friendly antifouling paints.
The cosmopolitan red alga Sphaerococcus coronopifolius is an unusually prolific source of secondary metabolites, mainly diterpenoids. So far, only a small number of reports have been published on the isolation and characterization of bioactive compounds from S. coronopifolius, and even more restricted information is available on their biological activity. Cafieri et al. (1982a and 1988) isolated the diterpenes sphaeopyrane and 12 S-hydroxybromosphaerol from S. coronopifolius; Etahiri et al. (2001) isolated two new bromoditerpenes that showed antibacterial activity against Gram positive bacteria. Recently, Smyrniotopoulos et al. (2010) tested the cytotoxicity of nine brominated diterpenes against human lung cancer cell lines, and the antibacterial activity of six bromoditerpenes against multidrug-resistant and methicillin-resistant Staphylococcus aureus strains.
In this work, we report on the evaluation of eight metabolites from S. coronopifolius as to the settlement and larvae mortality of the cirriped crustacean Amphibalanus amphitrite. The therapeutic ratio (TR), which indicates whether settlement inhibition is due to the toxicity of the compounds or related to other mechanisms, is also discussed (Rittschof et al. 1994; Vitalina et al. 1997; Clare et al. 1999; Faimali et al. 2005; Fig. 1).
Materials and Methods
Extraction and Isolation of Metabolites
S. coronopifolius was collected by SCUBA diving in Palaiokastritsa bay in the West coast of Corfu Island, Greece, at a depth of 10–15 m, in May 2002. A specimen is kept at the Herbarium of the Laboratory of Pharmacognosy and Chemistry of Natural Products, University of Athens (ATPH/MO/201).
S. coronopifolius was initially freeze-dried (291.4 g dry weight) and then exhaustively extracted with mixtures of CH2Cl2/MeOH (3/1 v/v) at room temperature. The combined extracts were concentrated to give a dark green residue (8.20 g), which was later subjected to vacuum column chromatography on silica gel, using a 10% step gradient of cyclohexane-EtOAc elution sequence. Fraction Ia (10% EtOAc in cyclohexane; 753.8 mg) was fractionated on silica gel isocraticly with 100% cyclohexane, to yield pure compound 1 (449.8 mg). Fraction IIa (20% EtOAc in cyclohexane; 4.01 g) was subjected to gravity column chromatography, using a 2%, initially, to 10% step gradient of cyclohexane-EtOAc. Part (50 mg) of the VIIb fraction (8% EtOAc in cyclohexane; 2.85 g) was subjected to normal phase HPLC chromatography, using 5% EtOAc in cyclohexane as a mobile phase to yield pure compounds 2 (18.7 mg) and 3 (21.0 mg). The CH3CN soluble portion (173.4 mg) of fraction XIb (50% EtOAc in cyclohexane) (199.6 mg) was subjected to reversed-phase HPLC chromatography, using CH3CN as mobile phase to yield pure compounds 5 (10.9 mg) and 6 (15.3 mg). The CH3CN soluble portion (306.7 mg) of fraction IVa (60% EtOAc in cyclohexane; 337.8 mg) was subjected to reversed-phase HPLC chromatography, using 100% CH3CN as a mobile phase. Peak XIc (retention time 12.52 min; 86.3 mg) was subjected again to reversed-phase HPLC chromatography, using CH3CN as mobile phase to yield pure compound 4 (54.4 mg). The CH3CN soluble part (323.4 mg) of fraction Va (70% EtOAc in cyclohexane) (419.8 mg) was subjected to reversed-phase HPLC chromatography, using CH3CN as a mobile phase to yield pure compound 7 (42.1 mg). Peak XIId (retention time 11.6 min; 48.1 mg) with HPLC normal phase purification, using 70% CHCl3 in n-hexane as mobile phase, yielded pure compound 8 (2.0 mg).
Compounds 1–8 were identified by comparison of their spectroscopic data (including NMR, MS, IR, UV) with previously reported literature values.
Settlement Inhibition Assays
Cypris larvae were obtained from laboratory cultures of the crustacean cirriped A. amphitrite brood stock. Twenty to thirty adult barnacles were reared in 800 mL aerated beakers containing filtered natural sea water (FNSW) at 20 ± 1°C, with a 16 h:8 h light:dark (L:D) cycle. They were fed every 2 days with nauplii of Artemia salina sp. (100 mL, 20–35 larvae mL−1), and Tetraselmis suecica (100 mL, 2 × 105 cells·mL−1). Twenty beakers containing adults reared under the above mentioned conditions produced nauplii throughout the year. Nauplii were collected with a 5-mL pipette by positioning the beaker near a light source and reared in 500-mL beakers containing 0.22 μm FNSW gently aerated at 28 ± 1°C with a 16 h:8 h L:D cycle. Nauplii were fed every 48 h with T. suecica (5 × 105 cells mL−1) until, after 5–6 days, they reached the cyprid stage.
Newly metamorphosed cyprids were filtered and maintained in filtered (0.22 μm) natural sea water at 6°C for 4 days before being used in settlement assays (Rittschof et al. 1992). Settlement tests were performed by adding 15–20 cyprids (for each replicate) to 24-well polystyrene plates containing 2 mL of bioactive metabolite solution at different concentrations (0; 0.1; 1; 10; 100 mg/L). Four replicates were prepared for each concentration of each tested metabolite and the reported results are the mean values of the four replicates. The 24-well plates were stored for 72 h at 28°C with a 16:8 L:D cycle. After 24, 48 and 72 h, the number of settled, not-settled, and dead larvae was measured under a stereomicroscope. EC50 (concentration of metabolite causing 50% settlement inhibition to exposed organisms) was calculated with results obtained after 72 h. Additionally, at the same time, LC50(cypris) was calculated, as the metabolite concentration causing 50% mortality to the exposed organisms.
Naupliar Toxicity Test
Acute environmental toxicity of metabolites was tested by using stage II nauplii of A. amphitrite. Nauplii were obtained from adult brood stock as described above, collected and immediately filtered in 0.22 μm FNSW. The toxicity assay was set within 2–4 h from nauplii collection. The test was performed by adding 15 to 25 nauplii II to 24-well polystyrene plates containing 2 mL of bioactive metabolite solution at different concentrations (0; 0.1; 1; 10; 100 mg/L). Four replicates were prepared for each concentration of each tested metabolite and the reported results are the mean values of the four replicates. The plates were stored for 48 h at 20°C with a 16:8 L:D cycle. After 24 and 48 h, the number of dead larvae was observed under a stereomicroscope. LC50(nauplii) was calculated as the concentration of metabolite causing 50% mortality to the exposed organisms after 48 h of contact.
Statistical Analysis
Settlement inhibition (EC50) at 72 h and mortality (LC50) values at 48 h (for nauplii) and 72 h (for cyprids) were calculated using trimmed Spearman–Karber analysis (Finney 1978). Therapeutic ratio is defined as LC50/EC50. This index was calculated using mortality values measured for larvae at naupliar stage (TRN) and for larvae at cypris stage (TRC).
Results
Results of settlement inhibition and cyprid mortality tests for all eight compounds are shown in Fig. 2. The 72 h values of EC50(settl) and LC50(cypris) are summarised in Table 1. For five of these metabolites (2, 3, 5, 6 and 7), EC50(settl) was lower than 5 mg/L. Very high activity was observed for metabolites 3 and 6, which showed EC50(settl) values lower than 0.5 mg/L. Concerning cyprids mortality, five compounds (1, 2, 3, 4 and 8) showed LC50(cypris) values higher than the maximum tested concentration (100 mg/L), while the remaining three compounds showed toxicity values in the range of 30 mg/L. A naupliar toxicity assay was performed and results for all compounds are shown in Fig. 3. It is evident that naupliar response to tested molecules is very different from cyprids; indeed, naupliar mortality occurs at lower concentrations than for cyprids, and all LC50 (nauplii) values referring to nauplii (see Table 1) are lower than 10 mg/L (except for 4). In Table 2, we report the Therapeutic Ratio values, calculated both for naupliar (TRN) and cyprids (TRC). High TR values, indicate low toxicity of the tested compound. With regard to TRN values, only 3 showed a quite high value (15.78), while for TRC three metabolites (2, 3, 6) had values higher than 50.
Discussion
Isolated metabolites, tested in pure form and showing antifouling activity, belong to the chemical classes of fatty acids, lipopeptides, amides, alkaloids, terpenoids, lactones, pyrroles, and steroids. Seaweeds produce a wealth of bioactive metabolites (Tringali 1997), which, among other ecological roles, are responsible for the protection against other settling organisms. Chemical investigations of the benthic macroalga Delisea pulchra resulted in the isolation and identification of halogenated furanones, which, when tested against the barnacle A. amphitrite (Steinberg et al. 1998) and the macroalga Ulva lactuca (Maximilien et al. 1998), showed significant inhibitory effects against both fouling organisms in both field and laboratory experiments (de Nys and Steinberg 2002). Phlorotannins, isolated from the Australian brown algae Ecklonia radiata, have also shown to be effective against the settlement and growth of the green alga Ulva sp. (Jennings and Steinberg 1997). Non-polar secondary metabolites—dictyol E and pachydictyol A—isolated from the brown alga Dictyota menstrualis, were found capable of inhibiting the settlement of Bugula neretina (Schmitt et al. 1995). Extracts of the marine alga Sargassum muticum were found to inhibit the development of fouling organisms in a non-toxic manner (Hellio et al. 2000), whereas the dichloromethane extract of the same alga was found effective against germination of U. lactuca (Bazes et al. 2009). Elatol and deschloroelatol, isolated from the red alga Laurencia rigida, were capable of inhibiting larval settlement of both A. amphitrite and B. neretina (de Nys et al. 1996; Konig and Wright 1997). Two halogenated monoterpenes, exhibiting deterrent effects against A. amphitrite, have been isolated from the Tasmanian red alga Plocamium costatum, (Konig et al. 1999). Furthermore, Lau and Qian (1997) reported that phlorotannins from Sargassum tenerrimum inhibited the metamorphosis of the polychaete Hydroides elegans. Larval settlement of A. amphitrite was found to be inhibited by crude extracts of the brown alga Bifurcaria bifurcata (Marèchal et al. 2004).
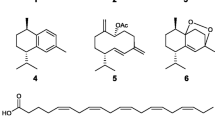
S. coronopifolius is known to produce halogenated analogues of diterpenoids (Dembitsky et al. 2002). The sesquiterpene alloaromadendrene (1) (De Rosa et al. 1988; Smyrniotopoulos et al. 2010) along with the brominated diterpenes sphaerococcenol A (2) (Fenical et al. 1976; Smyrniotopoulos et al. 2008a), bromosphaerol (3) (Fattorusso et al. 1976; De Rosa et al. 1988; Smyrniotopoulos et al. 2008a), 12R-hydroxybromosphaerol (4) (Cafieri et al. 1987; Smyrniotopoulos et al. 2008a), coronopifoliol (5) (Cafieri et al. 1985), bromotetrasphaerol (6) (Cafieri et al. 1988), 12 S-hydroxybromosphaerol (7) (Cafieri et al. 1982a; Smyrniotopoulos et al. 2008a) and 1 S-hydroxy-1,2-dihydro-bromosphaerol (8) (Cafieri et al. 1982b; Smyrniotopoulos et al. 2008b), have been previously isolated from various collections of S. coronopifolius collected from different areas of the Mediterranean Sea.
The algal and not microbial biogenetic origin of the above mentioned metabolites is strongly supported by their high percentage in the algal biomass. Specifically, the sesquiterpene alloaromadendrene (1) and the bromoditerpenes sphaerococcenol (2), bromosphaerol (3) and 12R-hydroxybromosphaerol (4), are major constituents of the alga, accounting for the 28.1% (w/w) of the organic extract (1: 8.1%, 2: 9.1%, 3: 10.4%, 4: 0.5%; Smyrniotopoulos, unpublished data). The coexisting minor diterpenoids (5–8) are structurally related to the main metabolites indicating a common biosynthetic origin from genanyl-geranylpyrophosphate.
Recently, diterpenoids extracted from the Mediterranean brown alga Dictyota sp. have been evaluated as antifouling substances against marine bacterial biofilm (Viano et al. 2009), while Culioli et al. (2008) investigated the antifouling activity against A. amphitrite of four meroditerpenoids isolated from the marine brown alga Halidrys siliquosa. Meroditerpenoids from the brown alga Cystoseira baccata also showed antifouling activity against the growth of microalgae and macroalgal settlement (Mokrini et al. 2008).
Antifouling diterpenes produced by the brown seaweed Canistrocarpus cervicornis strongly inhibited the establishment of the mussel Perna perna (Bianco et al. 2009) and a diterpene isolated from the gorgonian Junceella juncea showed potent antifouling activity against larval settlement of A. amphitrite at non-toxic concentrations (Qi et al. 2009).
Complying with the guidelines of the US Navy Program that require an EC50(settl) lower than 25 mg/L for a compound to be considered a promising natural antifoulant, all eight compounds evaluated in this study meet this requirement. The EC50 values obtained are comparable to seven of the nine reported by Qi et al. (2006), even if two of the nine diterpenoids isolated from the gorgonian J. juncea showed a very high antifouling activity against the barnacle A. amphitrite (EC50 = 0.004 mg/L). Levels of antifouling activity showed in this study can are also similar to those obtained from a marine-derived fungus Ampelomyces sp. (Kwong et al. 2006).
Bromosphaerol (3) is the most active among the eight tested metabolites; showing the lowest EC50(settl) value (0.23 mg/L) without toxic effects on the cypris larvae (LC50(cypris) > 100 mg/L). TRC value of 3, calculated considering cyprids mortality, is impressive (434.78), whereas its toxicity towards nauplii is relatively low (LC50 (nauplii) = 3.63 mg/L). When compared with all assayed compounds, 3 clearly results to be the most promising natural antifoulant candidate.
Besides 3, metabolites 2 (sphaerococcenol A) and 6 (bromotetrasphaerol) were significantly active compounds, showing EC50(settl) values of 1.53 and 0.38 mg/L, respectively, and a TRC (calculated with cyprids mortality) of 65.3 and 79.47. Their toxicity among naupliar stage is however high (LC50(nauplii) values of 0.32 and 0.39 mg/L, respectively).
Settlement inhibition levels of metabolites examined in this work are comparable to some of the most significant ones found in the literature for natural products such as those from Callyspongia truncata (EC50 = 0.24 mg/L) (Tsukamoto et al. 1997), Phyllidia pustulosa (EC50 = 0.17 mg/L) (Hirota et al. 1998), and Reniera sarai (EC50 = 0.27 mg/L) (Faimali et al. 2003).
Traditionally, the TR is calculated by taking into account naupliar mortality (Rittschof et al. 1994; Clare et al. 1999; Faimali et al. 2003). A recent review from Qian et al. (2010) underlined how the TR is commonly used as a yardstick of the potential antifouling activity of a compound. In this review, it is suggested that a compound with a TR > 15 can be considered as a non-toxic antifouling compound, even if a much higher TR is recommended when selecting candidate compounds. It is suggested that only small molecules with a TR > 50 and an EC50 < 5 mg/L against both hard and soft foulers should be considered.
Since the aim of TR is to determine whether the mechanism of settlement inhibition is based on toxic effect, it would be more appropriate to measure mortality on the same larval stage on which settlement is evaluated (competent larval stage). On the other hand, LC50 on nauplii is a good index of toxicity against non-target organisms, since nauplii can be considered a more representative class of zooplankton.
It is clear (see Table 2) that the proposed approach for therapeutic ratio calculation, using cyprids (TRC) instead of naupliar mortality (TRN), yields very different results. For instance, taking TRN of compound 2 (0.21), we can assert that this compound is characterised by good antifouling properties (EC50 = 1.53 mg/L), but acts with a toxic mechanism. However, looking at TRC (65.3) of the same compound, the conclusions are completely different, and this compound becomes a well-performing antifoulant, acting through a non-toxic mechanism. These considerations are also valid for the rest of the tested compounds. Settlement inhibition of compounds 2, 3 and 6 can be associated to a non-toxic mechanism, since its TRC is quite high (more than 60).
On the basis of settlement and mortality assay results, the tested compounds can be divided into three groups. Only compound 3 belongs to the first group, since it is able to inhibit settlement of A. amphitrite with a non-toxic mechanism and without toxicity to the naupliar stage. Indeed, TRC of metabolite 3 is 434.78, nearly five times higher than that of 6 and almost 100 times greater than that of 8 (which was the lowest among all the eight tested compounds). Furthermore, its toxicity towards nauplii is quite low, with LC50 (nauplii) amounting to 3.36 mg/L. The second group includes compounds 2, 5, 6 and 7, which showed relatively low EC50(settl) values (<5 mg/L), low toxicity towards cyprids, but were toxic to the naupliar stage. The third group contains metabolites 1, 4 and 8, which are characterised by low settlement inhibition (EC50 > 5 mg/L) and low toxicity towards cypris larvae and nauplii and, basically, are of no biological interest. It is important to note that this division into three groups would be very different if naupliar toxicity (TRN) was to be used for therapeutic ratio calculation. In this case, for example, compound 4 would appear as one of the best performing chemicals, even if showing a high EC50(settl) value (15.97 mg/L), while 2 would be considered as the worst compound, due to its high toxicity towards nauplii.
The results obtained in this study highlight a new perspective in the quest for environment-friendly antifouling agents, and encourage further field experiments. Finally, the issue of cultivation is worth mentioning, which is easier for macroalgae, than for other marine organisms, thus giving them a considerable advantage as potential sources of antifouling agents in the future.
References
Abarzua S, Jakubowski S, Eckert S, Fuchs P (1999) Biotechnological investigation for the prevention of marine biofouling II. Blue-green algae as potential producers of biogenic agents for the growth inhibition of microfouling organisms. Bot Mar 42:459–465
Ali MS, Saleem M, Yamdagni R, Ali MA (2002) Steroid andantibacterial steroidal glycosides from marine green alga Codium yengarii Borgesen. Nat Prod Lett 16:407–413
Almeida E, Diamantino TC, de Sousa O (2007) Marine paints: the particular case of antifouling paints (review). Prog Org Coat 59:2–20
Bazes A, Silkina A, Douzenel P, Fay F, Kervarec N, Morin D, Berge JP, Bourgougnon N (2009) Investigation of the antifouling constituents from the brown alga Sargassum muticum (Yendo) Fensholt. J Appl Phycol 21:395–403
Bianco EM, Rogers R, Teixeira VL, Pereira RC (2009) Antifoulant diterpenes produced by the brown seaweed Canistrocarpus cervicornis. J Appl Phycol 21:341–346
Burgess JG, Boyd KG, Armstrong E, Jiang Z, Yan L, Berggren M, May U, Pisacane T, Grammo A, Adams DR (2003) The development of a marine natural product-based antifouling paint. Biofouling 19:197–205
Cafieri F, Ciminiello P, Fattorusso E, Santacroce C (1982a) 12 S-hydroxybromosphaerol, a new bromoditerpene from the red alga Sphaerococcus coronopifolius. Experientia 38:298–299
Cafieri F, Ciminiello P, Santacroce C, Fattorusso E (1982b) (1S)-1, 2-dihydro-1-hydroxybromosphaerol, a minor bromoditerpene from the red alga Sphaerococcus coronopifolius. Phytochemistry 21:2412–2413
Cafieri F, Fattorusso E, Mayol L, Santacroce C (1985) Coronopifoliol, a diterpene based on an unprecedented tetracyclic skeleton from the red algae Sphaerococcus coronopifolius. J Org Chem 50:3982–3984
Cafieri F, Fattorusso E, Mayol L, Santacroce C (1987) Structure of bromotetrasphaerol, a further irregular diterpene from the red algae Sphaerococcus coronopifolius. Tetrahedron 42:4273–4276
Cafieri F, De Napoli L, Fattorusso E, Santacroce C (1988) Sphaeropyrane, a diterpene from the marine alga Sphaerococcus coronopifolius. Phytochemistry 27(2):621–623
Chambers LD, Stokes KR, Walsh FC, Wood RJK (2006) Modern approaches to marine antifouling coatings. Surf Coat Technol 201:3642–3652
Clare AS, Rittschof D, Gerhart DJ, Hooper IR, Bonaventura J (1999) Antisettlement and narcotic action of analogues of diterpene marine natural product antifoulants from Octocorals. Mar Biotechnol 1:427–436
Culioli G, Ortalo-Magne A, Valls R, Hellio C, Clare AS, Piovetti L (2008) Antifouling activity of meroditerpenoids from the marine brown alga Halidrys siliquosa. J Nat Prod 71(7):1121–1126
Da Gama BA, Pereira RC, Carvalho AGV, Coutinho R, Yoneshigue-Valentin Y (2002) The effects of seaweed secondary metabolites on biofouling. Biofouling 18:13–20
Dembitsky VM, Tolstikov AG, Tolstikov GA (2002) Natural halogenated diterpenoids. Chem sustainable development 10:253–264
De Nys R, Leya T, Maximilen R, Afsar A, Nair PSR, Steinberg PD (1996) The need for standardised broad scale bioassay testing: a case study using the red alga Laurencia rigida. Biofouling 10(1–3):213–224
De Nys R, Steinberg PD (2002) Linking marine biology and biotechnology. Curr Opin Biotechnol 13:244–248
De Rosa S, De Stefano S, Scarpelli P, Zavodnik N (1988) Terpenes from the red alga Sphaerococcus coronopifolius of the north Adriatic sea. Phytochemistry 27:1875–1878
Engel S, Jensen PR, Fenical W (2002) Chemical ecology of marine microbial defense. J Chem Ecol 28:1971–1985
Etahiri S, Bultel-Ponce V, Caux C, Guyot M (2001) New bromoditerpenes from the red alga Sphaerococcus coronopifolius. J Nat Prod 64(8):1024–1027
Faimali M, Sepcic K, Turk T, Geraci S (2003) Non-toxic antifouling activity of polymeric 3-alkylpyridinium salts from the mediterranean sponge Reniera sarai (Pulitzer-finali). Biofouling 19(1):47–56
Faimali M, Garaventa F, Mancini I, Sicurelli A, Guella G, Piazza V, Greco G (2005) Antisettlement activity of synthetic analogues of polymeric 3-alkylpyridinium salts isolated from the sponge Reniera sarai. Biofouling 21(1):49–57
Fattorusso E, Magno S, Santacroce C, Sica D, Di Blasio B, Pedone C, Impellizzeri G, Mangiafico S, Oriente G, Piattelli M, Sciuto S (1976) Gazz Chim Ital 106:779–783
Fenical W, Finer J, Clardy J (1976) Sphaerococcenol A; a new rearranged bromo-diterpene from the red alga Sphaerococcus coronopifolius. Tetrahedron Lett 17:731–734
Finney DJ (1978) Statistical methods in biological assay, 3rd edn. Griffin, London, pp 394–401
Fusetani N (2004) Biofouling and antifouling. Nat Prod Rep 21:94–104
Kwong T, Miao L, Li X, Qian PY (2006) Novel antifouling and antimicrobial compound from a marine-derived fungus Ampelomyces sp. Mar Biotechnol 8:634–640
Hellio C, Bourgougnon N, Le Gal Y (2000) Phenoloxidase from Mytilus edulis byssus gland: purification, partial characterization and application for screening products with potential antifouling activities. Biofouling 16:235–244
Hellio C, Pascal Berge J, Beaupoil C, Le Gal Y, Bougougnon N (2002) Screening of marine algal extracts for anti-settlement activities against microalgae and macroalgae. Biofouling 18:205–215
Hellio C, Tsoukatou M, Marechal JP, Aldred N, Beaupoil C, Clare AS, Vagias C, Roussis V (2005) Inhibitory effect of Mediterranean sponge extracts and metabolites on larval settlement of the barnacle Balanus amphitrite. Mar Biotech 7:297–305
Hirota H, Okino T, Yoshimura E, Fusetani N (1998) Five new antifouling sesquiterpenes from two marine sponges of the genus Axinyssa and the nudibranch Phyllidia pustulosa. Tetrahedron 54(46):13971–13980
Jennings JG, Steinberg PD (1997) Phlorotannins vs other factors affecting epiphyte abundance on the kelp Eclonia radiata. Oecologia 109:461–473
Konig GM, Wright AD (1997) Laurencia rigida: chemical investigation of its antifouling dichloromethane extract. J Nat Prod 60(10):967–970
Konig GM, Wright AD, Linden A (1999) Plocamium hamatum and its monoterpenes: chemical and biological investigations of the tropical marine red alga. Phtyochemistry 52:1047–1053
Kubanek J, Jensen PR, Keifer PA, Sullards MC, Collins DO, Fenical W (2003) Seaweed resistance to microbial attack: a targeted chemical defense against marine fungi. Proc Natl Acad Sci USA 100:6916–6921
Lau SCK, Qian PY (1997) Phlorotannins and related compounds as larval settlement inhibitors of the tube-building polychaete Hydroides elegans. Mar Ecol Progr Ser 159:219–227
Marèchal JP, Culioli G, Hellio C, Thomas-Guyon H, Callow ME, Clare AS, Ortalo-Magné A (2004) Seasonal variation in antifouling activity of crude extracts of the brown alga Bifurcaria bifurcate (Cystoseiracee) against cyprids of Balanus amphitrite and the marine bacteria Cobetia marina and Pseudoalteromonas haloplanktis. J Exp Mar Biol Ecol 313(1):47–62
Maximilien N, de Nys R, Holmstrom C, Gram L, Givskov M, Crass K, Kjelleberg S, Steinberg PD (1998) Chemical mediation of bacterial surface colonisation by secondary metabolites from the red alga Delisea pulchra. Aquat Microb Ecol 15:233–246
Mokrini R, Ben Mesaoud M, Daoudi M, Hellio C, Marèchal JP, El Hattab M, Ortalo-Magné A, Piovetti L, Culioli G (2008) Meroditerpenoids and derivatives from the brown alga Cystoseira baccata and their antifouling properties. J Nat Prod 71:1806–1811
Omae I (2003) General aspects of tin-free antifouling paints. Chem Rev 103:3431–3448
Paul VJ, Puglisi MP (2004) Chemical mediation of interactions among marine organisms. Nat Prod Rep 21:189–209
Pawlik J (1992) Chemical ecology of the settlement of benthic marine invertebrates. Oceanogr Mar Biol Ann Rev 30:273–335
Qi SH, Zhang S, Qian PY, Xiao ZH, Li MY (2006) Ten new antifouling briarane diterpenoids from the South China Sea gorgonian Junceella juncea. Tetrahedron 62:9123–9130
Qi SH, Zhang S, Quian PY, Xu HH (2009) Antifeedant and antifouling briaranes from the South China Sea gorgonian Junceella juncea. Chem Nat Compd 45:49–54
Qian PY, Xu Y, Fusetani N (2010) Natural products as antifouling compounds: recent progress and future perspectives. Biofouling 26(2):223–234
Ralston E, Swain G (2009) Bioinspiration—the solution for biofouling control? Bioinsp Biomim 4:1–9
Rittschof D (2001) Natural products antifoulants and coatings development. In: McClintock JB, Baker BJ (eds) Marine chemical ecology. Marine science series. CRC, London, pp 543–566
Rittschof D, Clare AS, Gerhart DJ, Mary SA, Bonaventura J (1992) Barnacle in vitro assays for biologically active substance: toxicity and settlement inhibition assay using mass cultured Balanus amphitrite amphitrite Darwin. Biofouling 6:115–122
Rittschof D, Sasikumar N, Murlless D, Clare AS, Gerhart D, Bonaventura J (1994) Mixture interactions of lactones and furans and a commercial biocide: toxicity and antibarnacle settlement activity. In: Thompson M-F, Nagabhushanam R, Sarojini R, Fingerman M (eds) Recent developments in biofouling control. Balkema, Rotterdam, pp 269–274
Schmitt TM, Hay ME, Lindquist N (1995) Constraints on chemically mediated coevolution: multiple functions for seaweed secondary metabolites. Ecology 76:107–123
Smyrniotopoulos V, Quesada A, Vagias C, Moreau D, Roussakis C, Roussis V (2008a) Cytotoxic bromoditerpenes from the red alga Sphaerococcus coronopifolius. Tetrahedron 64:5184–5190
Smyrniotopoulos V, Vagias C, Rahman MM, Gibbons S, Roussis V (2008b) Brominated diterpenes with antibacterial activity from the red alga Sphaerococcus coronopifolius. J Nat Prod 71:1386–1392
Smyrniotopoulos V, Vagias C, Bruyère C, Lamoral-Theys D, Kiss R, Roussis V (2010) Structure and in vitro antitumor activity evaluation of brominated diterpenes from the red alga Sphaerococcus coronopifolius. Bioorg Med Chem 18:1321–1330
Steinberg PD, de Nys R (2002) Chemical mediation of colonization of seaweed surfaces. J Phycol 38:621–629
Steinberg PD, De Nys R, Kjelleberg S (1998) Chemical inhibition of epibiota by Australian seaweeds. Biofouling 12:227–244
Swain GW, Kovach B, Touzot A, Casse F, Kavanagh CJ (2007) Measuring the performance of today’s antifouling coatings. J Ship Prod 23:164–170
Yebra MD, Kiil S, Dam-Johansen K (2004) Antifouling technology—past, present and future steps towards efficient and environmentally friendly antifouling coatings. Progr Org Coat 50:75–104
Thomas KV (2001) The environmental fate and behaviour of antifouling paint booster biocides: a review. Biofouling 17:73–86
Thomas KV, Brooks S (2010) Mini-review: the environmental fate and effects of antifouling paint biocides. Biofouling 26(1):73–88
Tringali C (1997) Bioactive metabolites from marine algae: recent results. Curr Org Chem 1:375–394
Tsukamoto S, Kato H, Hirota H, Fusetani N (1997) Seven new polyacetylene derivatives, showing both potent metamorphosis-inducing activity in ascidian larvae and antifouling activity against barnacle larvae, from the marine sponge Callyspongia truncata. J Nat Prod 60(2):126–130
Viano Y, Bonhomme D, Camps M, Briand JF, Ortalo-Magnè A, Blache Y, Piovetti L, Culioli G (2009) Diterpenoids from the Mediterranean brown alga Dictyota sp. evaluated as antifouling substances against a marine bacterial biofilm. J Nat Prod 72:1299–1304
Vitalina S, Avelin S, Rittschof D, Sarojini R, Nagabhushanam R (1997) Compounds from octocorals that inhibit barnacle settlement; isolation and biological potency. In: Thompson M-F, Sarojini R, Nagabhushanam R (eds) Bioactive compounds from marine organisms. Balkema, Rotterdam, pp 331–339
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Piazza, V., Roussis, V., Garaventa, F. et al. Terpenes from the Red Alga Sphaerococcus coronopifolius Inhibit the Settlement of Barnacles. Mar Biotechnol 13, 764–772 (2011). https://doi.org/10.1007/s10126-010-9337-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10126-010-9337-4