Abstract
Differential expression of genes is crucial to embryogenesis. The analysis of gene expression requires appropriate references that should be minimally regulated during the embryonic development. To select the most stable genes for gene normalization, the expression profiles of eight commonly used reference genes (ACTB, GAPDH, rpL17, α-Tub, EF1-α, UbcE, B2M, and 18S rRNA) were examined during Japanese flounder (Paralichthys olivaceus) embryonic development using quantitative real-time polymerase chain reaction. It was found that all seven mRNA genes appeared to be developmentally regulated and exhibited significant variation of expression. However, further analyses revealed the stage-specific expression stability. Hence when normalization using these mRNA genes, the differential and stage-related expression should be considered. 18S rRNA gene, on the other hand, showed the most stable expression and could be recommended as a suitable reference gene during all embryonic developmental stages in P. olivaceus. In summary, our results provided not only the appropriate reference gene for embryonic development research in P. olivaceus, but also possible guidance to reference gene selection for embryonic gene expression analyses in other fish species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Quantitative real-time polymerase chain reaction (PCR) is increasingly applied to investigate relevant changes in mRNA levels because of its advantage of sensitivity, large dynamic range, and the potential for high throughput as well as accurate quantification (Huggett et al. 2005). However, several variables, such as the amount of starting material, the quality of the RNA, and the efficiency of reverse transcription and PCR during the experiments, will introduce errors and make the analyses difficult (Vandesompele et al. 2002; de Kok et al. 2005). Fortunately, there are many strategies to control these errors. One simple and popular used method is to normalize the RNA fraction to an endogenous reference gene or housekeeping gene (Dheda et al. 2004). The ideal reference gene should keep a constant level of expression in all tissues independent of experimental conditions and at different developmental stages (Suzuki et al. 2000). However, such an ideal reference gene is almost nonexistent in practice. A feasible strategy is to choose the gene that has minimal variability or its variation is much less than target genes as the endogenous reference gene. For this purpose, many studies have been done to select and validate reference genes for data normalization in various experiments. More and more evidence has shown that many widely used classic reference genes that were thought constantly expressed, in fact, varied greatly in different tissues and under different experimental treatments (Radonic et al. 2004; Ingerslev et al. 2006; Jorgensen et al. 2006).
Embryonic development is a complicated process including cleavage, blastulation, gastrulation, body axis formation, tissue and organ formation, and so forth (Drasdo and Forgacs 2000). During the process, the activation and consumption of masked maternal RNA and the programmed differential expression of zygotic genes are considered key molecular events that result in the differentiation of different kinds of cells from the primary pluripotential fertilized egg.
Japanese flounder (Paralichthys olivaceus) is an economically important flatfish. A great number of studies on cloning and expression of various genes have been carried out throughout the world (Chen et al. 2005; Zhang et al. 2006; Takano et al. 2007). To investigate the differential expressions of those genes in different developmental stages, suitable reference genes are required. However, studies attempting to identify endogenous reference genes suited for early embryonic development focused mainly on rat (Al-Bader and Al-Sarraf 2005), mouse (Jeong et al. 2005), bovine (Goossens et al. 2005; Bettegowda et al., 2006), and Xenopun laevis (Sindelka et al. 2006). Similar research on fish species was recently reported only in zebrafish (Tang et al. 2007), but not yet in marine species. For this reason, in the present study, we selected eight widely used candidate reference genes: β-actin (ACTB), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), ribosomal protein L17 (rpL17), α-tubulin (α-Tub), elongation factor-1-α (EF1-α), ubiquitin-conjugating enzyme (UbcE), β-2-microglobulin (B2M), and 18S ribosomal RNA (18S rRNA), and made use of the 2−ΔCt (Livak and Schmittgen 2001) method to investigate the expression profiles of these genes during the whole embryonic developmental stages to determine the most suitable endogenous reference gene for P. olivaceus embryonic development research.
Materials and Methods
Embryo Preparation
Fertilized eggs of P. olivaceus were obtained by artificial fertilization and incubated at 16 °C in clean sea water with aeration. The embryonic stage for each sample was determined via microscopy. Ten developmental stages were selected: unfertilized egg, 64-cell (5 hpf), late blastula (10 hpf), neurula (23 hpf), eye-bud (27 hpf), tail-bud–forming (33 hpf), tail-bud (40 hpf), heart-beating (60 hpf), hatching (71 hpf) and 2-day-old larva (2 days after hatching/120 hpf) stage. Embryos at different stages were collected with a nylon net (80 mesh) and preserved in liquid nitrogen until used.
Selection of Reference Genes
A total of eight reference genes were selected for gene expression analysis. The full gene name, function, and accession number are provided in Table 1. The primers were designed with Primer Premier 5.0 software (PREMIER Biosoft International, Palo Alto, CA; Table 2). The theoretical optimal annealing temperature of each primer was about 60°C and the amplicons were within the range of 90–130 bp.
RNA Extraction and cDNA Synthesis
Total RNA was extracted from each pool of oocytes/embryos (n = 3 pools of 50 oocytes/embryos per developmental stage) using the Trizol reagent (Invitrogen) according to the manufacturer’s instructions. Fifteen 2-day-old larvae were pooled for RNA extraction. DNA contamination was removed by DNase I treatment, and verified by PCR using 18S rRNA gene specific primers. The quality of total RNA was assessed via 1% ethidium bromide agarose gel electrophoresis and the concentration was determined by UV spectrophotometry at A260. cDNA was generated with M-MLV reverse transcriptase (Promega, Madison, WI) and random hexamers. A total of 2 μg of RNA from different samples was reverse-transcribed in a final volume of 25 μl for 10 min at 25°C, 60 min at 40°C, and 5 min at 95°C. Finally, cDNA was diluted 1:20, and stored at –20°C until used.
Quantitative Real-Time PCR
All reactions were run on a FTC-2000 Real-Time PCR system (Funglyn BioTech, Shanghai, P.R. China). Each amplification was performed in a 15-μl final volume containing 1× SYBR Green Realtime PCR Master Mix (TOYOBO), 0.2 μM each of specific forward and reverse primers, and 4 ng of diluted cDNA for mRNA genes or 4 pg of diluted cDNA for 18S rRNA gene. The reaction conditions were as follows: an initial 1-min activation and denaturation step at 95°C, followed by 45 cycles of 5 s at 95°C, 10 s at 60°C, 20 s at 72°C. Fluorescent detection was performed after each extension step. A dissociation protocol was added after thermocycling to verify the specificity of the target amplification. The PCR efficiency (E%) of each assay was derived from serial dilutions of 40 ng of cDNA from 2-day-old larva. All samples were amplified in triplicate and the means were used for further analyses.
Data Analysis
The cycle threshold (C t) value is the number of PCR cycles at which the amount of amplified target reaches a fixed threshold. When the reaction efficiency approaches 100%, the gene expression can be calculated using the following equation: Fold difference = 2–ΔCt (Livak and Schmittgen, 2001). The expression changes of 7 mRNA genes were presented as 2–ΔCt for their high PCR efficiencies. However, the reaction efficiency of 18S rRNA gene was less than 90%. To reduce the errors, a standard curve of serially diluted plasmid containing 18S rRNA gene fragment was obtained to compute the amount of the template, and the expression variation was shown as ratio between the amount of copies of 18S rRNA at a particular developmental stage and that in unfertilized eggs. The association between different developmental stages and the levels of gene expression was analyzed via one-way ANOVA (analysis of variance). Individual mean comparisons were performed using Duncan’s test. Differences of P < 0.05 were considered significant.
Results
Quality and Quantity of Total RNA
The quality of the RNA is essential to the overall success of the gene expression analysis. Electrophoresis (Fig. 1) and the ratios of OD260nm/OD280nm (1.95–2.06) showed that high quality total RNA was extracted. Total RNA concentration per embryo remained at an approximately constant level (less than 1.5-fold change) before hatching, and then increased that the RNA abundance in 2-day-old larva was about 2.3 times higher than that in unfertilized eggs (Fig. 2).
Q-RT-PCR Efficiency and Intra- and Interassay Variability
When using the 2–ΔCt method, the crucial is the PCR efficiency that should approach 100%. Thus we first determined the PCR efficiency of each gene by measuring serial dilutions of 40 ng of cDNA from 2-day-old larva. The PCR efficiency was calculated according to the equation: PCR efficiency = (10–1/slope-1) × 100. All reactions displayed an efficiency between 95% and 100%, except for 18S rRNA gene, which exhibited an efficiency of 82% (Table 2).
Intraassay variation was <1.19% and interassay variation <0.98% in this study, indicating high experimental reproducibility.
Relative Abundance of 7 mRNA Genes in Different Embryonic Developmental Stages
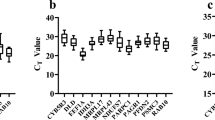
In quantitative real-time PCR, when the reaction efficiency is identical, the C t value can reflect the amount of template input initially in the PCR. The mean C t values of mRNA genes at different developmental stages are shown in Fig. 3. The transcripts of 7 mRNA genes were all detected in unfertilized eggs, reflecting the maternal transcripts stored in mature oocytes. In unfertilized eggs, B2M and rpL17 showed the relative low level of RNA storages with average C t = 27.65 and C t = 27.83, respectively, while others had relative high RNA abundance with average C t = 23.06 for α-Tub, C t = 22.94 for ACTB, C t = 23.46 for EF1-α, C t = 23.45 for GAPDH, and C t = 22.75 for UbcE. With the embryonic development, C t values of all these genes decreased, reflecting the elevation of the transcription levels. However, among all 7 mRNA genes, the B2M gene always displayed the lowest expression level throughout the embryo development, while the EF1-α gene showed the much higher transcription level, especially after late blastula stage.
The relative expression level of mRNA genes, presented as absolute C t value at different developmental stages: unfertilized egg (Unf), 64-cell (64C), late blastula (Lab), neurula (Neu), eye-bud (Eyb), tail-bud–forming (Tbf), tail-bud (Tab), heart-beating (Heb), hatching (Hat), and 2-day-old larva (2-D)
Expression Profiles of Reference Genes During Embryonic Development
The 2–ΔCt method was used to display the expression profile of each mRNA gene. As shown in Fig. 4, the expression of all studied mRNA genes varied significantly during embryonic development (P < 0.001). The mRNA levels of these genes were relatively low before the late blastula stage, and then increased greatly and displayed different temporal expression patterns.
Quantitative analyses of the expression profiles of eight reference genes at unfertilized egg (Unf), 64-cell (64C), late blastula (Lab), neurula (Neu), eye-bud (Eyb), tail-bud–forming (Tbf), tail-bud (Tab), heart-beating (Heb), hatching (Hat), and 2-day-old larva (2-D) stage. The expression of EF1-α (a), rpL17 (b), ACTB (c), α-Tub (d), B2M (e), GAPDH (f), and UbcE (g) were analyzed by 2−ΔCt method. As for 18S rRNA gene, the amounts of the templates were determined from the standard curve of serial dilution plasmid containing 18S rRNA gene fragment and the expression variance is showed as ratio between the number of copies of 18S rRNA at a particular developmental stage and that in unfertilized eggs (h). Data are shown as means ± SEM (n = 3)
Based on the -fold changes relative to unfertilized egg, seven mRNA genes could be divided into three groups: group A showed high variation with a -fold change >100, group B showed moderate variation with a -fold change between 25 and 100, and group C showed low variation with a –fold change <25. EF1-α and rpL17 belonged to group A. The expression levels of these two genes increased sharply after late blastula stage and reached the maximum level, which was about 230 times higher at the eye-bud stage for EF1-α (Fig. 4a) and 300 times higher at the tail-bud stage for rpL17 (Fig. 4b) than that in unfertilized egg, respectively. Then, the expressions of these two genes gradually declined. Group B included ACTB and α-Tub, which had moderate changes of transcription levels. Although both genes code for components of cell skeleton proteins, they showed very different transcription profiles. The ACTB transcripts increased throughout the entire embryonic period examined and reached the highest level at the 2-day larva stage, about 60-fold higher than in unfertilized eggs (Fig. 4c). In comparison, the expression of the α-Tub gene continuously increased, with a maximum at eye-bud stage, about 30-fold, and subsequently remained at an almost constant level (Fig. 4d). Group C included GAPDH, B2M, and UbcE genes, all of which showed less than a 25-fold change during the whole developmental stages examined. The expression profile of B2M had a shape similar to that of α-Tub except for a relative elevation at late blastula stage and a slight decrease at tail-bud stage (Fig. 4e). The expression pattern of GAPDH was similar to that of ACTB at the stages before tail-bud with the increasing mRNA level, and then followed by nearly constant transcription levels until 2-day larva stage (Fig. 4f). The UbcE gene displayed the least variation of all the seven tested mRNA genes (Fig. 4g). Its expression showed only about a 2-fold increase before neurula stage and very small elevation from eye-bud stage to hatching.
Figure 5 shows the variations expressed as -fold changes with box-and-whisker plots for selected reference genes throughout embryonic development. Unlike the mRNA genes described in the preceding text, 18S rRNA gene exhibited the most stable expression, with an average -fold change of <1.5 and a maximal variability of approximately 1.7-fold. Furthermore, individual comparisons of expression variation between different stages confirmed that 18S rRNA gene was regulated minimally during all embryonic developmental stages with no statistically significant difference (P = 0.351; Fig. 4h).
Discussion
Many maternal products in teleost eggs are produced endogenously by the oocyte itself during oogenesis, and are crucial to the early embryonic developments including fertilization, egg activation, the first cell division, the initiation of zygotic transcription, and so forth (Pelegri 2003). Here different levels of transcripts of all seven mRNA genes were detected in unfertilized eggs, indicating the maternal RNA stored in mature eggs. This was not surprising when considering the “housekeeping” functions of these genes.
The transcription levels of all these mRNA genes elevated significantly after the late blastula stage, reflecting the zygotic genes activation and the developmental regulation. This observation was consistent with the result obtained in zebrafish in which the accumulation of most newly synthesized zygotic transcripts was first detected at midblastula transition (Kane and Kimmel 1993). The transcription of the EF1-α gene during early embryogenesis of X. laevis commenced at mid-blastula transition, which was considered as an activation switch of the transcription of the embryonic EF1-α gene (Krieg et al. 1989; Sindelka et al. 2006). A similar result was also obtained in P. olivaceus, although the late blastula stage was used in this study, suggesting that such activation switch may also exist in embryogenesis of P. olivaceus. The expression of the rpL17 gene in P. olivaceus also increased sharply at the late blastula stage. This profile was similar to the results obtained in early development of X. laevis, in which the transcription of ribosome protein genes started at gastrulation and accumulated progressively afterwards (Pierandrei-Amaldi et al. 1982) and reflected the zygotic activation of ribosome protein genes.
The expression stability of the endogenous reference genes is crucial for normalization in quantitative real-time PCR analysis. Recently more and more studies have revealed that ACTB and GAPDH genes were not suitable as reference genes for their obvious expression variations in many conditions (Schmittgen and Zakrajsek 2000; Glare et al. 2002; Olsvik et al. 2005; Zhang and Hu 2007). However, as a traditional idea, these two genes were still used as endogenous reference genes in gene-expression analyses during the embryogenesis of fish species without any validation. Our results showed that both genes had considerable expression variations during embryonic development and were inappropriate as references in analyzing embryonic gene expression of Japanese flounder. This was especially obvious for ACTB, the expression of which kept increasing throughout the whole embryonic stages and reached about 45 times higher at hatching than in unfertilized egg. Although significant expression variations of these genes made them unsuitable as reference genes throughout embryonic development, the stage-specific expression stability existed. The transcription levels of α-Tub and rpL17 genes both displayed no significant change after the eye-bud stage. The GAPDH appeared to be suitable as a reference after the tail-bud stage and the B2M gene could be used as a normalizer after neurula stage. As for UbcE, two relative stable ranges existed: one was from unfertilized egg to neurula stage and the other was from eye-bud stage to hatching. Thus, considering the differential and stage-related expression, different developmental periods should be strictly confined when these mRNA genes are used for normalization.
18S rRNA, which composed the large amount of the total RNA, had often been used as an internal standard for its stability in many studies (Schmittgen and Zakrajsek 2000; Goidin et al. 2001). Recently, however, the rationality of 18S rRNA being used for normalization was questioned because of the conception that the transcription of 18S rRNA was carried out via a different RNA polymerase comparing with that of mRNA and the expression of 18S rRNA was shown to be regulated in some experimental situations (Solanas et al. 2001; Raaijmakers et al. 2002). Our results showed that 18S rRNA exhibited a very different expression pattern in comparison with those of mRNA genes discussed in the preceding text, displaying the highest stability with no more than a 2-fold change during the whole developmental stages. This profile strongly suggested that 18S rRNA could be suitable as a reference throughout embryonic development. Moreover, some researchers still recommended 18S rRNA as an internal standard for mRNA quantification study because mRNA variations were thought weak in comparison and cannot highly modify the total RNA level (Thellin et al. 1999).
The geNorm program was a recently developed mathematical model and widely used to determine the most stable reference genes from a set of tested genes in a given cDNA sample panel (Vandesompele et al. 2002). However, there were two reasons that would restrict the application of this program in embryonic development. The first was that the geNorm program provided only the ranking of the selected housekeeping genes, but not the detailed expression pattern of each gene during the embryogenesis. However, more information about expression would facilitate selection of suitable reference genes at different embryonic stages. The second, also critical reason, was that the geNorm program relied on the principle that the expression ratio of two ideal internal control genes was identical in all samples (Vandesompele et al. 2002), but did not consider the high variation of gene expression between the early developmental stages and the later developmental stages. This would result in an invalid analysis. As shown in Fig. 6, α-Tub and B2M were identified as the most stable reference genes throughout embryogenesis with geNorm analysis. This was obviously different from the result using the 2–ΔCt method and statistical analysis (one-way ANOVA), which showed that all mRNA genes varied significantly (P < 0.001) and unsuitable to be used as reference genes during embryonic development. The reason for this different result was that the geNorm program compared only the expression ratio of different genes, but did not consider the high expression variation of each gene in different developmental stages. For example, although α-Tub and B2M genes exhibited high expression variation during embryogenesis, the expression profiles of these two genes was much more similar than that of any other two genes. So, according the principle of geNorm analysis, these two genes were identified as the most stable reference genes. Similarly, the 18S rRNA gene ranked behind the rpL17 and EF1-α genes because of its very different expression pattern in comparison with those of mRNA genes, although statistical analysis showed that 18S rRNA gene was regulated minimally throughout the embryonic developmental stages with no statistically significant difference (P = 0.351). Therefore, although the geNorm analysis was widely used in many assays, it was not appropriate to be used in embryogenesis research and the 2–ΔCt method might be better.
In addition to normalizing to reference genes, the relative quantification method used in real-time PCR also employ total RNA content to normalize target gene expression as done in traditional Northern blot and/or sometimes in microarray data analysis. In P. olivaceus, total RNA concentration per embryo kept a relatively stable level throughout embryogenesis. This constant RNA abundance made normalization against total RNA a reliable choice in mRNA quantification. However, it should be realized that normalizing to total RNA cannot control for errors introduced at the reverse transcription or PCR steps (Huggett et al. 2005). Therefore, if possible, the verified suitable reference gene would be a preferable choice.
In conclusion, our data clearly demonstrated that 18S rRNA gene was minimally regulated during P. olivaceus embryogenesis and could be used as an appropriate reference gene for mRNA differential expression study in development. However, when the mRNA genes were used as references, the differential and stage-related expression should be well considered. These results also can be used as guidance to reference gene selection for embryonic gene expression analyses in other fish species.
References
Al-Bader MD, Al-Sarraf HA (2005) Housekeeping gene expression during fetal brain development in the rat-validation by semi-quantitative RT-PCR. Brain Res Dev Brain Res 156:38–45
Bettegowda A, Patel OV, Ireland JJ, Smith GW (2006) Quantitative analysis of messenger RNA abundance for ribosomal protein L-15, cyclophilin-A, phosphoglycerokinase, beta-glucuronidase, glyceraldehyde 3-phosphate dehydrogenase, beta-actin, and histone H2A during bovine oocyte maturation and early embryogenesis in vitro. Mol Reprod Dev 73:267–278
Chen YD, Zhang YB, Zhu R, Zhang FT, Jiang J, Shi Y, Zhang QY, Chen SL, Gui JF (2005) Inductive expression and characterization analysis of Paralichthys olivaceus pigment epithelium-derived factor in a virally infected cell line. Biochem Biophys Res Commun 335:799–809
de Kok JB, Roelofs RW, Giesendorf BA, Pennings JL, Waas ET, Feuth T, Swinkels DW, Span PN (2005) Normalization of gene expression measurements in tumor tissues: comparison of 13 endogenous control genes. Lab Invest 85:154–159
Dheda K, Huggett JF, Bustin SA, Johnson MA, Rook G, Zumla A (2004) Validation of housekeeping genes for normalizing RNA expression in real-time PCR. Biotechniques 37:112–119
Drasdo D, Forgacs G (2000) Modeling the interplay of generic and genetic mechanisms in cleavage, blastulation, and gastrulation. Dev Dyn 219:182–191
Glare EM, Divjak M, Bailey MJ, Walters EH (2002) beta-Actin and GAPDH housekeeping gene expression in asthmatic airways is variable and not suitable for normalising mRNA levels. Thorax 57:765–770
Goidin D, Mamessier A, Staquet MJ, Schmitt D, Berthier-Vergnes O (2001) Ribosomal 18S RNA prevails over glyceraldehyde-3-phosphate dehydrogenase and beta-actin genes as internal standard for quantitative comparison of mRNA levels in invasive and noninvasive human melanoma cell subpopulations. Anal Biochem 295:17–21
Goossens K, Van Poucke M, Van Soom A, Vandesompele J, Van Zeveren A, Peelman LJ (2005) Selection of reference genes for quantitative real-time PCR in bovine preimplantation embryos. BMC Dev Biol 5:27
Huggett J, Dheda K, Bustin S, Zumla A (2005) Real-time RT-PCR normalisation; strategies and considerations. Genes Immun 6:279–284
Ingerslev HC, Pettersen EF, Jakobsen RA, Petersen CB, Wergeland HI (2006) Expression profiling and validation of reference gene candidates in immune relevant tissues and cells from Atlantic salmon (Salmo salar L). Mol Immunol 43:1194–1201
Jeong YJ, Choi HW, Shin HS, Cui XS, Kim NH, Gerton GL, Jun JH (2005) Optimization of real time RT-PCR methods for the analysis of gene expression in mouse eggs and preimplantation embryos. Mol Reprod Dev 71:284–289
Jorgensen SM, Kleveland EJ, Grimholt U, Gjoen T (2006) Validation of reference genes for real-time polymerase chain reaction studies in Atlantic salmon. Mar Biotechnol 8:398–408
Kane DA, Kimmel CB (1993) The zebrafish midblastula transition. Development 119:447–456
Krieg PA, Varnum SM, Wormington WM, Melton DA (1989) The mRNA encoding elongation factor 1-alpha (EF-1 alpha) is a major transcript at the midblastula transition in Xenopus. Dev Biol 133:93–100
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 25:402–408
Olsvik PA, Lie KK, Jordal AE, Nilsen TO, Hordvik I (2005) Evaluation of potential reference genes in real-time RT-PCR studies of Atlantic salmon. BMC Mol Biol 6:21
Pelegri F (2003) Maternal factors in zebrafish development. Dev Dyn 228:535–554
Pierandrei-Amaldi P, Campioni N, Beccari E, Bozzoni I, Amaldi F (1982) Expression of ribosomal-protein genes in Xenopus laevis development. Cell 30:163–171
Raaijmakers MH, van Emst L, de Witte T, Mensink E, Raymakers RA (2002) Quantitative assessment of gene expression in highly purified hematopoietic cells using real-time reverse transcriptase polymerase chain reaction. Exp Hematol 30:481–487
Radonic A, Thulke S, Mackay IM, Landt O, Siegert W, Nitsche A (2004) Guideline to reference gene selection for quantitative real-time PCR. Biochem Biophys Res Commun 313:856–862
Schmittgen TD, Zakrajsek BA (2000) Effect of experimental treatment on housekeeping gene expression: validation by real-time, quantitative RT-PCR. J Biochem Biophys Methods 46:69–81
Sindelka R, Ferjentsik Z, Jonak J (2006) Developmental expression profiles of Xenopus laevis reference genes. Dev Dyn 235:754–758
Solanas M, Moral R, Escrich E (2001) Unsuitability of using ribosomal RNA as loading control for Northern blot analyses related to the imbalance between messenger and ribosomal RNA content in rat mammary tumors. Anal Biochem 288:99–102
Suzuki T, Higgins PJ, Crawford DR (2000) Control selection for RNA quantitation. Biotechniques 29:332–337
Takano T, Kondo H, Hirono I, Endo M, Saito-Taki T, Aoki T (2007) Molecular cloning and characterization of Toll-like receptor 9 in Japanese flounder, Paralichthys olivaceus. Mol Immunol 44:1845–1853
Tang R, Dodd A, Lai D, McNabb WC, Love DR (2007) Validation of zebrafish (Danio rerio) reference genes for quantitative real-time RT-PCR normalization. Acta Biochim Biophys Sin (Shanghai) 39:384–390
Thellin O, Zorzi W, Lakaye B, De Borman B, Coumans B, Hennen G, Grisar T, Igout A, Heinen E (1999) Housekeeping genes as internal standards: use and limits. J Biotechnol 75:291–295
Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3:RESEARCH0034
Zhang Y, Tan X, Zhang PJ, Xu Y (2006) Characterization of muscle-regulatory gene, MyoD, from flounder (Paralichthys olivaceus) and analysis of its expression patterns during embryogenesis. Mar Biotechnol 8:139–148
Zhang Z, Hu J (2007) Development and validation of endogenous reference genes for expression profiling of medaka (Oryzias latipes) exposed to endocrine disrupting chemicals by quantitative real-time RT-PCR. Toxicol Sci 95:356–368
Acknowledgments
We thank Dr. Yuanmin Wu, from Funglyn BioTech Co., Ltd., for technical assistance. This work was supported by grants from the National High Technology Research and Development Program of China (No. 2006AA10A404) and the National Natural Science Foundation of China (No. 30671624).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhong, Q., Zhang, Q., Wang, Z. et al. Expression Profiling and Validation of Potential Reference Genes During Paralichthys olivaceus Embryogenesis. Mar Biotechnol 10, 310–318 (2008). https://doi.org/10.1007/s10126-007-9064-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10126-007-9064-7