Abstract
Genetic variations within and between nine hatchery stocks and seven natural populations of abalone including Ezo-abalone (Haliotis discus hannai) and Kuro-abalone (H. d. discus) were assayed with nine microsatellite markers. Marked reductions of genetic variability in the hatchery stocks were recognized in the allelic diversity and mean heterozygosity compared with the natural populations. Thirteen of 16 significant HWE deviations in hatchery stocks revealed heterozygotes excess, while all natural populations did not show such a tendency. Highly significant F ST values were observed for all cases between the hatchery stocks, and between the hatchery stocks and natural populations. Genetic distance (D A) between each hatchery stock and the geographically proximal population (mean ± SD, 0.108 ± 0.035) were similar to those estimated for between the natural Ezo-abalone and Kuro-abalone (0.101 ± 0.021). The self-assignment test, which allocated individuals to their own stock with a high success rate, provided evidence of solid genetic differences among the nine hatchery stocks. These results suggests that the allelic composition and diversity in the natural populations was not necessarily reflected in the hatchery stocks owing to population bottleneck and genetic drift through seedling process, and thus the seedling and stocking practice of these hatchery stocks should take much notice of the results to conserve the genetic diversity of natural populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Production of many important fishery species has been decreasing as a result of destruction of habitats suitable for spawning and nursing environment, and over-fishing. To compensate for the reduced fishery resources, enhancement practices have been intensively continued to release hatchery stock into natural coastal areas. A reduced genetic diversity was observed in most hatchery stocks and has been related to a loss of adaptations for new environments (Allendorf and Ryman, 1987), and thus genetic monitoring for hatchery stocks and natural populations is recommended to preserve genetic variations in natural populations (FAO, 1993).
The Pacific abalone Haliotis discus species, including a cold-current type called Ezo-abalone and a warm-current type called Kuro-abalone, is widely distributed in coastal areas of East Asia (Ino, 1952) and is the most important abalone species for commercial fisheries resources owing to its high market value. Recent annual landing of this species has been reduced to less than the half volume (2004-2200 tons, Ministry of Agriculture, Forestry, and Fisheries, Japan, 2005) compared to the maximum catch level (1970-6500 tons). This has led to an increase in hatchery abalone production from the 1980s, and a large amount of hatchery abalone has been stocked intensively in coastal areas across Japan every year. In this situation, the genetic impact of stocked hatchery abalone on the natural resources is a growing concern for a sustainable fishery. Genetic variability of hatchery stocks in Pacific abalone has been researched using allozyme and microsatellite markers. Allozyme analysis allowed differentiation of allelic diversity between hatchery stocks and natural populations, but failed to estimate genetic compositions of hatchery stocks owing to the limited number of available polymorphic loci (Kijima et al., 1992). Six microsatellite markers could detect sensitively the reductions of genetic variability on allelic diversity and mean heterozygosity (Li et al., 2004), leaving the possibility that the use of additional loci would provide a more precise estimation of genetic relatedness between hatchery stocks. This study was conducted to assay the genetic diversity within and between hatchery stocks and natural populations of Pacific abalone both at population and individual levels based on nine microsatellite DNA markers.
Materials and Methods
Abalone Population Samples
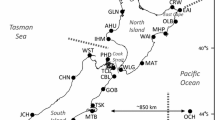
Pacific abalone (Ezo- and Kuro-abalone) samples surveyed in this study were collected from the geographical sites shown in Figure 1. Sampling locality with an abbreviated population name, date of sampling, and sample size of each population and founding records are shown in Table 1. The natural Ezo-abalone populations were derived from Iwanai in Hokkaido (NEA), Omoe (NEB), and Hirota (NEC) in Iwate Prefecture ,and Kinkasan Island in Miyagi Pref. (NED). The natural Kuro-abalone populations were collected from Ijika in Mie Pref. (NKE), Mikuni in Fukui Pref. (NKF) and Ryotsu in Niigata Pref. (NKG). All hatchery stocks (nine stocks) screened in this study were created using natural captives sampled from coastal areas geographically proximal to the sampling sites of the natural populations (Figure 1). The HEA and HEE stocks were founded using parents maintained in the hatchery over several generations, and the HEC was both natural caught parents and brood-stock reproduced in the hatchery. The other stocks (HEB, HED, HEF, HKG, HKH, and HKI) were composed of first-generation offspring. Unfortunately, there are no detailed records for all the stocks regarding how many number of parents actually contributed to the production of offspring.
Microsatellite Analyses
A DNA sample of each individual was extracted from a small part of shell foot muscles using the standard phenol/chloroform procedure (Sambrook et al., 1989). Nine microsatellite loci (Hd527–GenBank accession nos. AB178064; Hd535–AB178065; Hd553–AB178066; Hd601–AB178069; Hd604–AB178070; Hd680–AB178073; Hd715–AB178074; Hd731–AB178077; and Ahdh1147–AB17083) were amplified using previously reported primer pairs (Hara and Sekino 2005; Sekino and Hara, submitted). Polymerase chain reaction to amplify each microsatellite locus, and allele detection and designations were performed in the same manner as described in Hara and Sekino (2005).
Statistical Analysis
Genetic variability statistics within a population were calculated, which involved the number of alleles detected at each microsatellite locus (A); allelic richness (R s), which is an estimate of the number of alleles at each locus in each population independent of the sample size (El-Mousadik and Petit, 1996); and expected heterozygosity (H E), using the FSTAT version 2.9.3 (Goudet, 1995) and the CERVUS version 2.0 software package (Marshall et al., 1998). Test for Hardy-Weinberg’s equilibrium (HWE) at each locus was carried out based on a test analogous to Fisher’s exact test with the Markov-chain method (Markov-chain length, 100,000; dememorization, 10,000) using the ARLEQUIN version 3.0 package (Excoffier et al., 2005). Significance levels for all multiple tests were corrected following Rice (1989).
A multilocus estimate of genetic population heterogeneity for each pair of populations, F ST value (Weir and Cockerham, 1984), was estimated, and the significance of pairwise F ST values was evaluated through 10,000 permutation procedures. We also addressed the genetic relationship of population samples according to a genetic distance measure (modified Cavalli-Sforza chord distance: D A, Nei et al., 1983). Unrooted neighbor-joining tree (NJ tree, Saito and Nei, 1987) based on the D A, distance was constructed, and the tree was evaluated calculating bootstrap values through 1000 resamplings across loci on the DISPAN program http://iubio.bio.indiana.edu:7780/perl/custom/index.cgidir=/public/molbio/genetic/pop/Dispan). The trees were visualized using the TREE VIEW program (Page, 1996).
The self-assignment test was performed to examine the potential of the microsatellite markers for discrimination among the hatchery stocks at the individual level without parental genotype information. Each hatchery stock was allocated to the most probable population of origin using the GeneClass2 version 2.0 software (Piry et al., 2004) with the distance-based assignment option, which can be used in broader situation without a HWE agreement and gametic phase equilibrium. We adopted the D A distance to measure relatedness between individuals, and the probability of an individual belonging to a candidate population was calculated (Cornuet et al., 1999). The threshold probability value to reject the possibility that an individual belongs to a population was set at 0.01.
Results
Genetic Variability
Genetic variability indices for the seven natural populations and nine hatchery stocks in Ezo-abalone and Kuro-abalone are summarized in Table 2. The number of alleles per locus (A) and allelic richness per locus (Rs) ranged from 4.5 to 11.3 and from 4.4 to 10.0, respectively. We compared Rs values, rather than A values, between hatchery stocks and natural populations, as it is expected that the A value varies widely depending on the sample size of populations. The average of R s values in the nine hatchery stocks (4.4-8.2) were substantially lower than those in the seven natural populations (9.5-11.3; Kruskal-Wallis test, df = 1, H = 11.1, P < 0.001), and consequently the hatchery stocks showed 36% reduction of the R s values compared with those calculated for the natural populations. The R s value was not significantly different between the Ezo-abalone and Kuro-abalone in both the natural populations (Kruskal-Wallis test, df = 1, H = 0.5, P = 0.48) and hatchery stocks (df = 1, H = 3.2, P = 0.07). A comparison of mean H E values revealed that the values of the hatchery stocks (H E: 0.497-0.648) were significantly lower than the values of the natural populations (H E: 0.592-0.655; Kruskal-Wallis test, df = 1, H = 5.7, P = 0.02). As well as the case of the R S, there was no significance regarding the difference of mean H E values between the Ezo-abalone and Kuro-abalone in the natural populations (Kruskal-Wallis test, df = 1, H = 0.0, P = 0.96) and in the hatchery stocks (df = 1, H = 1.1, P = 0.30).
HWE testing revealed that 37 of 144 locus-population combinations were discordant with HWE at the uncorrected significance level (P < 0.05), and 17 combinations showed significant deviations from HWE after the Bonferroni correction (nine simultaneous tests, P < 0.006). Almost all the deviations were observed in the hatchery stocks except one instance (IWH, natural population) and 13 of 16 cases indicated heterozygote excess.
Genetic Relationships of Population Samples
Multilocus estimates of genetic population heterogeneity, F ST, were calculated to assess the genetic difference between 16 abalone popula tions (Table 3). Combinations of the 16 populations consistently yielded highly significant F ST values (0.001–0.259) for almost all cases (P < 0.0001) except for three combinations of natural populations (NEA-NEC, NEB-NED, and NKF-NKG), between which the geographical distances were relatively small. The D A genetic distance was calculated for all possible pairs of the 16 samples (Table 3). The D A values between the hatchery stocks (D A = 0.116–0.319) were obviously larger than those between the natural populations (D A = 0.021–0.148, Kruskal-Wallis test, df = 1, H = 33.1, P < 0.001). The D A between each hatchery stock and the geographically proximal natural population (D A, 0.066–0.155; mean ± SD, 0.108 ± 0.035) showed smaller distances than that between the hatchery stocks (df = 1, H = 15.1, P < 0.001), but showed larger distances than that between the natural populations (df = 1, H = 3.9, P = 0.049). The NJ tree constructed on the basis of the D A distances failed to allocate the 16 samples into any clear cluster. Almost all hatchery stocks were located at the tip of the tree with long branch lengths, and the natural populations were relatively congregated in the central part of the tree (Figure 2). This tree topology is consistent with a higher level of genetic divergence among the hatchery stocks compared to that among natural populations.
The results of individual assignment test also evidenced the high genetic divergence among the hatchery stocks (Table 4). This distance-based assignment method achieved a high success rate of individual assignment in the all hatchery stocks as 86% to 100% of individuals of each stock were correctly assigned to their origin. Also, relatively low proportions (3% to 12%) were rejected from its own stocks at the significant level (P < 0.01), furthermore 27 of all (4.9%) were rejected from the all hatchery stocks. These suggest the efficiency of the nine markers for discrimination among hatchery stocks.
Discussion
Substantial loss of allelic diversity was observed in all hatchery stocks compared to the natural populations, while little difference was found between Ezo- and Kuro-abalone hatchery stocks. Reduced allelic variabilities of microsatellites in hatchery-produced animals were reported in many species including the Pacific abalone (Coughlan et al., 1998, Was and Wenne, 2002; Li et al., 2004), which are attributable to population bottlenecks due to the small number of effective parents in most cases (Norris et al., 1999; Sekino et al., 2002). This is the case of hatchery stocks screened in this study, as six of the nine hatchery stocks were founded by less than 10 or15 candidate parents, although it is not possible to estimate the number of effective males and females. Consistent with the limited number of founders, the estimates of overall expected heterozygosity (H E) in hatchery stocks were lower than those in natural populations. However, our results are inconsistent with previous findings in an allozyme survey, in which the allelic diversity of Ezo-abalone hatchery stocks was significantly decreased with little reduction of heterozygosity (Kijima et al., 1992). This incongruence between the allozyme and microsatellite analysis is simply ascribable to the sensibility of microsatellites in detecting reductions of genetic diversity (Li et al., 2004). The HEA and HEE stocks showed the lowest level of genetic variations in terms of both mean heterozygosity and R S value. Because the two were created by founders maintained over four generations in the hatcheries, inbreeding as well as population bottlenecks would have precipitated the decay of genetic diversity in these stocks.
Significant deviations from HWE were observed in hatchery stocks but not in almost all the natural populations except for HEE stock. Thirteen of the 16 significant HWE deviations in hatchery stocks revealed heterozygote excess, and Li et al. (2004) showed, based on microsatellite analysis, that heterozygote excesses were observed in 8 of 10 significant HWE deviations in Ezo-abalone hatchery stocks. Population bottlenecks sometimes give rise to a reduction of homozygotes when the population of interest experienced a recent reduction in size, owing to sampling bias of alleles resulted from a small number of parents and differences in allele frequencies between the sexes (Spencer et al., 2000; Launey et al., 2001 and references therein). Alternatively, it is possible to ascribe a heterozygote excess in cultured stocks to overdominance phenomenon, which causes a lower survival of homozygotes (Sugita and Fujio, 1982; Fujio et al., 1985). This study was not designed to determine the cause of the heterozygote excesses; controlled crossbreeding experiments may uncover an underlying overdominance in Ezo-abalone (e.g., Launey and Hedgecock, 2001).
The high genetic distances (F ST and D A) estimated for between the hatchery stocks provided evidence of substantial genetic divergence of the hatchery stocks, consistent with a population bottleneck and the subsequent genetic drift. The high rate of assignment success for individuals from the hatchery stocks (86% to 100%) is also accounted for by the high genetic differentiation among the hatchery stocks because the success rate of individual assignment test depends largely on the magnitude of genetic differences among populations, from which individuals to be tested originate (Cornuet et al., 1999). These could be the result that the hatchery stocks are characterized with not only low genetic diversity but also heterogeneous allelic composition. It should be noted that based on the D A distance the extent of genetic differentiation between each hatchery stock and its geographically proximal natural population (i.e., the population closely related to the source population of each stock) is similar to those observed between natural Ezo-abalone (cold-current type) and Kuro-abalone (warm-current type) populations (D A, 0.083–0.148; mean ± SD, 0.101 ± 0.021). Nonetheless, there remains taxonomic uncertainty between Ezo- and Kuro-abalone; it is expected that they adapt biologically and genetically to the environmental condition of each habitat (Ino, 1952) and should be delineated genetically as separate management units (Hara and Sekino, 2005). If such hatchery stocks with genetic characteristics highly divergent from that of natural populations achieve a high reproductive success after the release, the stocking practices would be at risk of having a great genetic impact on natural abalone resources. Thus, it is necessary to find an allowable range of amount of release within which the alteration of genetic makeup in natural populations is minimized and the natural biomass is maximized. Answering this question requires the knowledge of reproductive contribution of released hatchery-produced abalone to the natural resources. The top priority of our future study should therefore be given to estimate the extent of reproductive success of released abalone under the natural environment, which can be addressed by collecting microsatellite genotype information of ensuing generations.
References
Allendorf FW, Ryman N (1987) “Genetic management of hatchery stocks.” In: Population Genetics and Fishery Management, Washington Sea Grant Program, Ryman N, Utter F, eds. (Seattle: University of Washington Press) pp 141–159
JM Cornuet S Piry G Luikart A Estoup A Solignac (1999) ArticleTitleNew methods employing multilocus genotypes to select or exclude populations as origins of individuals Genetics 153 1989–2000
JP Coughlan AK Imsland PT Galvin RD Fitzgerald G Naevdal TF Cross (1998) ArticleTitleMicrosarellite DNA variation in wild populations and farmed stocks of turbot from Ireland and Norway: a preliminary study J Fish Biol 52 916–922 Occurrence Handle10.1111/j.1095-8649.1998.tb00592.x
EI-Mousadik A, Petit RJ (1996) High level of genetic differentiation for allelic richness among populations of the argan tree (Argania spinosa (L.) Skeels) endemic to Morocco. Theor Appl Genet 92, 832–839
Excoffier L, Laval G, Schneider S (2005) ARLEQUIN ver. 3.0: An integrated software package for population genetics data analysis. Evolutionary Bioinformatics, Genetics and Biometry Laboratory, University of Geneva, Switzerland.
FAO (1993) Report of the expert consultation on utilization and conservation of aquatic genetic resources. FAO Fisheries Report No.491, Rome
Y Fujio N Sasaki M Sasaki A Koganezawa (1985) ArticleTitleGenetic aspect of natural and released population of plaice Bull Tohoku Reg Fish Res Lab 47 51–57
J Goudet (1995) ArticleTitleFSTAT (version 1.2): a computer program to calculate F-statistics J Heredity 86 485–486
M Hara M Sekino (2005) ArticleTitleGenetic difference between Ezo-awabi Haliotis discus hannai and Kuro-awabi H. discus discus populations: microsatellite-based population analysis in Japanese abalone Fish Sci 71 754–766 Occurrence Handle10.1111/j.1444-2906.2005.01025.x
T Ino (1952) ArticleTitleBiological study on the propagation of Japanese abalone (genus Haliotis) Bull Tokai Reg Fish Res Lab 5 1–102
A Kijima M Ikeda Y Fujio (1992) ArticleTitleGenetic characteristic of the artificial seed populations of abalone Fish Genet Breed Sci 18 53–63
S Launey D Hedgecock (2001) ArticleTitleHigh genetic load in the Pacific oyster Crassostrea gigas Genetics 159 255–265
S Launey M Barre A Gerard Y Naciri-graven (2001) ArticleTitlePopulation bottleneck and effective size in Bonamia ostreae-resistant populations of Ostrea edulis as inferred by microsatellite markers Genet Res Camb 78 259–270
Q Li C Park T Endo A Kijima (2004) ArticleTitleLoss of genetic variation at microsatellite loci in hatchery stocks of the Pacific abalone (Haliotis discus) Aquaculture 235 207–222 Occurrence Handle10.1016/j.aquaculture.2003.12.018
TC Marshall J Slate LEB Kruuk JM Pemberton (1998) ArticleTitleStatistical confidence for likelihood-based paternity inference in natural populations Mol Ecol 7 639–655 Occurrence Handle10.1046/j.1365-294x.1998.00374.x
Ministry of Agriculture, Forestry, and Fisheries, Japan (2005) Annual statistics report of fishery and aquaculture landings in Japan, Tokyo, p 272 (in Japanese)
M Nei F Tajima Y Tateno (1983) ArticleTitleAccuracy of estimated phylogenetic tree from molecular data, II: gene frequency data J Mol Evol 19 153–170 Occurrence Handle10.1007/BF02300753
AT Norris DG Bradley (1999) ArticleTitleMicrosatellite genetic variation between and within farmed and wild Atlantic salmon (Salmo salar) populations Aquaculture 180 247–264 Occurrence Handle10.1016/S0044-8486(99)00212-4
InstitutionalAuthorNamePage RDM (1996) ArticleTitleTreeview: an application to display phylogenetic tree on personal computers Comp Appl Bio Sci 12 357–358
S Piry A Alapetite JM Cornuet D Paetkau L Baudouin A Estoup (2004) ArticleTitleGeneClass2 : a software for genetic assignment and first generation migrations detection J Heredity 95 536–539 Occurrence Handle10.1093/jhered/esh074
WR Rice (1989) ArticleTitleAnalyzing table of statistical tests Evolution 43 223–225 Occurrence Handle10.2307/2409177
N Saito M Nei (1987) ArticleTitleThe neighbor-joining method: a new method for reconstructing phylogenetic tree Mol Biol Evol 4 406–425
Sambrook J, Fritsch EF, Manitaris T (1989) Molecular Cloning: A Laboratory Manual, 2nd ed (Plainview: Cold Spring Harbor Laboratory Press)
Sekino M, Hara M, Taniguchi N (2002) Loss of microsatellite and mitochondrial DNA variation in hatchery strains of Japanese flounder Paralichthys olivaceus. Aquaculture 213, 101–122
CC Spencer JE Neigel PL Leberg (2000) ArticleTitleExperimental evalution of the usefulness of microsatellite DNA for detecting demographic bottlenecks Mol Eco 9 1517–1528 Occurrence Handle10.1046/j.1365-294x.2000.01031.x
M Sugita Y Fujio (1982) ArticleTitleEffect of genotypes at the Aat-1 locus on the survival and growth rates in the cultured Oyster Tohoku J Agri Res 33 42–49
A Was R Wenne (2002) ArticleTitleGenetic differentiation in hatchery and wild sea trout (Salmo trutta) in the southern Baltic at microsatellite loci Aquaculture 204 493–506 Occurrence Handle10.1016/S0044-8486(01)00835-3
BS Weir CC Cockerham (1984) ArticleTitleEstimating F-statistics for the analysis of population structure Evolution 38 1358–1370 Occurrence Handle10.2307/2408641
Acknowledgments
We thank Mr. S. Kikuch, Dr. H. Hoshikawa, Dr. R. Sasaki, M. Yoshida, and N. Tanaka for providing the abalone samples and identification of abalone based on morphology. This work was supported by research project funds from Fisheries Research Agency of Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hara, M., Sekino, M. Genetic Differences Between Hatchery Stocks and Natural Populations in Pacific Abalone (Haliotis discus) Estimated Using Microsatellite DNA Markers. Mar Biotechnol 9, 74–81 (2007). https://doi.org/10.1007/s10126-006-6060-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10126-006-6060-2