Abstract
The purpose of the present study was to ascertain the tissue-specific expression of the water channel protein, aquaporin 3 (AQP3), during salinity acclimation and larval development of silver sea bream (Sparus sarba). A cDNA fragment encoding aquaporin 3 (aqp3) from silver sea bream gill was cloned and from the deduced amino acid sequence a polyclonal antibody was prepared. AQP3 was found to be present in gill, kidney, liver, brain, heart, and spleen but not in whole blood. The abundance of AQP3 was significantly highest in gills of hypoosmotic (6 ppt) and isoosmotic (12 ppt) acclimated sea bream when compared to seawater (33 ppt) and hypersaline (50 ppt)- acclimated sea bream. Spleen tissue also displayed significantly high levels of AQP3 protein in hypoosmotic and isoosmotic salinities whereas the AQP3 abundance in brain, liver, heart, and kidney remained unchanged across the range of salinities tested. The ontogenetic profile of AQP3 was also investigated from developing sea bream larvae and AQP3 was first detected at 14 days posthatch (dph) and increased steadily up to 28–46 dph. In conclusion, this study has demonstrated that AQP3 expression is modulated in gill and spleen tissue of salinity acclimated sea bream and that it can be detected relatively early during larval development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The aquaporin family of water channels are small, hydrophobic, intrinsic membrane proteins that have been implicated to play key roles in a number of physiological processes in animals, plants and bacteria (Borgnia et al., 1999). Most studies on aquaporins have been performed on mammals, and two subgroups are apparent- “aquaporins” that are highly selective for the passage of water (e.g., AQPs 1, 2, 4, 5, 6, and 8) and “aquaglyceroporins” that can transport water, glycerol, and other solutes such as urea (e.g., AQPs 3, 7, and 9) (Verkman and Mitra, 2000). Teleost fish are very informative models in which to study aquaporin expression and regulation because they continuously encounter challenges for maintaining both osmotic and ionic potential as the salinity of the aquatic environment changes through geographical position and seasonal fluctuations. Over recent years, several aquaporins have been cloned and identified from teleosts including a novel aquaglyceroporin (sbAQP) from gilthead sea bream (Santos et al., 2004), an AQP0 from the lens of killifish (Virkki et al., 2001), an AQP1 from the intestine of Japanese eel (Aoki et al., 2003), three AQP1 homologs from European eel (Martinez et al., 2005), and an AQP3 from gills of European eel (Cutler and Cramb, 2002), Mozambique tilapia (Watanabe et al., 2005), and Japanese dace (Hirata et al., 2003). To date, a large proportion of aquaporin studies, in teleosts, have focussed on AQP3 and immunohistochemical studies have localized this aquaporin, basolaterally, in gill chloride cells (Lignot et al., 2002; Watanabe et al., 2005), suggesting a key role for osmotic water flux and cell volume regulation. In addition to the aforementioned studies, a recent study using gilthead sea bream eggs has demonstrated that an AQP1-like water channel (SaAQP1o) is involved in fish oocyte hydration (Fabra et al., 2005, 2006), implying a key role for water transport processes during marine egg floatation. However, studies concerning aquaporin regulation and expression during posthatch development of fish have yet to be reported.
In the present study the tissue distribution of AQP3 in a marine fish, silver sea bream (Sparus sarba), is investigated. Tissue-specific expression profiles of AQP3 in sea bream acclimated to salinities ranging from hypoosmotic to isoosmotic to seawater and hypersaline conditions are presented. In addition, we also demonstrate the ontogenetic expression profile of AQP3 in larval sea bream from 1 to 46 days posthatch (dph). The data presented add to the accumulating studies on the role and importance of aquaporin for water transport in fish.
Materials and Methods
Experimental Fish and Holding Conditions
Adult silver sea bream (Sparus sarba) weighing between 200 and 300 g were purchased from a local fish farm and transferred to 1000-l seawater tanks. The water in the tanks was at a temperature of 24–25°C and kept fully aerated. Fish were fed ad libitum once daily with a formulated diet (Woo and Kelly, 1995) and were allowed to acclimate to experimental conditions for 3 weeks before salinity acclimation.
Salinity Acclimation
Fish were acclimated to salinities of 6 ppt (hypoosmotic), 12 ppt (isoosmotic), 33 ppt (seawater), and 50 ppt (hypersaline). Fish were initially maintained in four separate 1000-l seawater (33 ppt) tanks (n = 5–7 per group). Salinities of 6 ppt and 12 ppt were achieved via gradual flushing of seawater tanks with dechlorinated tap water over a period of 1 week. Hypersaline water was obtained by evaporating seawater to a salinity of 50 ppt followed by gradual flushing of seawater with hypersaline water over a period of 1 week, until the final salinity of 50 ppt was obtained. Fish were acclimated to the above salinities for 1 month and feeding was terminated 24 h before they were euthanized.
Larval Culture and Collection
Sexually mature silver sea bream (Sparus sarba) of both sexes (males: 200–250 g, females: 350–400 g) were obtained from a captive broodstock. Female silver sea bream were induced to spawn by administration of human chorionic gonadotropin (0.1 μg/g) daily, for 3 days, and eggs were stripped from females, transferred to a container containing a small amount of seawater (33 ppt), and fertilized with sperm from male silver sea bream. After 30 min all eggs were transferred to a glass cylinder and fertilized eggs were carefully removed from the surface of the liquid, leaving behind unfertilized eggs that had settled at the bottom. The fertilized eggs were transferred to a 250-l fibreglass tank containing seawater and observed for hatching. The larvae were observed and maintained according to previously defined procedures (Deane et al., 2003). Larvae were collected at incremental times from 1–46 dph and stored at –80°C until analysis.
Cloning of aqp3 cDNA Fragment
To obtain a gene fragment of the silver sea bream aqp3 low-stringency polymerase chain reaction (PCR) amplification of first-strand cDNA from gill tissue was performed. For this purpose, total RNA was extracted from gill filaments using Tri-Reagent (Molecular Research Center, Cincinnati, OH) treated with DNaseI (Invitrogen, Carlsbad, CA) and then quantified spectrophotometrically. For first strand cDNA synthesis, 1 μg of total RNA was added to a reaction mix (20 μl), containing 0.5 μg of oligo-DT primer (Pharmacia, LKB, Sweden), 2 μl of dithiothreitol (0.1 M), 1 μl of dNTP mix (10 mM, Pharmacia, LKB), 4 μl of reaction buffer, and 1 μl of Superscript II reverse transcriptase (Gibco-BRL, Gaithersburg, MD; 200 U/μl). First-strand cDNA synthesis was allowed to proceed at 42°C for 1 h, after which time the reaction was incubated at 70°C for 15 min. For amplification of aqp3 fragment, degenerate oligonucleotides designed from conserved amino acid regions between fish aqp3 genes were used. The amino acid sequences used for primer design were LAECLGTL (5′-tyngcngartgyytnggnacnyt-3′ sense) and TAGIFATYP (5′-ggrtangtngcraadatnccngcngt-3′ antisense) and primers were synthesized by Genset (Singapore). PCR reactions (50 μl) containing 2 μl of first-strand cDNA, 0.2 μl of Taq DNA polymerase (Promega, Madison, WI; 5 U/μl), 5 μl of MgCl2 (25 mM), 5 μl of reaction buffer, 0.5 μl of dNTP mix (10 mM), and 1 μl of each primer (50 pM) were prepared. PCR amplification was performed using a Mastercycler (Eppendorf, Westbury, NY) with cycle parameters of 94°C for 1 min, 45°C for 1 min, 72°C for 1 min, and a final extension of 72°C for 4 min. Reaction products were analyzed on a 2% w/v agarose gel and visualized by ethidium bromide staining. Putative gene fragments were subcloned into a T/A cloning vector (Invitrogen) and 10 clones were used for plasmid extraction. Plasmids were cycle sequenced using an ABI PRISM dye terminator kit with reaction products run on an ABI 310 Genetic Analyzer (Perkin Elmer, Wellesley, MA). The clones were sequenced on both strands and sequence data were analyzed using the Basic Local nt Search Tool Program (Altschul et al., 1990). It was confirmed that a 320- bp fragment, with 75–83% homology to a number of teleost aqp3 genes, was isolated and the sequence has been deposited on the GenBank under accession number DQ333306.
Antibody Preparation
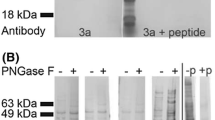
Using the deduced amino acid sequence from the aqp3 gene fragment, a peptide region (RWRKFPMYFLFQTIGAFFQ) was selected and this was used for subsequent antibody production. Peptide synthesis, peptide conjugation to keyhole limpet hemocyanin, rabbit polyclonal antibody production, and affinity purification were performed by Sigma-Aldrich (St. Louis, MO).
Tissue Sampling and Protein Extraction
For tissue distribution studies, fish (n = 2) were removed from seawater tanks, 1ml of whole blood was withdrawn from the caudal vessels, and fish were killed immediately by spinal transection. Brain, gill, heart, kidney, liver, and spleen tissues were removed and stored at −70°C until analysis. For protein extraction, approximately 5 mg of tissue was added to 2 ml of extraction buffer (4 M urea, 0.5% w/v sodium dodecyl sulfate [SDS], 10 mM EDTA, 2 mM phenylmethylsulfonyl fluoride [PMSF]) and homogenized for 1 min using an Ultra-Turrax T25 rotor stator homogenizer. The samples were then incubated at 94°C for 10 min, sonicated for 10 min, and then centrifuged at 10,000 g for a further 10 min. The supernatant was collected and total protein was quantified using the dye binding method of Bradford (1976). Protein samples from tissues were not combined.
Protein Gel Electrophoresis and Immunoblotting
One-dimensional sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was applied to resolve proteins of different molecular size according to the method of Laemmli (1975) using a 4% (stacking) and 12% (separating) polyacrylamide gel. For electrophoresis, 1.5 μg of rainbow molecular weight marker (Amersham, UK) and 10 μg of total protein from each tissue were electrophoresed for 1.5 h at 100V using a mini gel kit (Bio-Rad, Hercules, CA). After SDS-PAGE, the resolved proteins were transferred to Hybond ECL-nitrocellulose membrane (Amersham), using an electrotransfer cell, for 1 h at 150 V. After protein transfer, the membranes were rinsed briefly in 0.01 M phosphate-buffered saline (PBS; pH 7.2) containing 0.05% v/v Tween 20 (PBS-T) and then blocked in PBS-T/3% w/v skimmed milk powder overnight at 4°C. The membranes were rinsed for 1 h in PBS-T and then incubated for 1 h with rabbit AQP3 polyclonal antiserum diluted 1:8000 in PBS-T. The membranes were rinsed for a further 1 h before incubating with antirabbit IgG horseradish peroxidase (IgG-HRP) conjugate (Amersham) diluted 1:20,000. After a final rinse, immunoreactive bands were developed using an ECL reagent system (Amersham) and membranes were exposed to Hyperfilm (Amersham) for 2 min.
AQP3 Analysis Using Immuno-Dot Blot Analysis
After the size and tissue distribution of AQP3 were determined, an immuno-dot blot method was used to analyze tissue samples from salinity-acclimated groups and larval samples. The procedures used were as previously described with minor modifications (Deane et al., 2002). Hybond ECL-nitrocellulose membrane was soaked for 10 min in 0.1 M PBS (pH 7.2) and then placed in a Bio-Dot microfiltration manifold (Bio-Rad). Protein samples of 5 μg for tissues taken from fish acclimated to different salinities, or total protein equivalent to a single larva, were added to separate wells of the manifold and a vacuum was used to pass the samples through the membrane. The wells were washed with 0.2 ml of 0.1 M PBS and samples were fixed onto the membrane by air drying overnight. The membrane was blocked as previously described and then probed with primary antibody at a 1:8000 dilution and then secondary antibody at a 1:20,000 dilution. Immuno-dot blots were developed using the ECL reagent system and bands were visualized using a Lumi-Imager workstation (Roche, Germany) and quantified using Lumi-Analyst 3.1 software (Roche).
Statistical Analysis
The AQP3 values from each set of experiments were subjected to a one-way ANOVA to test for significance followed by a Student-Newman-Keuls test (Jandel scientific) to delineate significance among groups. Significant differences were accepted if p< 0.05.
Results
Tissue Distribution
Using Western blot analysis, AQP3 was found in all tissues analyzed except for whole blood (Figure 1). In kidney and liver tissue, two proteins of molecular masses 46 and 36 kDa were detected. In spleen, heart, and brain tissue only the 46-kDa form was present and in gill the 36 kDa form was predominant.
Salinity Acclimation Effects
Of the tissues that showed the presence of AQP3, only gill and spleen displayed modulated expression during salinity acclimation. In gills, AQP3 was approximately 5- to 8-fold greater in hypoosmotic and isoosmotic acclimated fish in comparison to seawater- and hypersaline-acclimated fish (Figure 2). Similarly, in spleen AQP3 was 3- to 4.5-fold greater in hypoosmotic and isoosmotic acclimated fish in comparison to seawater- and hypersaline-acclimated fish (Figure 3).
AQP3 expression in osmoregulatory important tissues for sea bream acclimated to hypoosmotic (6 ppt), isoosmotic (12 ppt), seawater (33 ppt), and hypersaline (50 ppt) conditions. Immunoanalysis was used for detection of AQP3 and OD [OD × area (mm2)] was quantified via Lumi Analyst 3.1 software. Values are expressed as mean (n = 6–7) ± SEM. Different letters above each bar indicate mean values that were found to be significantly different (p < 0.05).
AQP3 expression in nonosmoregulatory tissues for sea bream acclimated to hypoosmotic (6 ppt), isoosmotic (12 ppt), seawater (33 ppt), and hypersaline (50 ppt) conditions. Immunoanalysis was used for detection of AQP3 and OD [OD × area (mm2)] was quantified using Lumi Analyst 3.1 software. Values are expressed as mean (n = 6–7) ± SEM. Different letters above each bar indicate mean values that were found to be significantly different (p < 0.05).
Ontogenetic Profile
AQP3 could be detected from 14 dph onwards, during sea bream larval development. At 14–21 dph AQP3 increased 5–fold and a further 2.3-fold between 21 and 28 dph. From 28 to 46 dph AQP3 amounts remained relatively unchanged (Figure 4).
AQP3 expression in developing for sea bream larvae at incremental days following hatching. Immunoanalysis was used for detection of AQP3 and OD [OD × area (mm2)] per larva was quantified via Lumi Analyst 3.1 software. A dot blot figure for all samples (A) and corresponding data (B) is presented. Values are expressed as mean (n = 5) ± SEM. Different letters above each bar indicate mean values that were found to be significantly different (p < 0.05).
Discussion
Using the polyclonal antibody preparation that was designed from sea bream aqp3, a tissue distribution analysis was performed for sea bream and it was found that AQP3 was present in gill, kidney, spleen, heart, brain, and liver. Comparatively, transcript analysis showed aqp3 was present in brain, kidney, spleen, and gill but not liver in Mozambique tilapia (Watanabe et al., 2005) whereas high levels of aqp3 transcript were found in gills but not in kidney, brain, heart, or liver for European eel (Cutler and Cramb, 2002). Using a human tissue microarray, AQP3 was found in many tissues including kidney, colon, urinary bladder, trachea, esophagus, skeletal muscle, pancreas, ovary, and fetal membranes (Mobasheri et al., 2005). Transcript studies on rat demonstrated the presence aqp3 in kidney and spleen but not brain, liver, or heart (Echevarria et al., 1994; Ishibashi et al., 1994). These contrasting findings imply that AQP3 could have tissue-specific physiological functions in different species. It was also interesting to note that AQP3 was not found in sea bream whole blood preparations, suggesting the importance of other aquaporin family members such as AQP1 that has previously been demonstrated to be present in mammalian red blood cells (Borgnia et al., 1999). At present, there is a paucity of data on AQP3 protein distribution in teleost tissues although a study by Lignot et al. (2002) showed the presence of a major 24-kDa band alongside several bands of larger size, in European eel, whereas the present study defined two bands of 46 and 36 kDa. At present it is difficult to reconcile the differences in molecular sizes of AQP3 between sea bream and eel, but this may relate to either differential splicing and/or glycosylation effects. In the present study we did not examine the transcript size for aqp3 and this could be useful for future investigation; however, it is known that aquaporins contain a number of glycosylation sites (Verkman and Mitra, 2000) that could account for the appearance of multiple protein bands. The importance and implications for glycosylated versus nonglycosylated aquaporins in fish tissues are unknown and in mammalian tissues the findings are equivocal. Earlier studies demonstrated that glycosylation of aquaporins was not essential for membrane targeting (Zhang et al., 1993; Van Hoek et al., 1995; Baumgarten et al., 1998) but more recent studies have shown that glycosylation is important for aquaporin stabilization (Buck et al., 2004) and cell surface expression (Hendriks et al., 2004).
Silver sea bream is highly euryhaline, being able to tolerate salinities ranging from hypoosmotic to hypersaline with no appreciable changes in serum ions or tissue hydration (Woo and Kelly, 1995). In addition, it has been recently demonstrated that a number of molecular and biochemical changes associated with endocrine, stress, and osmoregulatory processes occur in sea bream tissues to allow for successful adjustments to wide ranges of salinity (Deane and Woo, 2004) and the ability of fish to alter their osmoregulatory strategies, dependent on the surrounding salinity, was outlined previously (McCormick, 2001). In hypoosmotic conditions, fish counteract the passive gain of water and loss of ions by producing dilute urine and actively taking up ions across the gills whereas in seawater and hypersaline conditions the passive gain of ions and loss of water is counteracted by increased drinking of seawater, absorbing water and ions across the gut, and secreting excess ions at the gills and kidney. Despite these generalizations, the role and importance of AQP3 in water regulation during hypoosmotic-hypersaline acclimation in sea bream still remain to be established. In the present study, it was found that AQP3 amounts were 5- to 8-fold higher in gills of hypoosmotic and isoosmotic acclimated sea bream in comparison to those maintained at seawater and hypersalinity. The extensive increase in AQP3 abundance in gills of sea bream acclimated to below seawater conditions suggests that increased water channels are needed as a protective response against osmotic swelling and subsequent gill cell damage. Cutler and Cramb (2001) hypothesized that such a mechanism would serve to release water entering the gill epithelia, externally, because cell volume regulation would be difficult to control under low salinity conditions. Conversely, the lowered AQP3 levels in seawater and hypersaline conditions would suggest a reduction in water channels in order to protect against water loss as well as gill cell shrinkage and damage. Findings similar to those in the present study were reported for European eel where gills taken from seawater-acclimated European eel had significantly low amounts of AQP3 in comparison to freshwater eels (Cutler and Cramb, 2002). Although well known for its role in water regulation, AQP3 is a multifunctional channel because it can be permeated by glycerol and small solutes including urea (Borgnia et al., 1999). In mammals, AQP3 is predominantly located in the basolateral membrane of renal collecting duct cells and it has been speculated that it could serve as an exit pathway for both water and urea during antidiuresis (Ishibashi et al., 1994). Whereas teleosts are mainly ammoniotelic (Anderson, 1995), it has been shown that urea production in sea bream is closely coupled to salinity as abrupt transfer from seawater to hypoosmotic conditions resulted in a transient elevation of serum urea (Kelly and Woo, 1999a). A major site of urea excretion in fish is through the gills, and concomitant alterations in chloride cell morphometrics on longer term hypoosmotic acclimation have also been reported for sea bream (Kelly and Woo, 1999b). Given that AQP3 was significantly highest in gills of low-salinity-acclimated sea bream, its role for facilitating branchial urea transport under such conditions cannot be overlooked.
Of the remaining sea bream tissues that showed AQP3 presence, only the spleen displayed expression changes related to external salinity, as it was found that hypoosmotic and isoosmotic acclimated sea bream had a 3- to 4.5-fold higher amount of spleen AQP3 than seawater and hypersaline-acclimated sea bream. The spleen is important for immune function and in fish this is related to aspects of nonspecific immunity through macrophage activity. In sea bream it has been shown that macrophage activity is highest at salinities below seawater, particularly under isoosmotic conditions (Narnaware et al., 2000) and it could be possible that the AQP3 changes detected in the present study may, in some way, be related to macrophage activity. In humans, AQP9 was found to be abundantly present in leukocytes that were permeable to both water and urea, and this aquaporin has been speculated to play a role during immunological responses and bactericidal activity (Ishibashi et al., 1998). Hormones such as prolactin and growth hormone are related to osmoregulation in fish (McCormick, 1995) and recently growth hormone has been shown to be elevated during low-salinity acclimation in sea bream (Deane and Woo, 2004). In addition, both prolactin and growth hormone stimulate fish macrophage activity (Sakai et al., 1996; Narnaware et al., 1998) and an alternative explanation for elevated spleen AQP3 may be related to elevated levels of these hormones.
Aquaporin (SaAQP1o) regulation has been shown to be important during fish egg hydration (Fabra et al., 2005); however, the profiles of aquaporin during posthatch development still remains to be elucidated and as the final part of this study the abundance of AQP3 was measured in sea bream larvae from 1 dph to 46 dph. The earliest signs of AQP3 were found in 14 dph larvae that coincide with flexion, caudal fin development, and upturning of the notochord (Deane et al., 2003). The detection of AQP3 at 14 dph was later than the appearance of ionic exchange mechanisms including Na+–K+–ATPase (Deane et al., 2003) and suggests that water and ionic control mechanisms are not in phase during sea bream larval development. A drastic increase of AQP3 occurred between 21 and 28 dph, which coincides with the transition of larvae to juveniles with a concomitant surge in thyroxine, triiodothyronine, and cortisol (Deane and Woo, 2003). Taken together these data show that AQP3 is developmentally regulated during the early stages of larval growth and the ontogenetic profile of AQP3 provides a clearer picture as to the approximate time of development. However, it still remains to be established as to how other aquaporin family members such as AQP1 are expressed during posthatch development in sea bream as well as other fish species, and future studies in this area would be useful to aid our understanding of fish larval development.
In summary, this study has shown how AQP3 is distributed and regulated in the euryhaline silver sea bream. The elevated gill AQP3 levels in hypoosmotic and isoosmotic conditions suggest a key role during low-salinity acclimation possibly related to protection against osmotic swelling. The appearance of AQP3 at 14 days after hatching gives us an approximate time of water regulation in early developing larvae, although the importance of other aquaporin family members still remains to be elucidated.
References
SF Altschul W Gish W Miller EW Myers D Lipman (1990) ArticleTitleBasic local alignment search tool J Mol Biol 215 403–410 Occurrence Handle10.1006/jmbi.1990.9999
PM Anderson (1995) Urea cycle in fish: molecular and mitochondrial studies CM Wood TJ Shuttleworth (Eds) Fish Physiology, Vol. 14 Academic Press San Diego 57–83
M Aoki T Kaneko F Katoh S Hasegawa N Tsutsui K Aida (2003) ArticleTitleIntestinal water absorption through aquaporin 1 expressed in the apical membrane of mucosal epithelial cells in seawater-adapted Japanese eel J Exp Biol 206 3495–3505 Occurrence Handle10.1242/jeb.00579
R Baumgarten MH Pol ParticleVan der JF Wetzels CH Os ParticleVan PM Deen (1998) ArticleTitleGlycosylation is not essential for vasopressin-dependent routing of aquaporin 2 in transfected Madin-Darby canine kidney cells J Am Soc Nephrol 9 1553–1559
M Borgnia S Nielson A Engel P Agre (1999) ArticleTitleCellular and molecular biology of the aquaporin water channels Annu Rev Biochem 68 425–458 Occurrence Handle10.1146/annurev.biochem.68.1.425
MM Bradford (1976) ArticleTitleA rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principles of protein dye binding Anal Biochem 72 248–252 Occurrence Handle10.1016/0003-2697(76)90527-3
TM Buck J Eledge WR Skach (2004) ArticleTitleEvidence for stabilization of aquaporin 2 folding mutants by N-linked glycosylation in endoplasmic reticulum Am J Physiol 287 C1292–C1299 Occurrence Handle10.1152/ajpcell.00561.2003
CP Cutler G Cramb (2001) ArticleTitleMolecular physiology of osmoregulation in eels and other teleosts: the role of transport isoforms and gene duplication Comp Biochem Physiol 130A 551–564
CP Cutler G Cramb (2002) ArticleTitleBranchial expression of an aquaporin 3 (AQP-3) homologue is downregulated in the European eel Anguilla anguilla following seawater acclimation J Exp Biol 205 2643–2651
EE Deane NYS Woo (2003) ArticleTitleOntogeny of thyroid hormones, cortisol, hsp70 and hsp90 during silver sea bream larval development Life Sci 72 805–818 Occurrence Handle10.1016/S0024-3205(02)02334-2
EE Deane NYS Woo (2004) ArticleTitleDifferential gene expression associated with euryhalinity in sea bream (Sparus sarba) Am J Physiol 287 R1054–R1063
EE Deane SP Kelly JCY Luk NYS Woo (2002) ArticleTitleChronic salinity adaptation modulates hepatic heat shock protein and insulin-like growth factor 1 expression in black sea bream Mar Biotechnol 4 193–205
EE Deane SP Kelly PM Collins NYS Woo (2003) ArticleTitleLarval development of silver sea bream (Sparus sarba): Ontogeny of RNA-DNA ratio, GH, IGF-1 and Na+−K+−ATPase Mar Biotechnol 5 79–91 Occurrence Handle10.1007/s10126-002-0052-7
M Echevarria EE Windhager SS Tate G Frindt (1994) ArticleTitleCloning and expression of AQP3, a water channel from the medullary collecting duct of rat kidney Proc Natl Acad Sci USA 91 10997–11001 Occurrence Handle10.1073/pnas.91.23.10997
M Fabra D Raldúa DM Power PMT Deen J Cerdà (2005) ArticleTitleMarine fish egg hydration is aquaporin-mediated Science 307 545 Occurrence Handle10.1126/science.1106305
M Fabra D Raldúa MG Bozzo PMT Deen E Lubzens J Cerdà (2006) ArticleTitleYolk proteolysis and aquaporin-1 o play essential roles to regulate fish oocyte hydration during meiosis resumption Dev Biol 295 250–262 Occurrence Handle10.1016/j.ydbio.2006.03.034
G Hendriks M Koudijs BWM Balkom ParticleVan V Oorshot J Klumperman PMT Deen P Sluijs ParticleVan der (2004) ArticleTitleGlycosylation is important for cell surface expression of the water channel aquaporin 2 but is not essential for tetramerization in the endoplasmic reticulum J Biol Chem 279 2975–2983 Occurrence Handle10.1074/jbc.M310767200
T Hirata T Kaneko T Ono T Nakazato N Furukawa S Hasegawa S Wakabayashi M Shigekawa MH Chang MF Romero S Hirose (2003) ArticleTitleMechanism of acid adaptation of a fish living in a pH 3.5 lake Am J Physiol 284 R1199–R1212
K Ishibashi S Sasaki K Fushimi S Uchida M Kuwuhara H Saito T Furukawa K Nakajima Y Yamaguchi T Gojobori F Marumo (1994) ArticleTitleMolecular cloning and expression of a member of the aquaporin family with permeability to glycerol and urea in addition to water expressed at the basolateral membrane of kidney collecting duct cells Proc Natl Acad Sci USA 91 6269–6273 Occurrence Handle10.1073/pnas.91.14.6269
K Ishibashi M Kuwuhara Y Gu Y Tanaka F Marumo S Sasaki (1998) ArticleTitleCloning and functional expression of a new aquaporin (AQP9) abundantly expressed in the peripheral leukocytes permeable to water and urea, but not to glycerol Biochem Biophys Res Commun 244 268–274 Occurrence Handle10.1006/bbrc.1998.8252
SP Kelly NYS Woo (1999a) ArticleTitleThe response of sea bream following abrupt hyposmotic exposure J Fish Biol 55 732–750 Occurrence Handle10.1111/j.1095-8649.1999.tb00714.x
SP Kelly NYS Woo (1999b) ArticleTitleCellular and biochemical charaterization of hyposmotic adaptation in a marine teleost, Sparus sarba Zool Sci 16 505–514 Occurrence Handle10.2108/zsj.16.505
UK Laemmli (1975) ArticleTitleCleavage of structural proteins during the assembly of the head of bacteriophage Nature 227 680–685 Occurrence Handle10.1038/227680a0
JH Lignot CP Cutler N Hazon G Cramb (2002) ArticleTitleImmunolocalisation of aquaporin 3 in the gill and the gastrointestinal tract of the European eel Anguilla anguilla (L.) J Exp Biol 205 2653–2663
AS Martinez CP Cutler GD Wilson C Phillips N Hazon G Cramb (2005) ArticleTitleCloning and expression of three aquaporin homologues from the European eel (Anguilla anguilla): effects of seawater acclimation and cortisol treatment on renal expression Biol Cell 97 615–627 Occurrence Handle10.1042/BC20040111
SD McCormick (1995) Hormonal control of gill Na+−K+−ATPase and chloride cell function CM Wood TJ Shuttleworth (Eds) Cellular and Molecular Approaches to Fish Ionic Regulation Academic Press New York 285–315
SD McCormick (2001) ArticleTitleEndocrine control of osmoregulation in teleost fish Am Zool 41 781–794 Occurrence Handle10.1668/0003-1569(2001)041[0781:ECOOIT]2.0.CO;2
A Mobasheri S Wray D Marples (2005) ArticleTitleDistribution of AQP2 and AQP3 water channels in human tissue microarrays J Mol Histol 36 1–14 Occurrence Handle10.1007/s10735-004-2633-4
YK Narnaware SP Kelly NYS Woo (1998) ArticleTitleStimulation of macrophage phagocytosis and lymphocyte count by exogenous prolactin administration in silver sea bream (Sparus sarba) adapted to hyper- and hypo-osmotic salinities Vet Immunol Immunopathol 61 387–391 Occurrence Handle10.1016/S0165-2427(97)00143-8
YK Narnaware SP Kelly NYS Woo (2000) ArticleTitleEffect of salinity and ration size on macrophage phagocytosis in juvenile black sea bream (Mylio macrocephalus) J Appl Ichthyol 16 86–88 Occurrence Handle10.1046/j.1439-0426.2000.00113.x
M Sakai M Kobayashi H Kawauchi (1996) ArticleTitleIn vitro activation of fish phagocytic cells by GH, prolactin and somatolactin J Endocrinol 151 113–118 Occurrence Handle10.1677/joe.0.1510113
CRA Santos MD Estêvão J Fuentes JCR Cardosa M Fabra AL Passos FJ Detmers PMT Deen J Cerdià DM Power (2004) ArticleTitleIsolation of a novel aquaglyceroporin from a marine teleost (Sparus auratus): function and tissue distribution J Exp Biol 207 1217–1227 Occurrence Handle10.1242/jeb.00867
AN Hoek ParticleVan MC Weiner JM Verbavatz D Brown RR Townsend H H Lipniunas AS Verkman (1995) ArticleTitlePurification and structure-function analysis of PNG ase F- and endo β galactosidase-treated CHIP28 water channels Biochemistry 34 2212–2219 Occurrence Handle10.1021/bi00007a015
AS Verkman AK Mitra (2000) ArticleTitleStructure and function of aquaporin water channels Am J Physiol 278 F13–F28
LV Virkki GJ Cooper WF Boron (2001) ArticleTitleCloning and functional expression of a MIP (AQP0) homolog from killifish (Fundulus heteroclitus) lens Am J Physiol 281 R1994–R2003
S Watanabe T Kaneko K Aida (2005) ArticleTitleAquaporin 3 expressed in the basolateral membrane of gill chloride cells in Mozambique tilapia Oreochromis mossambicus adapted to freshwater and seawater J Exp Biol 208 2673–2682 Occurrence Handle10.1242/jeb.01684
NYS Woo SP Kelly (1995) ArticleTitleEffects of salinity and nutritional status on growth and metabolism of Sparus sarba in a closed seawater system Aquaculture 135 229–238 Occurrence Handle10.1016/0044-8486(95)01003-3
R Zhang AN Hoek ParticleVan J Biwersi AS Verkman (1993) ArticleTitleA point mutation at cysteine 189 blocks the water permeability of rat kidney water channel CHIP28k Biochemistry 32 2938–2941 Occurrence Handle10.1021/bi00063a002
Acknowledgments
This research was supported by an Earmarked Grant, CUHK 4318/03M (Research Grants Council, Hong Kong) awarded to Dr. Norman Y.S. Woo.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Deane, E.E., Woo, N.Y. Tissue Distribution, Effects of Salinity Acclimation, and Ontogeny of Aquaporin 3 in the Marine Teleost, Silver Sea Bream (Sparus sarba). Mar Biotechnol 8, 663–671 (2006). https://doi.org/10.1007/s10126-006-6001-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10126-006-6001-0