Abstract
Enzymes responsible for digestion of food protein were evaluated and characterized in red lobster (Panulirus interruptus). Several tissues, organs, and body fluids were analyzed. The same composition of proteases was found in gastric juice, midgut gland, and intestinal contents. Using specific substrates and inhibitors, we indentified several isotrypsins and isochymotrypsins by gel electrophoresis. Protease activity was found at pH 3 and reduced by using pepstatin A. Operational variables of enzymes were characterized for management of future studies and potential biotechnologies. Types and activities of lobster digestive enzymes constitute background information to study the digestive abilities of the organism further, and will lead to understanding nutritional needs and feeding ecology, mainly because decapods display unique morphologic, metabolic, and behavioral changes during their life cycle. Also, such enzymes become alternative tools for use in biotechnologies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Gaining understanding of digestive enzymes and their operational variables in marine invertebrates is important for research on nutrition, feeding ecology, and potential biotechnologies. Digestion, with focus on enzymes, has been one of the more intensely studied areas of crustacean nutrition. Studies are available on characteristics of digestive enzymes in crustaceans (Galgani and Nagayama, 1987; Fang and Lee, 1992; and García-Carreño et al., 1994); variations in isozyme activities during larval development (Jones et al., 1997; Lemos et al., 1999); and change of enzyme activity by feeding, size, molting stage, environmental stress, and other factors (Lee et al., 1984; Shiau, 1998; Le Moullac et al., 1994; Rodriguez et al., 1994). In lobster species, studies have focused on digestive proteolytic enzymes such as trypsin, chymotrypsin, carboxipeptidase A and B, leucinaminopeptidase, elastase, and collagenase (Brockerhoff et al., 1970; Hoyle, 1973; Galgani and Nagayama, 1987; Glass and Stark, 1994, 1995). All this information has led to a model of multifactorial output in enzyme synthesis in crustaceans.
Several species of lobster from the west coast of the Baja California Peninsula have both ecological and economic interest. Panulirus interruptus is one of the most important fisheries of the regional economy. This study describes the first known work on the proteases for digesting food protein in this species. The main objective was to provide descriptions of enzyme class, type, composition, and other basic information as support for further study on potential uses of such biological reagents. To characterize digestive enzymes, we used test tube assays, substrate-SDS-PAGE techniques, and specific synthetic substrates and inhibitors. Another goal of the study was to explore the use of alternative body fluids as starting material for research of enzymes, to avoid the traditional approach of killing organisms and anatomizing the digestive glands.
MATERIALS AND METHODS
A local supplier provided 20 Panulirus interruptus (cephalothoracic length, 9.2 ± 4 cm) which were individually acclimatized in separate 40-L aquaria. Seawater was changed daily, and organisms were fed daily to satiation on thawed squid.
Specimens were killed by lowering the water temperature to 4°C. Digestive systems were dissected and separated individually into gastric chamber (GC) sometimes incorrectly called stomach, midgut gland (MG) or hepatopancreas, and intestine (It). Other samples included gastric juice (GJ) and intestinal contents (IC). Gastric juice was obtained with a canula attached to a hypodermic syringe. Individual gastric chambers were washed with distilled water before tissue analysis to eliminate enzymes regurgitated from the midgut gland. All samples were stored individually at −20°C until analysis. Thawed samples were homogenized with chilled distilled water at 10°C. Homogenates of tissues and liquid samples, as gastric juice and intestinal contents, were centrifuged for 20 minutes at 10,000g to eliminate tissue debris and particles. Supernatant was used as enzyme extract for further assays. The pH was 6.3 ± 0.2 for the midgut gland, 5.7 ± 0.1 for its homogenate with water, 6.4 ± 0.4 for gastric juice, and (6.8) for intestines. Protein concentration in the samples was evaluated according to Bradford (1976), using bovine serum albumin as standard.
Total protease activity was assayed using 1% azocasein in 50 mM Tris-HCl at pH 8.0 (García-Carreño, 1992a). Total acid protease activity was evaluated using 0.5% hemoglobin in 100 mM glycine-HCl buffer at pH 3. The pH for successive assays was chosen according to the observed optimum pH. Triplicates of enzyme extracts (5–20 μl) were mixed with 0.5 ml of buffer and 0.5 ml of substrate solution. Reaction mixtures were incubated for 10 minutes at 25°C. Proteolysis was stopped by adding 0.5 ml 20% trichloroacetic acid (TCA), and the mixture was centrifuged in microcentrifuge tubes for 5 minutes at 10,000g. The absorbance of supernatants at 366 nm (azocasein) and 280 nm (hemoglobin) was recorded.
The effect of pH on proteases was evaluated using universal buffer at pH 5 to 11 at 25°C (Stauffer, 1989); 100 mM glycine-HCl buffer was used for pH 2 to 4 at 25°C for acid proteases. Enzyme activity was evaluated from 10° to 70°C in 50 mM Tris-HCl buffer at pH 8.0. The effect of pH and temperature on residual enzyme activity was evaluated by incubating reaction mixtures up to 60 minutes at variable pH and temperature.
Class and type of enzymes were characterized using several synthetic substrates. Trypsin activity was measured with 1 mM N-benzoyl-DL-arginine p-nitroanilide (BAPNA; Erlanger et al., 1961). Chymotrypsin activity was evaluated using 0.1 mM Suc-Ala-Ala-Pro-Phe-p-nitroanilide (SAPNA; del Mar et al., 1979). BAPNA and SAPNA were dissolved in 1 ml dimethylsulfoxide (DMSO) and brought to 100 ml with 50 mM Tris-HCl at pH 8.0 containing 20 mM CaCl2. Triplicates of samples were mixed with 0.75 ml of substrate solution at 37°C. Changes in absorbance at 410 nm were recorded for 5 minutes. Each assay included blanks and commercial enzymes (1 mg/ml) as internal controls. Total protease, trypsin, and chymotrypsin units of activity were expressed as change in absorbance per minute per milligram of enzyme protein (Δabs min−1 mg protein−1). Absorbance was recorded in a PerkinElmer spectrophotometer.
Class and type of proteases were studied further, as described in García-Carreño (1992b). Phenylmethylsulfonyl fluoride (PMSF) and soybean trypsin inhibitor (SBTI) were used as serine class protease inhibitors. Nα- p-Tosyl-L-lysine chloromethyl ketone (TLCK) was used as a specific inhibitor of trypsin, N-tosyl-L-phenylalanine chloromethyl ketone (TPCK) was used as a specific inhibitor of chymotrypsin, and pepstatin A was used as specific inhibitor of pepsin. EDTA and EGTA are Ca2+ and Mg2+ chelators and, hence, prevent activation of cation-dependent proteases. Aliquots of 10 μl of each inhibitor were mixed separately with the enzyme extracts (1:1) and incubated for 60 minutes at 25°C, before use as enzyme preparation for activity as described above. Assays included solutions with distilled water instead of inhibitor as controls. Residual activity was reported as percentage of inhibition. Activity measured in the absence of inhibitor was considered as 100%. Assays were run in triplicate.
Sodium dodecyl sulfate, 12% polyacrylamide gel electrophoresis (SDS-PAGE) was performed according to Laemmli (1970). Enzyme preparations, containing 10 mU of samples diluted 1:2 with sample buffer, were loaded into individual gel wells at 4°C in a vertical electrophoresis device. Low molecular mass standards (4 μl each from Sigma Chemical Co.) were loaded on each gel. After electrophoresis, gels were stained with 0.05% Coomassie Brillant Blue R-250 for at least 24 hours, then destained. Protease composition and molecular weight were studied after SDS-PAGE in twin gels (García-Carreño et al., 1993). Gels were immersed in 3% casein in 50 mM Tris-HCl at pH 8.0 for 30 minutes at 5°C to allow substrate to diffuse into the gel. The temperature was then raised to 25°C for 90 minutes. Gels were washed in water and immediately fixed and stained with Brillant Blue R. When evaluating activity at acid pH, the substrate was 0.25% hemoglobin in 100 mM Gly-HCl buffer at pH 3 for 30 minutes at 5°C and 90 minutes at 37°C.
To characterize class, type, and composition of proteases in different samples by SDS-PAGE, enzyme extracts were incubated with protease inhibitors. Solutions of TLCK, TPCK, PMSF, SBTI, and pepstatin A were separately added to enzyme extracts containing 10 mU, in a ratio of 1:2 inhibitor–extract and incubated at 25°C for 60 minutes. Distilled water replaced inhibitors in controls. Samples were mixed in sample buffer, as described above, and loaded into gels. The bands of enzymes mixed with protease inhibitors were compared with those of controls without inhibitors to identify inhibitory effects on active bands.
Data are expressed as mean plus or minus standard deviation. Analysis of variance (ANOVA) and Tukey tests were used to analyze differences among means. In all cases, the level of significance was set for P < 0.05.
RESULTS
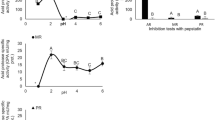
Enzyme activities were found in gastric juice, midgut gland, and intestinal contents when evaluated with azocasein at pH 8.0, and no activity was found in gastric chamber and intestine. When evaluated as specific activity (U/mg protein), azocaseinolytic activity was highest in gastric juice, followed by that in the midgut gland and the intestinal contents. Enzyme activity at acid pH was also found in the gastric juice. The maximum activities, both at acid (hemoglobin as substrate) and at alkaline (azocasein as substrate) pHs, are shown in Figure 1 as 100%. Because 2 different substrates and buffer systems were used, activities at acid and alkaline pH are shown as discontinuous lines.
The optimum pH values for enzymes in gastric juice and midgut gland were close. The highest activity was found between pH 8 and 9 (Figure 1). It is noteworthy that between pH 5 and 8, activity was higher in the gastric juice than in the midgut gland. Between pH 9 and 12, activities in both enzyme extracts were close. Activities for both enzyme extracts were 50% of the optimum at pH 10.5. Activity remained 50% of the optimum at pH 7 in midgut gland enzyme extract, while the same activity was found at more acid pH (5.7) in the gastric juice enzyme preparation. Another maximum of activity was found at pH 3. The effect of pH on activity of the main proteases in the gastric juice is shown in Figure 2. Some enzymes, mainly those around 29 kDa, were sensitive to extreme alkaline pH. At pH 4 and 9, enzyme activities were reduced by 20%. Caseinolytic activities in the gastric juice were stable up to 60 minutes at pH 5 to 9, and remained above 95% (Figure 3, a). Caseinolytic activity in the midgut gland enzyme preparation was stable (>90%) for up to 60 minutes at pH between 5 and 8 (Figure 3, b). At pH 4, 15% of the activity was lost after 60 minutes. At pH 12, enzyme extract activity decreased 90% at 10 mintues.
Protease activities were sharply higher from 10°C to 50°C (Figure 4), following the rule of Q10 = 2. It decreased abruptly at 60°C, for gastric juice and midgut gland enzyme preparations, indicating denaturation of enzymes and consequent loss of activity. Caseinolytic activity was resistant up to 50°C in the gastric juice (Figure 3, c) and midgut gland (Figure 3, d) enzyme preparations. Activity declined 60% after 30 minutes at 60°C and almost 90%after 15 minutes at 70°C. Enzymes sensitive to extreme alkaline pH (see Figure 2) were also sensitive to mild temperatures.
When evaluating enzyme type using specific synthetic substrates, the presence of trypsin or chymotrypsin was evident (Table 1). In enzyme extracts, trypsin activity was higher in all samples (0.3 to 1.5 specific activity) than chymotrypsin activity (0.1 to 0.5 specific activity). Trypsin and chymotrypsin activity was higher in the gastric juice than in the midgut gland or intestinal contents. In the gastric juice, trypsin activity was as much as triple that of chymotrypsin in all specimens. When assaying intestinal contents, trypsin and chymotrypsin activities were not found in any of the samples. However, in those samples with measured proteinolytic activities, trypsin activity was higher than chymotrypsin activity.
Electrophoresis analysis showed protein composition of enzyme extracts. Mainly proteins between 10 to 66 kDa were found with this technique. Protein compositions of gastric juice, midgut gland, and Intestinal contents were similar (Figure 5). Figure 5 also revealed a band of 24 kDa with activity at pH 3 when hemoglobin was used as substrate. Substrate SDS-PAGE also demonstrated an important variation in protease composition among individuals, mostly in those enzymes between 20 and 10 kDa.
To further characterize class and type of proteases in the samples, they were challenged with synthetic and natural inhibitors. The combined results from synthetic substrates and inhibitions indicate that serine proteases are the most active enzymes, with some activity of a putative aspartic protease, with trypsin, chymotrypsin, and pepsin activity. Results from test tube assays were confirmed by substrate SDS-PAGE. In all samples, PMSF inhibited bands of 60, 48, 35, 33, and 20 kDa; TLCK, bands of 22, 18, 16, and 10 kDa; and SBTI, bands of 60, 48, 35, 33, 22, 20, 18, 16, 10, and 9 kDa. EDTA and EGTA did not affect band activity (Figure 6).
DISCUSSION
Little is known about physiologic biochemistry in the lobster species Panulicus interruptus. This study is a first step in understanding the physiology of emersion, in which lobsters are subjected to stressful conditions that affect all systems during transport for sale. So far, the effects of emersion on the digestive system remain unknown, so the first step in understanding the phenomena is to establish which enzymes are involved. In other crustaceans, like Pleuroncodes planipes, the digestive gland disintegrates rapidly during emersion and digestive proteases hydrolyze the surrounding tissues, including the tail muscle (García-Carreño, 1992a). Digestive enzymes in shrimp reduce their value as seafood (Ezquerra et al., 1997). Autolysis of muscle protein in crustaceans, like shrimp, crab, and krill, occurs rapidly after harvest and has been attributed to digestive proteases (Salem et al., 1970). These characteristics of digestive enzymes point to high activity under diverse environmental conditions and can be treated as potential biological reagents.
A variety of proteases synthesized by the digestive gland of lobster were found. Most of them catalyze hydrolysis of natural and synthetic substrates at alkaline pH. Some enzymes catalyze hydrolysis of natural substrates at mildly acidic pH; even proteolytic activity was found at pH 3. Trypsin and chymotrypsin were identified with specific synthetic substrates and inhibitors. Trypsins have molecular masses between 10 and 22 kDa. Data on molecular mass are consistent with data for other crustaceans like the shrimp Penaeus vannamei and Penaeus paulensis. Shrimp have trypsins between 17.7 and 22 kDa (Lemos et al., 1999; Muhlia-Almazán and García-Carreño, 2002). Lobster chymotrypsins had a broader range of molecular mass than that of their trypsins. Chymotrypsins had molecular masses between 21 and 60 kDa, in contrast to those of chymotrypsins reported for other crustaceans. Shrimp chymotrypsin molecular masses are between 24 and 30 kDa (Muhlia-Almazán and García-Carreño, 2002). Trypsin is the most important protease involved in hydrolysis of food protein in lobster, with chymotrypsin as the second enzyme responsible for digestion of food protein (Figure 6).
The presence of trypsin and chymotrypsin is fortunate because trypsin splits peptide bonds next to the carboxylic side of positively charged amino acid residues like Lys and Arg. Chymotrypsin splits peptide bonds next to the carboxylic side of aromatic amino acid residues like Phe, Trp, Tyr, and Leu. Proteins are split with only 2 enzymes and left exposed in the carboxylic side of newly formed polypeptides in 6 of the 10 essential amino acids. These amino acids are then freed by carboxypeptidases. The activities of trypsin and chymotrypsin measured in vitro show that trypsin activity was higher than chymotrypsin, suggesting that digestion of food protein is achieved mainly by an enzyme that frees basic amino acids on the carboxylic side of a newly formed peptide. In crustaceans, both trypsin and chymotrypsin are responsible for collagenase activity (Chen et al., 1991; García-Carreño et al., 1994). In a previous study, we demonstrated that both trypsin and chymotrypsin are responsible for collagen hydrolysis in 2 decapods: langostilla and crayfish (García-Carreño and Haard, 1993). Sakharov et al. (1994) showed that crab also has collagenolytic activity which is dependent on serine proteases. This is interesting from a physiologic viewpoint because a third function is accomplished by combining 2 enzymes. Because lobsters feed on animal food, their digestive systems have to process connective tissue containing huge amounts of collagen. Lobsters have a protease of low molecular weight, around 10 kDa, whose identity remains unknown. Low molecular weight proteases seem to occur frequently in lobsters (Brockerhoff et al., 1970; Glass and Stark, 1994). The function of low molecular weight proteases has been discussed, but as far we know, no conclusion has been widely accepted.
Lobster digestive proteases have characteristics similar to orthologue enzymes in crustacea. Lobsters have a protease that has molecular mass of 24 kDa, has optimum activity at pH 3, and is inhibited by pepstatin A, which has a Ki of 45 pM. This makes it highly specific for pepsin. The function of acid protease in this species is not known. The pH of gastric juice is lightly acidic, and generally food alkalinizes the gastric juice during intake. Further investigation is needed to address the subject.
Compositions of proteases were similar in gastric juice, midgut gland, and intestinal contents. This seems to be a consequence of nonselective regurgitation of midgut gland secretion to the gastric chamber. After mixing with food, enzymes remain active during the migration of chyme to the intestine. The highest enzyme activity was found in the gastric juice, so it seems that food protein is exposed to a high concentration of enzymes when forming chyme, and hydrolysis proceeds during passage through the digestive system. Because enzyme activity was found in the intestinal contents, hydrolysis of protein apparently continues in the intestine. Since no physiologic function is currently associated with this finding, an ecologic function is likely, but unproven. It is not surprising that enzymes remain active in feces, as recently found in shrimp (Córdova et al., 2003). Electrophoretic analysis confirmed that protease composition was similar in gastric juice, midgut gland, and intestinal contents.
Since protease composition was the same in the midgut gland and the gastric juice, sampling is possible without killing the organisms. An other advantage of such sampling is for use in sequential studies that follow effects on an individual organism. This way, each organism becomes a replica for the study. The relevance of this point is clear when marked variation is found in the protease pattern among individuals (data not shown). We do not yet know if the bands observed in substrate SDS-PAGE are individual enzymes derived from individual genes, or if they are a phenotypic characteristic derived from heterozygocity (McCarthy et al., 1994), as was observed in the shrimp Penaeus vannamei (Sainz et al. personal communication).
Because enzymes should be analyzed in the laboratory to obtain the highest activities with the least amount of sample, and because they should serve as potential catalysts in biotransformations, we studied the effects of pH and temperature on both activity and stability in midgut gland and gastric juice extracts. The results showed slight differences in the stability of midgut gland and gastric juice enzymes, as much in response to pH as to temperature. We speculate that some salts present in the gastric juice can influence stability, because it has been demonstrated that some salts have a positive effect in the activity of digestive proteases (Tsai et al., 1991).
Characterization of the types and activities of lobster digestive enzymes constitutes background information to study further the digestive abilities of the organism. This information will lead to understanding their nutritional needs and feeding ecology, mainly because decapods have unique morphologic, metabolic, and behavioral changes during their life cycles. Also, this information encourages one to think of them as biological reagents. Because of their catalytic properties, such enzymes can be used to hydrolyze protein for commercial purposes, mainly for producing protein hydrolysates for food industry. Further investigation is needed to fully understand their industrial potential.
References
M. Bradford (1976) ArticleTitleA rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72 248–254 Occurrence Handle10.1006/abio.1976.9999 Occurrence Handle1:CAS:528:DyaE28XksVehtrY%3D Occurrence Handle942051
R. Brockerhoff R.J. Hoyle P.C. Hwang (1970) ArticleTitleDigestive enzymes of the American lobster (Homarus americanus). J Fish Res Bd Can 27 1357–1370 Occurrence Handle1:CAS:528:DyaE3MXktFc%3D
Y.L. Chen P. Lu I. Tsai (1991) ArticleTitleCollagenolytic activity of crustacean midgut serine proteases: comparison with the bacterial and mammalian enzymes. Comp Biochem Physio 100 763–768 Occurrence Handle10.1016/0300-9629(91)90405-2 Occurrence Handle1:STN:280:By2C2crpvFU%3D
J. Córdova-Murueta F. García-Carreño M. Navarrete Toro Particledel (2003) ArticleTitleDigestive enzymes present in crustaceans feces as tool for biochemical, physiological, and ecological studies. J Exp Mar Biol Ecol 297 43–56 Occurrence Handle10.1016/S0022-0981(03)00355-1
E.G. Mar Particledel C. Largman J.W. Brodrick M.C. Goekas (1979) ArticleTitleA sensitive new substrate for chymotrypsin. Anal Biochem 99 316–320 Occurrence Handle574722
B. Erlanger N. Kokowsky W. Cohen (1961) ArticleTitleThe preparation and properties of two new chromogenic substrates of trypsin. Arch Biochem Biophy 95 271–278 Occurrence Handle1:CAS:528:DyaF38XkslOmtg%3D%3D
J.M. Ezquerra F.L. García-Carreño N.F. Haard (1997) ArticleTitleEffects of feed diets on digestive proteases from the hepatopancreas of white shrimp (Penaeus vannamei). J Food Biochem 21 401–419 Occurrence Handle1:CAS:528:DyaK1cXlslahsw%3D%3D
L. Fang B. Lee (1992) ArticleTitleOntogenic changes in digestive enzymes in Penaeus monodon. Comp Biochem Physiol 103B 1033–1037 Occurrence Handle1:CAS:528:DyaK3sXjs1ansA%3D%3D
F. Galgani F. Nagayama (1987) ArticleTitleDigestive proteinases in the Japanese spiny lobster Panulirus japonicus. Comp Biochem Physiol 87B 889–893 Occurrence Handle1:CAS:528:DyaL2sXlvFektL8%3D
F. García-Carreño (1992a) ArticleTitleThe digestive proteases of langostilla (Pleuroncodes planipes, Decapoda): their partial characterization and the effect of feed on their composition. Comp Biochem Physiol 103B 575–578
F. García-Carreño (1992b) ArticleTitleProtease inhibition in theory and practice. Biotechnol Ed 3 145–150
F. García-Carreño N. Haard (1993) ArticleTitleCharacterization of proteinase classes in langostilla (Pleuroncodes planipes) and crayfish (Pacifastacus astacus) extracts. J Food Biochem 17 97–113
F. García-Carreño N. Haard N. Dimes (1993) ArticleTitleSubstrate-gel electrophoresis for composition and molecular weight of proteinases or proteinaceous proteinase inhibitors. Anal Biochem 214 65–69 Occurrence Handle10.1006/abio.1993.1457 Occurrence Handle8250256
F.L. García-Carreño M.P. Hernández-Cortés N. Haard (1994) ArticleTitleEnzymes with peptidase and proteinase activity from the digestive system of a fresh water and a marine decapod. J Agric Food Chem 42 145–146
H. Glass J. Stark (1994) ArticleTitleProtein digestion in the European lobster Homarus gammarus (L). Comp Biochem Physiol 108B IssueID(2 225–235 Occurrence Handle1:CAS:528:DyaK2cXkslyktr8%3D
H. Glass J. Stark (1995) ArticleTitleCarbohydrate digestion in the European lobster Homarus gammarus (L). J Crust Biol 15 IssueID3 424–433
R.J. Hoyle (1973) ArticleTitleDigestive enzyme secretion after dietary variation in the American lobster Homarus americanus. J Fish Res Bd Can 30 1647–1653
D.A. Jones M. Kumlu L. Le Vay D.J. Fletcher (1997) ArticleTitleThe digestive physiology of herbivorous, omnivorous and carnivorous crustacean larvae: a review. Aquaculture 155 285–295 Occurrence Handle10.1016/S0044-8486(97)00129-4
U.K. Laemmli (1970) ArticleTitleCleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227 680–685 Occurrence Handle5432063
P.G. Lee L.L. Smith A.L. Lawrence (1984) ArticleTitleDigestive proteases of Penaeus vannamei Boone: relationship between enzyme activity, size and diet. Aquaculture 42 225–239 Occurrence Handle10.1016/0044-8486(84)90103-0 Occurrence Handle1:CAS:528:DyaL2MXhtlSrur8%3D
D. Lemos M.P. Hernández-Cortés A. Navarrete F.L. García-Carreño V.N. Phan (1999) ArticleTitleOntogenetic variation in digestive proteinase activity of larvae and postlarvae of the pink shrimp Farfantapenaeus paulensis (Crustacea: Decapoda: Penaeidae). Mar Biol 135 653–662 Occurrence Handle10.1007/s002270050666 Occurrence Handle1:CAS:528:DC%2BD3cXnsFymtg%3D%3D
G. Le Moullac A.V. Wormhoudt ParticleVan . AQUACOP (1994) ArticleTitleAdaptation of digestive enzymes to dietary protein, carbohydrate and fibre levels and influence of protein and carbohydrate quality in Penaeus vannamei larvae (Crustacea, Decapoda). Aquat Living Resour 7 203–210
I.D. McCarthy D.F. Houlihan C.G. Carter (1994) ArticleTitleIndividual variation in protein turnover and growth efficiency in rainbow trout, Oncorhynchus mykiss (Walbaum). Proceed R Soc Lond Series B 257 141–147 Occurrence Handle1:CAS:528:DyaK2cXmt1KrtL8%3D
A. Muhlia-Almazán F.L. García-Carreño (2002) ArticleTitleInfluence of molting and starvation on the synthesis of proteolytic enzymes in the midgut gland of the white shrimp Penaeus vannamei. Comp Biochem Physiol 133 IssueID3 383–394
A. Rodriguez L. Le Vay G. Mourente D.A. Jones (1994) ArticleTitleBiochemical composition and digestive enzyme activity in larvae and postlarvae of Penaeus japonicus during herbivorous and carnivorous feeding. Mar Biol 118 45–51 Occurrence Handle1:CAS:528:DyaK2cXhtValurg%3D
H. Salem A. Youssef A. El-Nakkadi M. Bekeit (1970) ArticleTitleProteolytic decomposition of shellfish muscle proteins under different conditions. J Agricult Res 18 61–66 Occurrence Handle1:CAS:528:DyaE3MXktVCit7o%3D
I. Sakharov E. Litvin O. Mitkevich G. Samokhin Z. Bespalova (1994) ArticleTitleSubstrate specificity of collagenolytic proteases from the king crab Paralithodes camtschatica. Comp Biochem Physiol 107B 411–417 Occurrence Handle1:CAS:528:DyaK2cXkslKqsb4%3D
S.Y. Shiau (1998) ArticleTitleNutrient requirements of penaeid shrimps. Aquaculture 164 77–93 Occurrence Handle10.1016/S0044-8486(98)00178-1
C. Stauffer (1989) Effect of pH on activity Enzyme Assay for Food Scientists AVI Press New York NY
I.H. Tsai P.J. Lu J.L. Chuang (1991) ArticleTitleThe midgut chymotrypsins of shrimps Penaeus monodon, P. japonicus, P. penicillatus. Biochim Biophysi Acta 1080 59–67 Occurrence Handle10.1016/0167-4838(91)90112-D Occurrence Handle1:CAS:528:DyaK38XlsVWksg%3D%3D
Acknowledgments
The authors thank Cinthya Aldana for technical support and Consejo Nacional de Ciencia y Technologia of Mexico for funding the project (CONACYT grants 28257-B and E130) given to F.L.G.C., and for scholarship to L.C.G. Suggestions from Dr. Manuel Díaz, UAL, Spain, are appreciated. CIBNOR editing staff kindly reviewed the language. Experiments using organisms comply with the current laws of Mexico.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Celis-Guerrero, L.E., García-Carreño, F.L. & del Toro, M.A.N. Characterization of Proteases in the Digestive System of Spiny Lobster (Panulirus interruptus). Mar. Biotechnol. 6, 262–269 (2004). https://doi.org/10.1007/s10126-003-0032-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10126-003-0032-6