Abstract
Guppy is a popular ornamental fish owing to its diverse body and fin coloration. More than 40 established color varieties have been selectively bred. The complementary DNAs for 2 enzymes that are involved in the de novo synthesis of pteridines and purines, which are important for the production of color pigments, were cloned from the caudal fin. Two cDNA isoforms for 6-pyruvoyl tetrahydropterin synthase (PTPS), with an open reading frame of 130 and 147 amino acids, respectively, were cloned from the Red Tail variety. The deduced amino acid sequence of the longer isoform shows an overall identity of about 65% to the mammalian PTPS sequences. The cDNA for xanthine dehydrogenase (XDH) was cloned from the Yellow Tail variety, and consists of an open reading frame of 1331 amino acids. Although it shows a higher overall identity to bovine aldehyde oxidase (AO; 54%) than to chicken XDH (51%), it has a NAD-binding domain that is specific to XDHs. Northern blot analysis indicated that both PTPS and XDH messenger RNAs were highly expressed in the liver, but absent in the muscle. In the caudal fins, guppy varieties with a higher proportion of xanthophores and erythrophores showed higher expression of PTPS, while XDH mRNA levels were too low to indicate obvious differential expression among the color guppy varieties. The results implied that high expression of PTPS is correlated with the biosynthesis of pteridines in the erythrophores and xanthophores, while the association between the putative guppy XDH with specific chromatophores is less clear.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The spectacular coloration of poikilothermal vertebrates plays a vital role in their survival. Bright coloration protects animals from aggressive predators, affects sexual behavior, or assists visual communication in a group (Needham, 1974; Fox, 1976; Fujii, 1993; Houde, 1997). Poikilothermal vertebrates have 5 types of chromatophores, blackish melanophores, reddish erythrophores, yellowish xanthophores, as well as iridescent leucophores and iridophores (Bagnara and Hadley, 1973). These distinct chromatophores could either enclose a specific predominant pigment, or each individual pigment cell could contain several different types of pigments. Carotenoids, for instance, are a critical group of pigment components that are responsible for the conspicuous yellow, orange, and red colors, and are found predominantly in erythrophores and xanthophores, while iridescent purine crystals of uric acid, guanine, xanthine, adenine, and hypoxanthine reside in leucophores and iridophores, giving rise to metallic and silvery coloration (Bagnara and Hadley, 1973; Fujii, 1993; Oliphant and Hudon, 1993). Most animals, however, derive the carotenoids from their diet, as they have lost the ability to synthesize them de novo (Goodwin, 1984; Schiedt, 1980). Interestingly, numerous animal taxa have evolved the ability to synthesize yellow, orange, and red pteridines from purines, as alternative color pigment substitutes to the carotenoids (Bagnara and Hadley, 1973; Henze et al., 1977; Fujii, 1993; Oliphant and Hudon, 1993).
5,6,7,8-Tetrahydrobiopterin (BH4) functions as a critical cofactor for aromatic amino acid hydroxylases and nitric oxide synthase (Thony et al., 2000). BH4 is also involved in neurotransmitter biosynthesis, immunity control, antioxidation, and melanogenesis in mammals and other vertebrates (Harada et al., 1993; Schallreuter et al., 1994a, 1994b; Thony et al., 2000). In addition, the de novo biosynthetic pathway of BH4 has been associated with the synthesis of pteridine pigments in the goldfish and zebrafish (Matsumoto, 1965; Masada et al., 1990; Ziegler et al., 2000; Guyader and Jesuthasan, 2002). Three enzymes are involved in this pathway, GTP cyclohydrolase I (EC 3.5.4.16; GCH), 6-pyruvoyl tetrahydropterin synthase (EC 4.6.1.10; PTPS), and sepiapterin reductase (EC 1.1.1.153; SR). They catalyze the conversion of GTP to dihydroneopterin triphosphate, dihydroneopterin triphosphate to 6-pyruvoyl tetrahydropterin, and 6-pyruvoyl tetrahydropterin to BH4, respectively (Thony et al., 2000). Of the 3, GCH and PTPS are the rate-limiting enzymes (Werner et al., 1990; Hatakeyama et al., 1992). The enzymatic activities of both GCH and PTPS were found to be significantly higher in the erythrophores than in the fibroblast-like cells in goldfish (Masada et al., 1990).
Xanthine dehydrogenase (EC 1.1.204; XDH) catalyzes the conversion of hypoxanthine and xanthine to xanthine and uric acid, respectively (Parks and Granger, 1986). It also participates in the catabolic pathway of BH4, including the conversion of 7,8-dihydropterin to 7,8-dihydroxanthopterin, xanthopterin to leucopterin, and pterin to isoxanthopterin and drosopterin (Hille and Nishino, 1995; Blau et al., 1996; Ziegler et al., 2000). In poikilothermal vertebrates and insects, XDH also controls the synthesis of pigments for metallic and yellow or orange colorations (Oliphant and Hudon, 1993). For example, the chemical inhibition of XDH by allopurinol changed the wild-type axolotl to a melanoid mutant by reducing the number of xanthophores and iridophores (Frost and Bagnara, 1979). In Drosophila, XDH deficiency gives rise to brownish-colored eyes, while wild-type flies with normal XDH levels have red eyes (Reaume et al., 1991). A deficiency of XDH in a silkworm mutant makes the larva body translucent compared to the silvery wild-type (Yasukochi et al., 1998).
In our study of genes involved in bright pigmentation in poikilothermal vertebrates, a tropical ornamental fish, guppy (Poecilia reticulata), was used as the animal model. Male guppies have brilliant and intense coloration on their bodies and caudal fins, while females have gray color and dull patterns. The diverse color patterns of domesticated color varieties of guppy are determined chiefly by sex chromosomal X- and Y-linked genes (Phang et al., 1985, 1989; Khoo et al., 1999a, 1999b, 1999c, 2003). We are interested in the development of color patterns, which is a complex process of chromatophore differentiation, migration, and pigmentation. Pigment biosynthesis is the final downstream step within this process. The cloning of PTPS and XDH cDNA from guppy and their expression levels in the caudal fins of various domesticated color varieties of guppy are reported here. Our eventual goal is to study the expression of all the enzymes involved in the pteridine biosynthesis and metabolism in the guppy. Our preliminary study concentrates on PTPS, which is the main rate-limiting enzyme in the pteridine biosynthesis pathway (Thony et al., 2000), and XDH, which is largely responsible for the catabolism of 7,8-dihydropterin to pigmented pteridines, including isoxanthopterin, xanthopterin, and leucopterin (Oliphant and Hudon, 1993; Hille and Nishino, 1995; Ziegler et al., 2000).
MATERIALS AND METHODS
Domesticated Guppy Varieties
Twelve different color varieties of guppy were used. The pigmentation phenotypes of adult males and the dominant chromatophores present in the caudal fins are summarized in Table 1. The adult male and female individuals utilized were 2 to 3 months old. Juveniles used were 2 weeks old. They were purchased from several fish farms in Singapore.
The dominant types of chromatophore present in the color varieties were determined as described (Chan and Phang, 1990; Khoo et al., 1992). Scales (approx. 10 to 20) from the tail fin of the various color varieties were detached using fine forceps and individually mounted on glass slides in teleost physiological saline, TPS (6.5 g NaCl, 0.4 g KCl, 0.15 g CaCl2 · 2H2O, and 0.15 g MgSO4 · 7H2O in 1 L of distilled water, pH 7.3). Similarly, sections of tail fin (approx. 5 to 10) were removed and mounted in TPS. These whole mounts were examined at 100 to 200 times magnification under an Olympus BH2 light microscope. Since the color patterns of the guppy color varieties are extremely complex and highly variable among individuals, we decided to use a ranking scheme. The chromatophores were ranked as dominant when their numbers exceeded 60% for each of the whole mount specimens studied.
Total RNA Isolation and RT-PCR
Tissue samples were removed from freshly killed fish, and total RNA was extracted with Trizol reagent (Life Technologies). The RNA samples were then treated with DNase I (GenHunter) to remove genomic DNA contamination. First-strand cDNAs were synthesized with either oligo(dT) or gene-specific primers and MMLV reverse transcriptase (RT, GenHunter). Subsequent polymerase chain reaction (PCR) was carried out with a volume of 50 µl. The first-strand cDNA templates, obtained from 40 to 50 ng of total RNA, were mixed with PCR buffer, 0.2 mM of dNTP mix, and 1.25 U of Taq polymerase (Qiagen). After the mixture was heated to 85°C on a thermal cycler, 25 pmol of each of the primers was added. Thirty-five amplification cycles, with denaturation at 94°C for 30 seconds, annealing ranging from 48° to 60°C (depending on the PCR primer pairs) for 30 seconds, and extension at 72°C for 180 seconds, were carried out using a thermal cycler. A final 10-minute extension step at 72°C was also included.
Cloning of the PTPS and XDH cDNAs by RT-PCR
Total RNA from the Red Tail guppy variety was used to clone the PTPS cDNA. First-strand cDNA synthesis was carried out with a gene-specific primer, PTPS-1 (5′-ACDATRTTYTTGTCRGTTTC-3′). Degenerate PCR primer designs were based on the alignment of known PTPS gene sequences from human (D17400), mouse (AF043225), rat (M77850), and salmon (Hauer et al., 1992). The initial PCR reaction was carried out with PTPS-1 and PTPS-2 (5′-GCGCANGYCAYCGNYTGCA-3′), and followed by a second round of heminested PCR, using PTPS-1 and PTPS-3 (5′-CCYCTTGATCAYAAGAAYCTG-3′) as PCR primers. Based on the sequence of this 180-bp fragment, a gene-specific reverse primer, PTPS-4 (5′-GTGAATCCGGATCTCGTAGGG-3′) was designed and used with PTPS-2 ta obtain a 338-bp cDNA fragment.
The XDH cDNA was cloned using total RNA from the Yellow Tail variety. First-strand cDNA synthesis was carried using the primer XDH-1 (5′-AAHCCSGTDGCNGAYAARCT-3′). The degenerate primer designs were based on known sequences of XDH genes from human (NM_000379), mouse (X62932), bovine (X98491), chicken (P47990), zebrafish (AI657925, AI657926), and Drosophila (P22811). Based on an initial 300-bp fragment obtained using XDH-1 and XDH-2 (5′-CAYGGDGGNACWGAGATGGGYCAAGG-3′), a gene-specific reverse primer, XDH-3 (5′-GATCTTCTGCATATGAGCCTCCTTGA-3′), was designed and used with the degenerate primer XDH-4 (5′-GGTGGMCARGASCAYTTCTAYCTGGA-3′) to amplify a 1167-bp DNA fragment.
Full-length cDNAs were isolated using the SMART RACE cDNA amplification kit (BD Biosciences Clontech). The 5′ and 3′ cDNA ends of PTPS were isolated using the primer pairs, PTPS-5 (5′-TCACCTCCACCTTGTAGTTGTG- TCCATG-3′) with NUP (5′-AAGCAGTGGTAACAACGCAGA-3′), and PTPS-6 (5′-ATAAGGACGTTCCGTACTTTG-3′) with Anchor T25VN primer, respectively. The XDH-specific primers used were XDH-5 (5′-CACAGCGAACAGCTCGGTTGGTTTTCC-3′) for cloning the 5′ cDNA end, and XDH-6 (5′-CGACGTCAAAGATCTTCCTCAGTG-3′) for the 3′ cDNA end.
Cloning and Sequence Analysis
The PCR products of interest were recovered from agarose gel with the QIAEXII kit (Qiagen). The extracted PCR products were then cloned into pGEM-T (Promega). The ligated products were used to transform competent cells prepared from the E. coli strain DH10B. The plasmids were prepared with the QIAprep Spin Miniprep Kit (Qiagen). The insert size was determined by restriction endonuclease digestion. The insert sequence was determined using the ABI PRISM Dye Terminator Cycle Sequencing Ready Reaction Kit and ABI Prism 377 DNA Sequencer (PerkinElmer). Homology search was performed using Basic Local Alignment Search tool (BLAST) (available at http://www.ncbi.nlm.nih.gov ). The PTPS and XDH amino acid sequences were aligned using CLUSTAL X (Version 1.8.3). Maximum parsimony (MP) analysis was performed using PAUP 4.0b10 (Swofford, 1998).
Northern Blot Analysis
Thirty micrograms of total RNA was denatured at 50°C for 30 to 60 minutes with 15 µl of glyoxal/DMSO. The denatured RNA was separated on 1.2% phosphate agarose gel. After treatment with 0.05 M of NaOH for 20 minutes, the separated RNAs were transferred to a positively charged nylon membrane and cross-linked onto the membrane with a UV crosslinker (XL-1500, Spectronics Corporation) using a radiation dose of 1.2 × 105 µJ/cm2. The membranes were prehybridized in 10 ml of ExpressHyb hybridization buffer (BD Biosciences Clontech) for 3 hours, and then hybridized with the [32P]-labeled DNA probes at a concentration of l06 cpm/ml for 2 hours at 65°C. The PTPS and XDH cDNA probes were randomly labeled with 32P-dCTP using the Rediprime II kit (Amersham Pharmacia Biotech). The labeled DNA probes were then purified with the QIAquick nucleotide removal kit (Qiagen). After washing according to the manufacturer’s instructions, the membrane was then exposed to BioMax film β (Kodak).
RESULTS
Molecular Cloning of PTPS cDNA from Red Tail Guppy Variety
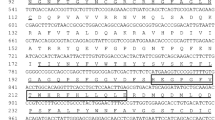
The complete nucleotide and deduced amino acid sequence for the putative guppy PTPS is shown in Figure 1(A). Two cDNA isoforms (AY034101 and AY034102) were cloned from the Red Tail guppy variety using RT-PCR and total RNA prepared from the caudual fin tissues. Isoform 1 contains a 68-bp 5′ untranslated region (UTR), a 301-bp 3′ UTR, and a 444-bp open reading frame (ORF) encoding a 147 amino acid polypeptide. The amino acid sequence of the Red Tail guppy PTPS isoform 1 is about 80% identical to the other known mammalian PTPS genes. In contrast, the shorter guppy PTPS isoform 2, which is deduced based on a 307-bp fragment amplified using PTPS-2 and PTPS-4 as the PCR primers, has a 39-bp nonconserved region and a 30-bp deletion within the ORF. The nonconserved region of PTPS isoform 2 would have altered the catalytic triad motif and renders it nonfunctional. The first 20 amino acid residues at the NH2 terminus of PTPS are highly variable, but the other portions of the protein are conserved among vertebrates such as human, mouse, rat, and guppy, and Drosophila (Figure 1B,). Besides the 27 amino acid extension, the Drosophila PTPS also differs from the vertebrate PTPS at the COOH terminus. All 4 four functional motifs specific for the PTPS can be observed in the guppy PTPS isoform 1.
A: The complete nucleotide and deduced amino acid sequences of 2 PTPS isoforms in Red Tail guppy variety. The start and stop codons are in bold. The polyadenylation signal is underlined. The 5′ UTR and 3′ UTR are in italics. A 39-bp nonconserved region and a 30-bp deletion in the ORF between the 2 isoforms are boxed, and isoform 2 is indicated inbold. The putative polyadenylation site is underlined. B: Multiple alignment of known vertebrate PTPS protein sequences. S21 is phosphorylated. The histidine residues, marked with an asterisk, are the binding sites for Zn2+-ion. Threonines marked with “♦” are the acceptor site for the pyrimidine ring of the substrate. The aminoacids marked with “▼” are catalytic triad motif. C: A single MP gene tree indicating the relationship of PTPS among different mammals and guppy PTPS isoform 1 was obtatined. The Drosophila PTPS is included as the outgroup. Bootstrap percentages are given (1000 replicates) above the branches.
PTPS mRNA Expression in Caudal Fins of Different Color Varieties of Guppy
Different guppy varieties have different predominant types of chromatophores on their caudal fins: for instance, xanthophores in the Yellow Tail, melanophores in the 3/4 Black Tail, and erythrophores in the Red Tail (Table 1). The remaining color varieties have a different combination of chromatophores. To elucidate the potential association between the PTPS mRNA expression and pigment cell types in the caudal fins of guppy, the PTPS mRNA levels of various guppy varieties were compared using Northern blot (Figure 2A,). A single predominant and highly expressed PTPS mRNA species of approximately l kb was observed.
A: Northern hybridization analysis of PTPS mRNA levels of caudal fins of 12 guppy color varieties. B: Northern hybridization analysisof guppy PTPS mRNAs in the liver, muscle, and caudal fin. Thirty micrograms of total RNA was separated on 1.2% agarose gel and transferred onto nylon membrane. Hybridization was carried out with [32P-dCTP]-labeled PTPS cDNA probe at high stringency. The 18S RNA profile of the total RNA samples prior to the Northern blotting is included as loading control. DS, Doublesword.
Among the 12 different guppy varieties that were included in the Northern blot analysis, Yellow Tail, Leopard, and Yellow Snakeskin contain xanthophores in decreasing density in the caudal fin, and the PTPS mRNA levels were found to decrease in the same order. Red Tail, Red Snakeskin, and Red Metallic represent guppy varieties with predominantly erythrophores, and PTPS mRNA levels were highly expressed among them. Purple Tail, Diamond, and Blue Metallic have silvery iridescent caudal fins resulting mainly from the presence of iridophores. Purple Tail looks more yellowish than Diamond and Blue Metallic, while Blue Metallic has deeper blue iridescence than Diamond. The PTPS mRNA levels were moderately expressed in these guppy varieties and appeared to be related to the density of the xanthophores. The lowest levels of PTPS mRNA were found in the Blue Tail and 3/4 Black Tail varieties, in which melanophores are abundant.
Adult females and juveniles of the Doublesword variety, which lack pigment cells on their caudal fins, were used as negative controls. PTPS mRNAs were expressed at very low levels. In constrast, the male Doublesword contains predominantly xanthophores and iridophores in the caudal fins. We were able to demonstrate that the PTPS mRNAs were very weakly expressed in the female and juvenile Doublesword but highly expressed in the male. Thus, the PTPS mRNA level appeared to be proportional to the density of xanthophores and erythrophores in the caudal fins of the various guppy varieties investigated.
Northern Blot Analysis of PTPS mRNA in Specific Tissues of Guppy Varieties
In rat, PTPS mRNA is highly expressed in the liver but has little or no expression in muscle (Hirayama and Kapatos, 1995). We wanted to determine if the expression level of PTPS in liver follows the expression level as previously established for the caudal fins. Five varieties were selected, Yellow Tail, Red Tail, 3/4 Black Tail, Blue Metallic, and adult colorless female Doublesword. As shown in Figure 2(B), PTPS mRNA levels in the caudal fins of the 5 varieties were consistent with the results we obtained earlier. As expected, no signals were detected in the muscle tissues. However, the liver PTPS mRNA levels among the 5 guppy varieties were similar. Thus, the relationship established for the PTPS mRNA levels and xanthophore and erythrophore density in the caudal fin does not extend to the liver.
Molecular Cloning of XDH Gene from Yellow Tail Guppy Variety
The complete nucleotide sequence of the putative guppy XDH (AY034103), cloned from Yellow Tail guppy, has been determined. The full-length guppy XDH cDNA is 4657 bp long and contains a 25-bp 5′ UTR, a 612-bp 3′ UTR, and an ORF of 1331 amino acids. The putative guppy XDH amino acid sequence is 54% identical to the bovine aldehyde oxidase (AO) and 51% to the chicken XDH. AO and XDH belong to a small family of molybdoflavoproteins. Both utilize a flavin besides the molybdenum cofactor to oxidize their respective substrates. Structurally, the putative guppy XDH shares resemblances with both AO and XDH. The NH2 terminus of the guppy XDH contains only the 2Fe-2S domain and shows greater homology to members of AO family, while its COOH terminus is more similar to the XDH family. Phylogenetic analysis by the MP approach was carried out using known XDH and AO genes. As shown in Figure 3(A), the putative guppy XDH appears to be more related to members of the AO family.
Phylogenetic analysis and alignment of functional domains of XDH and AO. A: A single MP phylogenetic tree is generated using various XDH and AO amino acids sequences. Only bootstrap percentages that give less than 100% support are given above the branches (1000 replicates). GenBank accession numbers of XDHs: human (hXDH), NP_000370; mouse (mXDH), CAA44705; rat (rXDH), NP_058850; bovine (bXDH), P80457; chicken (cXDH), P47990; guppy (PrXDH), AAK59699; Drosophila (DmXDH), P22811; and Calliphora (CvXDH), P08793. GenBank accession numbers of AOs: human (hAO), Q06278; mouse (mAO), AAF98385; rat (rAO), NP_062236; and bovine (bAO), P48034. The 2 AO and XDH sequences used as outgroups come from Culex (CpXDH), AAF87601 and Arabidopsis (AtAO), NP_180283. B: Alignment of the FAD+ binding domain. C: Alignment of the molybdopterin cofactor binding domain.
The alignment analysis of XDH and AO indicates a common distribution of 13 conserved cysteines at the N-terminal fragment (data not shown). This cysteine-rich N-terminal region of XDH was proposed to consist of 2 iron-sulfur binding centers, the 2Fe/2S domain (Amaya et al., 1990; Thoenes et al., 1994; Sato et al., 1995; Terao et al., 1997). Moreover, a conserved XDH-specific NAD+-binding domain, FFIGFGKTILKPE 406, that is absent in all AOs, is observed in the guppy XDH (Figure 3B,). Since NAD+ could react with FAD, the FAD center was proposed around the NAD+-associated region (Schopfer et al., 1988; Thoenes et al., 1994; Sato et al., 1995). The molybdopterin cofactor binding domain (Figure 3C,) is located at the C-terminal polypeptide chain (Thoenes et al., 1994; Xu et al., 1994; Terao et al., 1997).
Northern Blot Assay of XDH mRNA in Specific Tissues of Guppy Varieties
In bovine, XDH mRNA is expressed in the liver, but not in the muscle, while AO expression is high in both liver and muscle (Terao et al., 1997). The XDH expression levels in liver, muscle, and caudal fin of 3 guppy varieties, Yellow Tail, 3/4 Black Tail, and Blue Metallic, were compared by Northern blot at high stringency. As shown in Figure 4(A), the putative XDH mRNAs were highly expressed in the liver tissues of all 3 varieties. No detectable XDH signal was found in their muscle tissues, while in the caudal fins, XDH levels were weak. There was, however, a difference in the size of the dominant XDH mRNA species in the liver (4.5 kb) compared with that in the caudal fin (4.9 kb). Interestingly, the expression levels of XDH in liver and caudal fin appeared not to be correlated.
A: Northern hybridization for guppy XDH mRNA in liver, muscle, and caudal fin of 3 guppy varieties. Thirty micrograms of total RNA was separated on 1.2% agarose gel and transferred onto nylon membrane and hybridized with 32P-labeled guppy XDH cDNA. The 2.37-kb and 4.40-kb RNA markers are indicated. The 4.9-kb XDH mRNA species expressed in the caudal fins is indicated by an arrow. The 18S RNA profile of the total RNA samples prior to the Northern blotting is included as loading control. B: Northern analysis of guppyXDH mRNA in 12 guppy varieties. The 4.4-kb RNA marker is shown on the left.
XDH Expression in Caudal Fins of Different Color Varieties of Guppy
Northern blot analysis indicated that XDH expression was considerably lower than PTPS expression in the guppy caudal fins (Figure 4B,). Like PTPS, the XDH mRNAs appeared to be differentially expressed among the 12 varieties. The caudal fins of Purple Tail and Diamond had higher XDH expression than did Blue Metallic, Blue Tail, and 3/4 Black Tail. Among these varieties, Purple Tail and Diamond have a higher density of iridophores. Among the predominantly red guppy varieties, Red Metallic had the highest level of XDH expression, and again it has the highest density of iridophores. The XDH level in Yellow Tail was higher than that in Leopard and Yellow Snakeskin, although Yellow Snakeskin is the only yellow variety that has a reasonably high density of iridophores. Yellow Tail has the highest density of xanthophores, and the XDH level in the yellow varieties is likely to be correlated to the density of xanthophores rather than iridophores. Finally, the XDH expression in male Doublesword, which has a high density of iridophores, was higher than in juvenile and female Doubleswords. In conclusion, caudal fin XDH mRNA levels appear to be higher in guppy varieties that have a higher density of iridophores, with the exception of the Yellow Snakeskin.
DISCUSSION
The finding that PTPS mRNA expression is proportional to the density of xanthophores and erythrophores on the guppy is in line with a study on goldfish (Masada et al., 1990). In this particular study, the activities of GCH and PTPS were found to be significantly higher in erythrophores than in melanophores, and they were barely detectable in nonpigment cells such as fibroblast-like cells. These results implied that there might be significant production of sepiapterin and other pteridines in yellow-red chromatophores of fish relative to other pigment and nonpigment cells. Our results provide evidence to support the hypothesis that a de novo BH4 biosynthetic pathway is involved in pteridine biosynthesis in xanthophores and erythrophores. Since the PTPS expression level in Blue Metallic is similar to that in the colorless female Doublesword, the association of PTPS mRNA levels with iridophores is not evident. The PTPS expression in 3/4 Black and Blue Tail is, however, significantly higher than in the colorless female Doublesword.
Three possibilities could explain this result. First, 3/4 Black Tail and Blue Tail have xanthophores and erythrophores in the caudal fins but are masked by a thick layer of melanophores. Second, de novo synthesized pteridines could accumulate in the melanophores as a less abundant pigment. This is consistent with the study by Masada et al. (1990), which showed that melanogenesis in goldfish was accompanied by pterinogenesis. Third, BH4 functions as a critical cofactor of phenylalanine hydroxylase, which converts L-phenylalanine to L-tyrosine (Schallreuter et al., 1994a, 1994b), a precursor of melanin synthesis. Thus, PTPS expression in melanophores is necessary to supply a regulatory cofactor for melanogenesis. The homogeneous expression of PTPS in livers, in contrast to its differential expression in caudal fins of guppy varieties, implies that the high expression of PTPS in liver may not be associated with the pigmentation patterns of the caudal fins. It also further suggests that pteridine biosynthesis in xanthophores and erythrophores is not related to that in the liver.
The homology analysis indicates that the putative guppy XDH is only slightly more similar to AO (54%) compared to XDH (51%), making a firm conclusion difficult. AO and XDH belong to the same group of molybdenum-containing hydroxylases, which are involved in the reaction: RH + H2O → ROH + 2e− + 2H+. Functional enzymes are dimers comprising 2 identical subunits, each of which has 2 Fe-S redox centers, 1 FAD center, and 1 molybdopterin cofactor center (Hille and Nishino, 1995). Aldehyde oxidase oxidizes the N-heterocyclic compounds. Its exact physiological functions are not known. The mRNA tissue profile of AO is different from that of XDH. The mRNA of AO is high in the striated muscle and duodenum, but XDH mRNA is absent from both (Terao et al., 1997). It implies that AO has different physiological functions from XDH.
The primary reaction difference between oxidase and dehydrogenase is that NAD+ is the electron acceptor of the dehydrogenase. XDH has a specific amino acid domain for NAD binding that does not exist in AO (Figure 4C,). In the XDH of mammals and chicken, the motif is Phe-X-Gly-Tyr*-Arg-(Lys or Arg)-Thr-(Leu or Ile)-X-X-Pro-Glu (Amaya et al., 1990; Sato et al., 1995; Terao et al., 1997). Tyr* was proposed to be responsible for NAD binding through a hydrogen bond (Nishino and Nishino, 1987, 1989). The corresponding location in AO is Leu-(Ser or Arg)-(Arg or Lys)-Cys*-Pro-X-(Ala or Ser)-Asp-Leo-Lys-Pro-Gln. The putative XDH gene we isolated has a motif of Phe-Ile-Gly-Phe*-Gly-Lys-Thr-Ile-Leu-Lys-Pro-Glu, which is quite similar to a NAD-binding domain. At the proposed NAD-binding site, Tyr* is replaced by Phe* in the putative XDH gene of guppy. A point mutation might have occurred at the proposed NAD-binding site, converting Tyr (UAU C) to Phe (UUU C). Whether this mutation has disrupted its NAD-binding function remains to be established. This speculation is supported by the Northern result showing that the putative XDH gene is highly expressed in liver but absent in muscle. However, there is another possible explanation for this domain. As indicated by phylogenetic tree analysis, this may be an AO gene with a NAD-binding domain. The final classification for the putative guppy XDH as either XDH or AO must await further enzymatic analysis.
XDH is involved in the biosynthesis of certain pterins and purines. Isoxanthopterin and xanthopterin are colorless pigments present in the xanthophores of medaka (Hama, 1975), while isoxanthopterin is a yellow pigment in isopods (Nakagoshi et al., 1992) and xanthopterin is a yellow pigment in zebrafish (Guyader and Jesuthasan, 2002). The leucopterin and 7,8-dihydroxanthopterin are reflective pigments in gizzard shad and percids (Oliphant and Hudon, 1993). In wild-type axolotl, the inhibition of XDH with allopurinol induced the reduction of xanthopterin, isoxanthopterin, biopterin, and sepiapterin in the xanthophores. Furthermore, they showed a significant reduction in xanthophores and iridophores, while melanogenesis was enhanced (Frost and Bagnara, 1979; Thorsteinsdottir and Frost, 1986). In zebrafish, there is a peridine pathway associated with xanthophore pigments (Ziegler et al., 2000). An alternative XDH pathway, which catalyzes 7-oxobiopterin synthesis from 7,8-dihydrobiopterin and biopterin from the precursor sepiapterin, has also been established.
The observation that the inhibition of XDH activity enhanced melanin synthesis in conjunction with the reduction of xanthophores and iridophores in axolotl suggests that the down-regulation of pigmentation in these chromatophores is accompanied by the up-regulation of melanogenesis. This linkage was also observed in the zebrafish mutant nacre, in which the gene encoding the microphthalmia transcription factor carries a null mutation. The mutant has a body pattern of 40% more iridophores and an absence of melanophores (Lister et al., 1999). In the guppy, our Northern blot analysis indicated different signal intensities in caudal fins, but the expression levels were too low in some varieties to be meaningfully compared. Guppy varieties that lack iridophores consistently show lower levels of XDH expression. In contrast, it appears that XDH is more highly expressed in guppy varieties that have a high proportion of iridophores. However, this association is not consistent, particularly in the Red and Yellow Snakeskin, and Blue Metallic guppy varieties, in which the XDH expression levels remain low despite the presence of substantive iridophores.
With the 2 full-length PTPS and XDH cDNAs, and ongoing efforts to clone the sepiaterin reductase and GTP cyclohydrolase I from the guppy, future experiments could be carried out to determine the detailed expression pattern of these genes in juvenile guppies during the prepigmentation and postpigmentation stages. Functional analyses of gene products as well as the transcriptional regulation of the pteridine biosynthetic pathway will also be elucidated.
References
Y. Amaya K. Yamazaki M. Sato K. Noda T. Nishino T. Nishino (1990) ArticleTitleProteolytic conversion of xanthine dehydrogenase from the NAD-dependent type to the O2-dependent type. Amino acid sequence of rat liver xanthine dehydrogenase and identification of the cleavage sites of the enzyme protein during irreversible conversion by trypsin. J Biol Chem 265 14170–14175 Occurrence Handle1:CAS:528:DyaK3MXitVSksA%3D%3D Occurrence Handle2387845
J.T. Bagnara M.E. Hadley (1973) The nature of pigmentation. Chromatophores and Color Change, the Comparative Physiology of Animal Pigmentation. Prentice-Hall Press Englewood Cliffs, N.J. 4–45
N. Blau J.B. de Klerk B. Thony C.W. Heizmann L. Kierat J.A. Smeitink M. Duran (1996) ArticleTitleTetrahydrobiopterin loading test in xanthine dehydrogenase and molybdenum cofactor deficiencies. Biochem Mol Med 58 199–203 Occurrence Handle10.1006/bmme.1996.0049 Occurrence Handle1:CAS:528:DyaK28XltVKqsLc%3D Occurrence Handle8812740
S.Y. Chan V.P.E. Phang (1990) Pigment patterns in six color varieties of the platy, Xiphophorus maculatus. R. Hirano I. Hanyu (Eds) The Second Asian Fisheries Forum. Asian Fisheries Society Manila, Philippines 483–489
D.L. Fox (1976) Animal Biochromes and Structural Colors, 2nd ed. Berkeley University of California Press
S.K. Frost J.T. Bagnara (1979) ArticleTitleAllopurinol-induced melanism in the tiger salamander (Ambystoma tigrinum nebulosum). J Exp Zool 209 455–466 Occurrence Handle1:CAS:528:DyaL3cXjsVKn Occurrence Handle490138
R. Fujii (1993) ArticleTitleCytophysiology of fish chromatophores. Int Rev Cytol 143 191–253 Occurrence Handle1:CAS:528:DyaK3sXks1yns7Y%3D
T.W. Goodwin (1984) The Biochemistry of the Carotenoids. Chapman and Hall London, U.K.
S.L. Guyader S. Jesuthasan (2002) ArticleTitleAnalysis of xanthophore and peterinosome biogenesis in zebrafish using methylene blue and pteridine autofluorescence. Pigm Cell Res 15 27–31 Occurrence Handle10.1034/j.1600-0749.2002.00045.x
T. Hama (1975) Chromatophores and iridocytes. T. Yamamoto (Eds) Medaka (Killifish): Biology and Strains. Keigaku Pub. Co. Tokyo, Japan 138–153
T. Harada H. Kagamiyama K. Hatakeyama (1993) ArticleTitleFeedback regulation mechanisms for the control of GTP cyclohydrolase I activity. Science 260 1507–1510 Occurrence Handle1:CAS:528:DyaK3sXkt1ent7k%3D Occurrence Handle8502995
K. Hatakeyama T. Harada H. Kagamiyama (1992) ArticleTitleIMP dehydrogenase inhibitors reduce intracellular tetrahydrobiopterin levels through reduction of intracellular GTP levels: indications of the regulation of GTP cyclohydrolase I activity by restriction of GTP availability in the cells. J Biol Chem 267 20734–20739 Occurrence Handle1:CAS:528:DyaK38XlsVOktLc%3D Occurrence Handle1356983
C.R. Hauer W. Leimbacher P. Hunziker F. Neuheiser N. Blau C.W. Heizmann (1992) ArticleTitle6-Pyruvoyl tetrahydropterin synthase from salmon liver: amino acid sequence analysis by tandem mass spectrometry. Biochem Biophys Res Commun 182 953–959 Occurrence Handle1:CAS:528:DyaK38XhvFSisr0%3D Occurrence Handle1734893
M. Henze G. Rempeters F. Anders (1977) ArticleTitlePteridines in the skin of xiphohorine fish (Poeciliidae). Comp Biochem Physiol B 56 35–46 Occurrence Handle10.1016/0305-0491(77)90219-X Occurrence Handle1:CAS:528:DyaE2sXhtVegs7g%3D Occurrence Handle830468
R. Hille T. Nishino (1995) ArticleTitleXanthine oxidase and xanthine dehydrogenase. FASEB J 9 995–1103 Occurrence Handle1:CAS:528:DyaK2MXns1Smsbk%3D Occurrence Handle7649415
K. Hirayama G. Kapatos (1995) ArticleTitleExpression and regulation of rat 6-pyruvoyl tetrahydropterin synthase mRNA. Neurochem Int 26 601–606 Occurrence Handle10.1016/0197-0186(94)00175-T Occurrence Handle1:CAS:528:DyaK2MXmt1Sgu74%3D Occurrence Handle7545485
A.E. Houde (1997) Choosy females and competing males: mechanisms of sexual selection. Sex, Color, and Mate Choice in Guppies. Princeton University Press Princeton, N.J. 45–79
G. Khoo V.P.E. Phang T.M. Lim (1992) ArticleTitleThe confocal laser scanning microscope as a tool for studying xanthophores of the swordtail (Xiphophorus helleri). Zool Sci 9 665–669
G. Khoo T.M. Lim W.K. Chan V.P.E. Phang (1999a) ArticleTitleThe genetic basis of the variegated tail pattern in the guppy, Poecilia reticulata. Zool Sci 16 431–437
G. Khoo T.M. Lim W.K. Chan V.P.E. Phang (1999b) ArticleTitleSex-linkage of the black caudal-peduncle and red tail genes in the tuxedo strain of the guppy, Poecilia reticulata. Zool Sci 16 629–638
G. Khoo T.M. Lim W.K. Chan V.P.E. Phang (1999c) ArticleTitleLinkage analysis and mapping of three sex-linked color pattern genes in the guppy, Poecilia reticulata. Zool Sci 16 893–903 Occurrence Handle1:CAS:528:DC%2BD3cXhtlyks7k%3D
G. Khoo M.H. Lim S. Haridas D.K.Y. Gan K.F. Lim F. Chen W.K. Chan T.M. Lim V.P.E. Phang (2003) ArticleTitleGenetic linkage maps of the guppy (Poecilia reticulata): assignment of RAPD markers to multipoint linkage groups. Mar Biotechnol Occurrence Handle10.1007/s10126-002-0056-3 Occurrence Handle1:CAS:528:DC%2BD3sXit1Sgs7w%3D
J.A. Lister C.P. Robertson T. Lepage S.L. Johnson D.W. Raible (1999) ArticleTitle Nacre encodes a zebrafish microphthalmia-related protein that regulates neural-crest-derived pigment cell fate. Development 126 3757–3767 Occurrence Handle1:CAS:528:DyaK1MXmtlOitrw%3D Occurrence Handle10433906
M. Masada J. Matsumoto M. Akino (1990) ArticleTitleBiosynthetic pathways of pteridines and their association with phenotypic expression in vitro in normal and neoplastic pigment cells from goldfish. Pigm Cell Res 3 61–70 Occurrence Handle1:CAS:528:DyaK3MXltlahsbk%3D
J. Matsumoto (1965) ArticleTitleRole of pteridines in the pigmentation of chromatophores in cyprinid fish. Jpn J Zool 14 45–94 Occurrence Handle1:CAS:528:DyaF28XitFCksA%3D%3D
M. Nakagoshi S. Takikawa S. Negishi M. Tsusue (1992) ArticleTitlePteridines in the yellow-colored chromatophores of isopod, Armadillidium vulgare. Biol Chem Hoppe Seyler 373 1249–1254 Occurrence Handle1:CAS:528:DyaK3sXnsleksg%3D%3D Occurrence Handle1292511
A.E. Needham (1974) The Significance of zoochromes. Springer Berlin, Germany
T. Nishino T. Nishino (1987) ArticleTitleEvidence for the existence of a tyrosyl residue in the nicotinamide adenine dinucleotide binding site of chicken liver xanthine dehydrogenase. Biochemistry 26 3068–3072 Occurrence Handle1:CAS:528:DyaL2sXitFSjtb8%3D Occurrence Handle3475129
T. Nishino T. Nishino (1989) ArticleTitleThe nicotinamide adenine dinucleotide-binding site of chicken liver xanthine dehydrogenase. J Biol Chem 264 5468–5473 Occurrence Handle1:CAS:528:DyaL1MXhvVyntLs%3D Occurrence Handle2925614
L.W. Oliphant J. Hudon (1993) ArticleTitlePteridines as reflecting pigments and components of reflecting organelles in vertebrates. Pigm Cell Res 6 205–208 Occurrence Handle1:CAS:528:DyaK2cXhsF2iuro%3D
D.A. Parks N.D. Granger (1986) ArticleTitleXanthine oxidase: biochemistry, distribution and physiology. Acta Physiol Scand Suppl 548 87–99 Occurrence Handle1:CAS:528:DyaL28XkslKktbw%3D Occurrence Handle3529824
V.P.E. Phang O.K. Chow A.A. Fernando (1985) ArticleTitleGenetic analysis of scale chromatophores of two domesticated varieties of guppy, Poecilia reticulata. J Sing Nat Acad Sci 14 1–5
V.P.E. Phang L.N. Ng A.A. Fernando (1989) ArticleTitleInheritance of snakeskin color pattern in the guppy, Poecilia reticulata. J Hered 80 393–399 Occurrence Handle1:STN:280:By%2BD3M%2FgsVw%3D Occurrence Handle2794473
A.G. Reaume D.A. Knecht A. Chovnick (1991) ArticleTitleThe rosy locus in Drosophila melanogaster: xanthine dehydrogenase and eye pigments. Genetics 129 1099–1109 Occurrence Handle1:CAS:528:DyaK38XotVKquw%3D%3D Occurrence Handle1783294
A. Sato T. Nishino K. Noda Y. Amaya T. Nishino (1995) ArticleTitleThe structure of chicken liver xanthine dehydrogenase. J Biol Chem 270 2818–2826 Occurrence Handle10.1074/jbc.270.6.2818 Occurrence Handle1:CAS:528:DyaK2MXjs1Kmurc%3D Occurrence Handle7852355
K.U. Schallreuter J.M. Wood M.R. Pittelkow M. Gutlich K.R. Lemke W. Rodl N.N. Swanson K. Hitzemann I. Ziegler (1994a) ArticleTitleRegulation of melanin biosynthesis in human epidermis by tetrahydrobiopterin. Science 263 1444–1446 Occurrence Handle1:CAS:528:DyaK2cXitFeku7s%3D
K.U. Schallreuter G. Buttner M.R. Pittelkow J.M. Wood N.N. Swanson C. Korner (1994b) ArticleTitleCytotoxicity of 6-biopterin to human melanocytes. Biochem Biophys Res Commun 204 43–48 Occurrence Handle1:CAS:528:DyaK2cXmsFaitL4%3D
K. Schiedt (1980) New aspects of carotenoid metabolism in animals. N.I. Krinsky M.M. Mathews-Roth R.F. Taylor (Eds) Carotenoids: Chemistry and Biology, Plenum Press New York, N.Y. 247–268
L.M. Schopfer V. Massev T. Nishino (1988) ArticleTitleRapid reaction studies on the reduction and oxidation of chicken liver xanthine dehydrogenase by the xanthine/urate and NAD/NADH couples. J Biol Chem 263 13528–13538 Occurrence Handle1:CAS:528:DyaL1cXmtVOnsrc%3D Occurrence Handle3166459
D.L. Swofford (2002) PAUP: Phylogenetic Analysis Using Parsimony, Version 4.0b10. Sinauer Sunderland, Mass.
M. Terao M. Kurosaki S. Zanotta E. Garattini (1997) ArticleTitleThe xanthine oxidoreductase gene: structure and regulation. Biochem Soc Trans 25 791–796 Occurrence Handle1:CAS:528:DyaK2sXmtFCkt74%3D Occurrence Handle9388547
U. Thoenes O.L. Flores A. Neves B. Devreese J.J.V. Van Beeumen R. Huber R.J. Romao J. Legall J.J.G. Moura C. Rodrigues-Pousada (1994) ArticleTitleMolecular cloning and sequence analysis of the gene of the molybdenum-containing aldehyde oxidoreductase of Desulfovibrio gigas. Eur J Biochem 220 901–910 Occurrence Handle1:CAS:528:DyaK2cXkt1yitbs%3D Occurrence Handle8143744
B. Thony G. Auerbach N. Blau (2000) ArticleTitleTetrahydrobiopterin biosynthesis, regeneration and functions. Biochem J 341 1–16 Occurrence Handle10.1042/0264-6021:3470001
S. Thorsteinsdottir S.K. Frost (1986) ArticleTitlePigment cell differentiation: the relationship between pterin content, allopurinol treatment, and the melanoid gene in axolotls. Cell Differ 19 161–172 Occurrence Handle10.1016/0045-6039(86)90092-8 Occurrence Handle1:CAS:528:DyaL28Xmt1eiu7Y%3D Occurrence Handle3791419
E.R. Werner G. Werner-Felmayer D. Fuchs A. Hausen G. Reibnegger J.J. Yim W. Pfleiderer H. Wachter (1990) ArticleTitleTetrahydrobiopterin biosynthetic activities in human macrophages, fibroblasts, THP-1, and T 24 cells. GTP-cyclohydrolase I is stimulated by interferon-gamma, and 6-pyruvoyl tetrahydropterin synthase and sepiapterin reductase are constitutively present. J Biol Chem 265 3189–3192 Occurrence Handle1:CAS:528:DyaK3cXhslKlu7g%3D Occurrence Handle2154472
P. Xu T.P. Huecksteadt R. Harrison J.R. Hoidal (1994) ArticleTitleMolecular cloning, tissue expression of human xanthine dehydrogenase. Biochem Biophys Res Commun 199 998–1004 Occurrence Handle10.1006/bbrc.1994.1328 Occurrence Handle1:CAS:528:DyaK2cXksFSqurw%3D Occurrence Handle8135849
Y. Yasukochi T. Kanda T. Tamura (1998) ArticleTitleCloning of two Bombyx homologues of the Drosophila rosy gene and their relationship to larval translucent skin color mutants. Genet Res Camb 71 11–19 Occurrence Handle10.1017/S0016672397003078 Occurrence Handle1:CAS:528:DyaK1cXksFegsr4%3D
I. Ziegler T. McDonaldo C. Hesslinger I. Pelletier P. Boyle (2000) ArticleTitleDevelopment of the pteridine pathway in the zebrafish, Danio rerio. J Biol Chem 275 18926–18932 Occurrence Handle10.1074/jbc.M910307199 Occurrence Handle1:CAS:528:DC%2BD3cXksVGktL0%3D Occurrence Handle10770954
Acknowledgements
We thank Mr. Goh Kim Jew and Mrs. Toh Seah Liew for purchase of fish and reagent. This work was supported by a research grant from the National University of Singapore (R-154-000-056-112).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ben, J., Lim, T., Phang, V.P. et al. Cloning and Tissue Expression of 6-Pyruvoyl Tetrahydropterin Synthase and Xanthine Dehydrogenase from Poecilia reticulata . Mar. Biotechnol. 5, 568–578 (2003). https://doi.org/10.1007/s10126-002-0121-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10126-002-0121-y