Abstract
This systematic review assessed if photobiomodulation of human dental pulp tissue improved cell viability, proliferation, and/or differentiation compared with a placebo. This systematic review was conducted in line with PRISMA. PICO question was established; inclusion and exclusion criteria were established before a search had begun. A literature search was conducted through PubMed, Scopus, and Cochrane. Studies were included if published within the last 20 years in English language, or where translation was available; laser parameters were mentioned; human dental pulp tissue was studied in vitro. Studies were excluded if non-human dental pulp tissue was studied and where the study was an in vivo study. Out of the total 121 studies found, 109 were excluded. Of the twelve included studies, three full-text articles were not available despite attempts made to contact the respective authors, leaving nine studies. Four of the included studies reported the use of stem cells derived from human deciduous teeth (SHEDs), and five used those from human permanent teeth (DPSCs). Most included studies utilized InGaAlP laser with wavelengths 660 nm, and one study with 610 nm. Other types of lasers included LED InGaN, and GaAlAs. Out of all included studies, two had a moderate risk of bias, and the rest had a low risk of bias. All studies confirmed positive effects on proliferation. One study also found improved osteogenic differentiation of the stem cells derived from stem cells of deciduous teeth. After assessing SHEDs and DPSCs separately, it is found that photobiomodulation improved cell proliferation in both subgroups. Due to heterogeneity in design protocols and laser parameters, it was not possible to compare the studies together. However, this study indicated that cell viability and proliferation did improve with photobiomodulation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tissue defects in the craniofacial region have serious costs financially, psychologically, and physiologically. Reconstruction, therefore, is highly desired. There have been significant developments over the last few decades in this area of tissue engineering, both in terms of research and clinical protocols [1, 2]. It now focuses on three factors: regenerative cells, cell scaffolds, and bioactive substances. In terms of regenerative cells, the focus has been on those derived from bone marrow. However, stem cells make up 0.001–0.01% of all cellular components in bone marrow, and the patient is still required to undergo an invasive procedure to acquire these cells. Therefore, when Gronthos et al. in 2000 isolated and cultured human dental pulp stem cells (DPSCs) from permanent teeth, and later in 2003 Miura et al. did the same from deciduous teeth, there have been a growing number of studies looking into proliferative and differentiation properties of these cells [1, 2].
There are multiple types of stem cells derived from the dentoalveolar tissues—dental pulp stem cells (DPSCs), dental pulp tissue of deciduous teeth (SHEDs), and periodontal ligament (PDLSCs). The difference in proliferative and differentiation properties between these three types of stem cells has not yet been conclusively elucidated. It has, however, been shown that these cells are more proliferative than human BMSCs (hBMSCs). They are capable of differentiating into osteoblasts, adipocyte chondrocytes, and neurons [3]. There is also some in vivo evidence to suggest that bone regeneration occurs with SHEDs and DPSCs on par with BMSCs [3].
Upon transplantation, stem cells have demonstrated a low percentage of viability and proliferation [1]. This has been attributed to multiple drawbacks in the current tissue engineering process. One of the main drawbacks is nutritional deficiency, identified as lack of blood supply. Photobiomodulation therapy has previously successfully demonstrated its effectiveness in improving proliferation, migration, and differentiation of cells, and activation of growth factors, as well as the acceleration of protein synthesis. Therefore, photobiomodulation has been applied in tissue engineering to improve cell viability, proliferation, and differentiation of stem cells. All three processes, improving cell viability, improved proliferation, and induction or acceleration of differentiation of stem cells can dictate the success of repair of tissue defects [1, 2]. Hence, there is a need to conclusively prove that photobiomodulation can improve the said processes in DPSCs and SHEDs.
Two recent systematic reviews in 2016 and one narrative review in 2017 have considered photobiomodulation and dental-derived mesenchymal stem cells [4,5,6]. The narrative review conducted in 2017 did not include studies identified in the previous systematic review, and only included studies that the authors identified, without justification, as important [5]. This reduces the external validity of the narrative review. The systematic review by Farahani (2016) identified studies with the outcome measure to be proliferation of human dentoalveolar–derived stem cells alone, and excluded studies that considered cell viability and differentiation of the stem cells [5]. This exempts the consideration of the complete effect of Photobiomodulation on dental-derived stem cells [5]. Another criticism of this systematic review, and that of Marques et al. (2016), was that they considered more than one type of stem cell for comparison—dental pulp stem cells (DPSCs), dental pulp tissue of deciduous teeth (SHEDs), and periodontal ligament (PDLSCs) [4, 5]. The resulting conclusion therefore does not point unequivocally towards the synthesis of current evidence for photobiomodulation and DPSCs or SHEDs.
Farahani (2016) did not conduct a risk of bias assessment, and only included one comment about the possible source of bias in a table dedicated to outcomes [5]. Marques et al. (2016) did conduct a risk of bias assessment; however, the constructed tool in the review was deemed inadequate [4]. Risk of bias assessment evaluates internal validity. This is a test of study design and its credibility to link the exposure and outcome. Inadequate risk of bias assessment leads to a reduction in the level of evidence possible from the systematic review. In addition, comment on external validity was found lacking in the two systematic reviews [7,8,9]. Furthermore, the 2016 systematic review also included one study without a complete description of laser parameters despite the recommendation by the World Association for Laser Therapy (WALT) [4]. This undermines reproducibility as well as comparison and synthesis of data. These problems compromise the level of evidence present from the existing evidence.
In addition, multiple new in vitro studies have been identified since 2016 dictating more concrete evidence and future direction for research. The need for an update was therefore identified. This systematic review aims to answer the null hypothesis that photobiomodulation does not improve the cell viability, proliferation, and/or differentiation of dentally derived stem cells, under the question:
-
In human dental pulp tissue (population), does photobiomodulation (intervention) improve cell viability, proliferation, and/or differentiation (outcome) compared with a placebo (control)?
Methods
This systematic review was written and conducted to comply with Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) [10]. A systematic search strategy was conducted using Medical Subject Heading (MeSH) terms [dental pulp] and [low-level laser therapy]. The search was conducted through PubMed, Scopus, and Cochrane up until 11 September 2019.
Inclusion and exclusion criteria were decided prior to searching in accordance with the PRISMA protocol and were registered on PROSPERO to avoid duplication of results. These criteria were as follows:
Inclusion criteria:
-
Laser parameters are mentioned
-
Published in English language or English translation is available
-
Published in the last 20 years
-
Human dental pulp tissue is studied
-
In vitro study
Exclusion criteria
-
Non-human dental pulp tissue is studied
-
Non–in vitro study
Risk of bias assessment
As no formal risk of bias assessment tool was identified for these in vitro cell culture studies, the last used tool in the 2016 systematic review was critiqued and built upon to construct the tool as displayed in Table 1 [4]. The systematic review identified some parameters to assess the study design and comment on risk of bias. However, these were deemed inadequate.
The key features of the study design were identified using the list recommended by the Cochrane Handbook for Systematic Reviews Chapter 13 [7]. The following parameters were identified as being imperative for assessment of internal validity and hence were added to the risk of bias assessment table: handling of cell culture between establishment and measurement, reporting of all outcome measures as stated, number of lost wells with reason, and reporting of all laser parameters. Two independent researchers evaluated the studies and reported ‘Y’ if the study had reported that parameter, and ‘N’ if the study did not. More than five ‘N’s were considered to have a high risk of bias, between 2 and 5 ‘N’s were considered to have a moderate risk of bias, and 1 or 0 ‘N’s were considered to have a low risk of bias.
Results
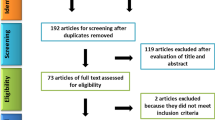
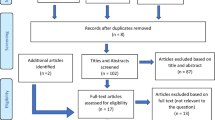
Once the systematic search strategy was constructed, and the said databases were searched, duplicates were identified and eliminated. Two independent researchers (S. K., R. G.) reviewed the titles and abstracts to allocate the studies to ‘included’ and ‘excluded’ folders in the citation manager Endnote X8® (Clarivate Analytics, Boston, MA, USA). Any that were left in the ‘unsure’ folder, were later discussed with a third researcher (M. M.) to include or exclude it. This selection process can be seen in Fig. 1.
The study characteristics have been reported in Tables 2 and 3. The source of stem cells is reported in Table 2. Four of the included studies reported the use of stem cells derived from human deciduous teeth (SHEDs), and five used those from human permanent teeth (DPSCs) [11,12,13,14,15,16,17,18,19]. No comparative study was found to analyse the different effects of photobiomodulation between SHEDs and DPSCs. One study found higher proliferative activity of SHEDs compared with bone marrow mesenchymal cells and DPSCs without any stimulation, as well as greater expression of runt-related transcription factor 2 (Runx2) and alkaline phosphatase (ALP) genes [3]. Runx2 expression indicates osteoblastic differentiation and ALP is a matrix mineralisation marker, both indicating possibly greater osteoblastic differentiation potential compared with DPSCs [3]. Due to this difference, studies with SHEDs were compared amongst themselves, separately to those with DPSCs.
Laser parameters in the included studies (Table 3)
Most included studies utilized InGaAlP laser with wavelengths 660 nm, and one study with 610 nm [12,13,14,15,16, 18]. Other type of lasers included LED InGaN, and GaAlAs [14, 19].
This may not be of great significance as a recent study found no difference in cell viability on myoblasts when InGaAlP was compared with GaAlAs and control [20]. However, no comparative study on dentally derived stem cells was found to confirm this finding. It is important to note that there are some differences between LED and lasers. LEDs have a larger bandwidth, meaning they can be applied to a larger tissue size. The coherent characteristic of the lasers, unlike other LED-based light sources, could be the difference in the photobiomodulated stimulatory effect on cells. The coherent light from laser can result in an interference pattern due to tissue imperfections, and the resultant stimulation is theorised to affect mitochondrial activity [21]. Similarly, it is reported that pulsed lasers have different photobiomodulatory effects compared with continuous wave therapy [22]. Given these differences, there is a potential source of heterogeneity between the studies that used LED and those that used lasers.
In addition, Kim et al. (2017) did not state the duration of exposure, which determines the energy induced into the cells; therefore, that study does not remain comparable [17].
Risk of bias and study methodology assessment
Out of all included studies, two had a moderate risk of bias, and the rest had a low risk of bias [12, 17] (Table 1). All included studies reported cell culture techniques, handling techniques, laser parameters, cell passages, and also reported on all outcomes as specified prior in their methods section. One study did not report all laser parameters, specifically the duration of each laser session [17]. They also did not report the stem cells passage, nor did they report whether the characterisation was done [17]. Cell passage is the number of times the cells were subcultured and although this does not have an impact on the effect of photobiomodulation, lack of recording and reporting of cell passage questions the handling of cells during the experiment. This, if not recorded or reported, adds to increase the risk of bias. In addition, the lower the number of cell passages, the closer it is to the primary cell culture. A higher number, on the other hand, means the cells might have a finite life span and more prone to changes in their proliferative and differentiation ability [23]. Cell characterisation, on the other hand, can be conducted using flow cytometry and is important to determine as this allows comparison of cells of the same lines. Failure to report the cell line and whether characterisation was done adds to the increased risk of bias. Eduardo et al. (2008) failed to report the source of the cells, posing a significant problem in validating the study [12]. This means that the cell type was not clearly identified in this study, making it difficult to conclude on the effect of PBM on the cells in relation to other studies.
Outcome measures for SHEDs
Although all four studies using SHEDs studied the main effect of proliferation under the influence of photobiomodulation, two studies added specific conditions. One study assessed the effect on nitric oxide production [11]. Another study created a nutritionally deficient environment as a simulator for stress. All studies confirmed positive effects on proliferation [11, 15, 16, 18]. One study also found improved osteogenic differentiation [18]. A study evaluated the effect of varying energy densities and output power on the photobiomodulatory effects on cell viability and proliferation in SHEDs [16]. It found that any energy density between 0.5 and 4 J/cm2 improved cell viability. Higher energy density has been reported to potentially reduce the photobiomodulatory effect by damaging the photoreceptors. However, that study did not find any statistically significant difference by varying output power [16].
Outcome measured for DPSCs
Five studies used permanent teeth as the source of dental stem cells [12,13,14, 17, 19]. One study, although agreeing with other studies that photobiomodulation improves proliferation, found that the proliferation rate of cells improved as time passed from 1 to 2 weeks [14]. Kim et al. (2017) went on to test pulse frequency dependency of photobiomodulation on biostimulation and concluded that 300 Hz was more effective for enhanced alkaline phosphatase activity, compared with 3 Hz. This, and a new testing mechanism of biostimulation with detection of biophoton emission, adds to the new areas of research but does not form part of our question and systematic review. The new testing mechanism used in this study is called delayed luminescence, where emissions of photons are determined after the light source is switched off. This is based on the theory that oxidative metabolic reactions in cells emit these photos, and photobiomodulation increases these reactions. Therefore, testing for these emitted photos allows testing the effectiveness of light sources on cells [24]. Eduardo et al. (2008) found that 20 mW was more effective than 40 mW as output power in stimulating proliferation of DPSCs [12]. This was done under the 660 nm InGaAlP. Sivakumar et al. (2019) also concurred with the study by Eduardo et al. (2008) and showed that there might be more benefits with two sessions of laser therapy instead of one [12, 19]. Comparing SHEDs and DPSCs was not done by any of the included studies.
Discussion
Tissue engineering has varied applications in the orofacial region from the repair of craniofacial defects to repair of dental tissues. Stem cell research has been of increasing interest over the last two decades. The aim of the present systematic review was to add to the existing systematic reviews upon finding new in vitro studies, as well as to expand on the flaws identified from previous systematic reviews. Out of three reviews, two were systematic, and one was a narrative review [4,5,6]. The narrative review had a high selection bias as studies were included without any justification [6]. Although the other two reviews were conducted with a systematic search strategy and inclusion and exclusion criteria, they had a few flaws. One of them did not report a risk of bias assessment, and when the other did report a tool, it was deemed inadequate as not all factors in the design of study were added to it [4, 5]. The present systematic review built on that tool to make it more comprehensive in its assessment for risk of bias. The systematic reviews also combined the results of different types of stem cells—DPSCs, SHEDs, as well as PDLSCs. This present systematic review separately analysed the two groups of studies, SHEDs and DPSCs.
One of the most significant findings is that there is a therapeutic window of laser parameters of varying energy densities [16], as evidenced by one study, and supported by other studies [4, 12, 19]. This is in line with the Arndt-Schulz law which states that small doses can stimulate, moderate doses inhibit, and large doses kill cells. The existence of an effective window could be the reason why the lowest (0.05 J/cm2) and the highest (42 J/cm2) energy densities reported no effects [4]. In this range of energy densities, positive effects of photobiomodulation therapy were observed, as has been demonstrated in other cell types [4, 12,13,14, 17, 19]. The effects of photobiomodulation analysed on cell activities relevant for tissue regeneration were mostly cell viability and proliferation and odonto/osteogenic differentiation. When in vitro survival, viability, and proliferation in response to photobiomodulation therapy was analysed in this systematic review, as was the case in the previous reviews, positive effects were obtained.
The red laser (660 nm) was the most used wavelength. The wavelength of laser used does not require a debate as a range of wavelengths from 660 to 855 nm have all shown to be effective, and this is in line with the absorption range of cells [11, 16, 18]. Beam divergence however is a major issue in all studies with laser therapy. Four studies performed irradiations from the top of the wells, which could have beam divergences that should be further considered in the calculation of energy density deposited on the cell monolayer [11, 13, 14, 25]. In some studies, the contact mode was used by irradiating the cells through the bottom of the wells, where the distance between the cell monolayer and the laser source is < 1 mm, and the beam divergence is negligible [11, 15]. In accordance, the calculations of energy densities were more accurate in these cases, as the irradiation spot sizes were the same as those of the laser tips. Although all studies found positive results, in future studies, it is important to note this design flaw of beam divergence to better evaluate photobiomodulation.
Additionally, some included studies used near-infrared lasers (780–1100 nm) [11, 14, 17, 19]. Both red and near-infrared lasers have some similar properties. They both have been found to increase intracellular ATP level, as well as increase cell proliferation, and display biphasic dose-dependent response. Literature also reports an increase in intracellular matrix metalloproteinase levels and reduced ROS levels [26]. This is consistent with an included study which found reduced ROS and NO levels when subjected to near-infrared radiation [11]. An important condition to consider when determining the effect of irradiation is the number of mitochondria in a cell as they tend to be the initial site of light absorption. Cytochrome c oxidase (a light absorbing enzyme in mitochondria) (CCO) is the most important chromophore in photobiomodulation effects and has two different absorption bands—one corresponding to red and another near-infrared laser wavelengths. Although both wavelengths produce positive effect, the depth of penetration is different. In a clinical situation, tissue penetration would determine which wavelength should be used. Red wavelengths can penetrate 0.5–1 mm and wavelengths in the range of 780–1100 nm can penetrate 2 mm before losing 37% of its intensity [27]. Therefore, deeper tissues such as bone have shown better response with near-infrared lasers [27].
Characteristics and gene expression related to the mineralisation processes, innervation, tissue formation, vascularization, and immune response are different in dental pulp cells from primary and permanent teeth. Thus, cells from these different sources may demonstrate different levels of effect to photobiomodulation. Therefore, this systematic review compared SHEDs and DPSCs separately. The key outcome measure was cell viability and/or proliferation. In both groups of studies, positive evidence was found, with some distinct findings as detailed below.
SHEDs
It is known that children will lose their deciduous teeth between 6 and 12 years of age. Therefore, harvesting the stem cells from deciduous teeth is more sustainable and less painful for the patient. Studies have shown there is no difference in cell viability between stem cells from deciduous teeth and permanent teeth [4, 14]. Currently, to harvest stem cells from deciduous teeth, the teeth should have a pulp that appears red and at least two thirds of the root is remaining [26]. These teeth should be disease-free and extracted in a sterile environment. After rinsing with phosphate-buffered saline and alcohol, they are transferred for harvestation of cells. The pulp is harvested in the laboratory or in the dental clinic with a sterile barbed broach and then trypsinized and cultured to acquire different colonies of stem cells. After sorting the colonies of cells with fluorescence activated system, the identified mesenchymal stem cells are then stored under cryopreservation [28].
Four of the included studies used SHEDs [11, 15, 16, 18]. Although Montoro et al. (2014) studied different energy densities and their effect on nitric oxide production, it still concluded that all resulted in improved cell viability [11]. Nitric oxide is produced as a free radical and although beneficial in some quantity, initially for vasodilation, it is known to be cytotoxic and reduction in NO is therefore a desirable mechanism to improve cell viability. NO production also is known to increase in the presence of bacteria. Therefore, Montoro et al. (2014) demonstrated that photobiomodulation can be effectively used in the region of transplanted cells in tissue defects with present bacteria to allow for increased cell viability [11]. After creating nutritional deficiency as a simulator for stress, due to poor blood supply, in in vivo conditions, Moura-Netto et al. (2016) reported that photobiomodulation could effectively improve cell viability and proliferation for both their protocols (3 J/cm2 and 5 J/cm2) [15]. In the future, this simulated stress should be combined with a Montoro et al. style study on NO production and cell viability and proliferation testing [11]. Clinically, both of these situations present themselves whether through bacterial infiltration or reduced blood flow, and it is a key finding of these studies that photobiomodulation can be beneficial in those situations.
DPSCs
Kim et al. (2017) and Eduardo et al. (2008) did not state how the cell characterisation was done, nor what the cell passage was [12, 17]. Kim et al. (2017) also did not state the duration of their lesser sessions [17]. This was a problem with comparison as cell passages determine their proliferative and differentiation properties [29]. The duration of the laser session also has an impact on the effectiveness of laser—and this meant that the comparison in this subgroup of DPSCs was flawed in two studies with bias. This means that both the internal validity of those two studies was affected and so was the overall external validity of those studies, therefore leaving the conclusion for this subgroup weaker in strength than that for SHEDs.
Conclusion
This systematic review was conducted in line with PRISMA. PICO question was established; inclusion and exclusion criteria were established before a search had begun. However, the data limited the scope of the study. No previous study compared the effect of photobiomodulation on these two types of stem cells. In the subgroup of SHEDs, there was a low risk of bias, and cell viability and proliferation did improve with photobiomodulation. Similar results can be reached for the other subgroup DPSCs, but with a moderate level of bias. Due to heterogeneity in design protocols and laser parameters, it was not possible to compare the studies together.
References
Neel EAA, Chrzanowski W, Salih VM, Kim HW, Knowles JC (2014) Tissue engineering in dentistry. J Dent 42(8):915–928 [cited 2019 Sep 11]
Diniz IMA, Carreira ACO, Sipert CR et al (2018) Photobiomodulation of mesenchymal stem cells encapsulated in an injectable rhBMP4-loaded hydrogel directs hard tissue bioengineering. J Cell Physiol 233(6):4907–4918
Kunimatsu R, Nakajima K, Awada T, Tsuka Y, Abe T, Ando K et al (2018) Comparative characterization of stem cells from human exfoliated deciduous teeth, dental pulp, and bone marrow-derived mesenchymal stem cells. Biochem Biophys Res Commun 501(1):193–198 [cited 2019 Sep 11]
Marques MM, Miyagi de Cara SPH, Abe GL, Lima PLV, Moreira MS (2016) Photobiomodulation of dental derived mesenchymal stem cells: a systematic review. Photomed Laser Surg 34(11):1–9 [cited 2019 Sep 11]
Farhani AB (2016) Effect of low-level laser irradiation on proliferation of human dental mesenchymal stem cells: a systematic review. J Photochem Photobiol B 162:577–582 [cited 2019 Sep 11]
Staffoli S, Romeo U, Amorim RNS, Migliau G, Palaia G, Resende L et al (2017) The effects of low level laser irradiation on proliferation of human dental pulp: a narrative review. Clin Ter 168(5):320–326 [cited 2019 Sep 11]
Higgins J, Green S (2011) Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration. [cited 2019 Sep 11]; Available from: www.cochrane-handbook.org [accessed 3 February 2013]
IOM (Institute of Medicine) (2011) Finding what works in health care: Standards for systematic reviews. The National Academies Press, Washington, DC, p 318 [cited 2019 Oct 3]. Available at: http://www.nap.edu/openbook.php?record_id=13059
Viswanathan M, Ansari M, Berkman ND, Chang S, Hartling L, McPheeters LM et al (2013) Assessing risk of bias and confounding in observational studies of interventions or exposures: further development of the RTI item bank. Agency for Healthcare Research and Quality (AHRQ) 13 [cited 2019 Sep 11]. Available at: http://www.effectivehealthcare.ahrq.gov/ehc/products/414/1612/RTI-item-bank-bias-precision-130805.pdf
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6(7):e1000097 [cited 2019 Sep 11]
Montoro LA, Turrioni AP, Basso FG, de Souza Costa CA, Hebling J (2014) Infrared LED irradiation photobiomodulation of oxidative stress in human dental pulp cells. Int Endod J 47(8):747–755 [cited 2019 Sep 11]
Eduardo Fde P, Bueno DF, de Freitas PM, Marques MM, Passos-Bueno MR, Eduardo Cde P et al (2008) Stem cell proliferation under low intensity laser irradiation: a preliminary study. Lasers Surg Med 40(6):433–438 [cited 2019 Sep 11]
Zaccara IV, Ginani F, Mota-Filho HG, Henriques ACG, Barboza CAG (2015) Effect of low-level laser irradiation on proliferation and viability of human dental pulp stem cells. J Lasers Med Sci 30:2259–2264 [cited 2019 Sep 11]
Tabatabaei FS, Torshabi M, Nasab MM, Khosraviani K, Khojasteh A (2015) Effect of low-level diode laser on proliferation and osteogenic differentiation of dental pulp stem cells. Laser Phys 25 [cited 2019 Sep 11]
Moura-Netto C, Ferreira LS, Maranduba CM, Mello-Moura ACV, Marques MM (2016) Low-intensity laser phototherapy enhances the proliferation of dental pulp stem cells under nutritional deficiency. Braz Oral Res 30(1) [cited 2019 Sep 11]
Marques NCT, Neto NL, Prado MTO, Vitor LLR, Oliveira RC, Sakai VT et al (2017) Effects of PBM in different energy densities and irradiance on maintaining cell viability and proliferation of pulp fibroblasts from human primary teeth. J Lasers Med Sci 32(7):1621–1628 [cited 2019 Sep 11]
Kim HB, Baik KY, Choung PH, Chung JH (2017) Pulse frequency dependency of photobiomodulation on the bioenergetic functions of human dental pulp stem cells. Sci Rep 7(1):15927 [cited 2019 Sep 11]
Pinheiro CCG, de Pinho MC, Aranha AC, Fregnani E, Bueno DF, Low Power Laser Therapy (2018) A strategy to promote the osteogenic differentiation of deciduous dental pulp stem cells from cleft lip and palate patients. Tissue Eng A 24(7–8):569–575 [cited 2019 Sep 11]
Sivakumar TT, Muruppel AM, Joseph AP, Reshmi JA, Ramachandran R, Nair PD et al (2019) Photobiomodulatory effect delivered by low-level laser on dental pulp stem cell differentiation for osteogenic lineage. Laser Dent Sci 3:175 [cited 2019 Sep 11]
Ferreira MP, Ferrari RA, Gravalos ED, Martins MD, Bussadori SK, Gonzalez DA et al (2009) Effect of low-energy gallium-aluminum-arsenide and aluminium gallium indium phosphide laser irradiation on the viability of C2C12 myoblasts in a muscle injury model. Photomed Laser Surg 27(6):901–906 [cited 2019 Sep 11]
Heiskanen V, Hamblin MR (2018) Photobiomodulation: lasers vs. light emitting diodes. Photochem Photobiol Sci 17(8):1003–1017 [cited 2019 Sep 11]
Hashmi JT, Huang YY, Sharma SK, Kurup DB, De Taboada L, Carroll JD et al (2010) Effect of pulsing in low-level light therapy. Lasers Surg Med 42:450–466 [cited 2019 Sep 19]
Public health England (2017) Passage numbers explained. European Collection of Authenticated Cell Cultures. [cited 2019 Sep 12]. Available at: https://www.phe-culturecollections.org.uk/products/celllines/generalcell/detail.jsp?refId=92020424&collection=ecacc_gc
Gerschman JA, Ruben J, Gebart-Eaglemont J (1994) Low level laser therapy for dentinal tooth hypersensitivity. Aust Dent J 39:353–357 [cited 2019 Sep 11]
Pereira LO, Longo JP, Azevedo RB (2012) Laser irradiation did not increase the proliferation or the differentiation of stem cells from normal and inflamed dental pulp. Arch Oral Biol 57(8):1079–1085 [cited 2019 Sep 11]
Wang Y, Huang Y, Wang Y, Lyu P, Hamblin MR (2017) Red (660 nm) or near-infrared (810 nm) photobiomodulation stimulates, while blue (415 nm), green (540 nm) light inhibits proliferation in human adipose-derived stem cells. Sci Rep 7:7781 [cited 2020 May 5]
Zein R, Selting W, Hamblin MR (2018) Review of light parameters and photobiomodulation efficacy: dive into complexity. J Biomed Opt 23(12):120901 [cited 2020 May 5]
Sunil PM, Manikandan R, Muthumurugan M, Yoithapprabhunath TR, Sivakumar M (2015) Harvesting dental stem cells – overview. J Pharm Bioallied Sci 7(2):384–386 [cited 2019 Sep 11]
Milward MR, Hadis MA, Cooper PR, Gorecki P, Carroll JD, Palin W (2015) Biomodulatory effects of laser irradiation on dental pulp cells in vitro. Proc SPIE 9309:930908–930901 [cited 2019 Sep 11]
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kulkarni, S., Meer, M. & George, R. The effect of photobiomodulation on human dental pulp–derived stem cells: systematic review. Lasers Med Sci 35, 1889–1897 (2020). https://doi.org/10.1007/s10103-020-03071-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-020-03071-6