Abstract
To evaluate the efficacy of selective retina therapy (SRT) in patients with diabetic macular edema (DME) based on pretreatment central foveal thickness (CFT). Seventy-two eyes of 63 patients with DME who had previously undergone SRT were included. Patients were divided into two groups based on the CFT at baseline. Group 1 was composed of 35 eyes with CFT < 400 μm and group 2 was composed of 37 eyes with CFT ≥ 400 μm. Changes in best corrected visual acuity (BCVA) and CFT were measured at baseline, 3 and 6 months after SRT. A single-session retreatment was performed at 3-month posttreatment if there was no reduction in CFT. Rescue treatment with intravitreal anti-VEGF injections was performed if persistent DME or vision loss of 1 ≥ logMAR VA line was observed by 6 months after initial SRT. Six months after SRT, group 1 showed reduction of 45.9 μm in mean CFT (P < 0.001) and gain of 0.13 logMAR in mean BCVA (P < 0.001), whereas group 2 experienced no significant change in CFT or BCVA. In group 1, retreatments were performed in 6 eyes (17.1%), and rescue treatment was performed in 1 eye (2.9%), whereas in group 2, retreatment was performed in 17 eyes (45.9%), and rescue treatments were administered in 27 eyes (73%) during a 6-month follow-up. Although SRT had limited effects as a treatment for severe DME, SRT monotherapy for mild DME was effective in improving BCVA and reducing CFT during a 6-month follow-up period.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Diabetic macular edema (DME), an advanced complication of diabetic retinopathy, is the leading cause of visual loss in diabetic patients [1, 2]. The causative mechanism of DME is thought to be the abnormal permeability of retinal capillaries and fluid accumulation between 2 plexiform layers [3, 4]. If left untreated, 25–30% of affected patients will experience a 15-letter (three line) decrease in visual acuity on the Early Treatment of Diabetic Retinopathy Study (ETDRS) eye chart within 3 years [5]. The ETDRS trial showed that conventional laser photocoagulation (CLP) reduced the risk of severe visual loss at least 50% in patients with clinically significant DME over a 3-year period when compared with untreated patients. However, limited functional improvement was achieved after modified grid laser photocoagulation for center-involving diffuse DME [6]. In addition, CLP is associated with significant destruction of retinal tissue, and heat conduction beyond retinal pigment epithelium (RPE) induces various complications, such as symptomatic scotoma, irreversible photoreceptor damage, choroidal neovascularization, and scar enlargement [7, 8].
In order to overcome these disadvantages of continuous-wave (CW) CLP, new laser modalities, such as subthreshold micropulse laser (SMPL), retinal rejuvenation therapy (2RT), and selective retina therapy (SRT) have been developed to target the RPE while sparing the photoreceptors and choroid by “chopping” a CW beam into repetitive, short “micro” pulses [9, 10]. These new reduced-power laser treatments, so-called restorative retinal laser therapy (RRLT), confine the peak temperature increase to the RPE. Since the new selective RPE-targeting laser can spare photoreceptors, RRLT produces “no instantly visible” or “barely visible” changes in the treated lesions, depending on the therapeutic endpoints of the laser system. RRLT increases the RPE-selectivity by using the specified titration algorithm or micropulsing technology. To obtain RPE selectivity, SMPL uses a duty cycle (the ratio of “on” and “off” pulses), and the laser energy is reduced by lowering the duty cycle [10,11,12]. In contrast, 2RT uses ultra-short-pulse nanosecond lasers, and SRT uses a Q-switched frequency-doubled Nd:YLF laser [10, 13, 14]. The new lasers have yielded favorable clinical outcomes in the treatment of DME in several previous studies [11,12,13,14,15] and have demonstrated therapeutic effects equal to those of CLP in previous randomized trials [16, 17]. While the beneficial effect of CLP is associated with increasing oxygen influx from the choriocapillaris into the retina through the destruction of photoreceptors that consume oxygen [18], the therapeutic effect of the new RPE-targeting lasers is related to the release of RPE-derived factors, such as matrix metalloproteinases (MMPs) or heat shock protein-70 (HSP70), according to the specific laser modality [19,20,21,22]. This was demonstrated in rabbit experiments when HSP70 expression after 577 nm laser irradiations did not significantly differ between the non-damaging 30% endpoint management setting and other more damaging energy settings. Additionally, HSP70 expression did not increase with a 40% setting of damaging irradiation, while the tissue damage was increased. Since the purpose of the new non-damaging laser is to maximize release of HSP70, an anti-inflammatory mediator, while minimizing tissue damage, it seems to be unnecessary to use damaging irradiation for no benefit in HSP70 expression [20].
SMPL does not induce any retinal damage, and various imaging and angiographic techniques have not detected SMPL lesions after treatment [17]. Unlike SMPL, SRT and 2RT lesions can be detected by fundus fluorescein angiography (FFA) because of selective RPE damage [14, 15]. While 2RT (532 nm Q-switched YAG laser) delivers ultra-short pulses of 3 ns that damages the RPE, SRT (527 nm Q-switched Nd-YLF laser) uses 15 ramping micropulses, with a pulse duration of 1.7 μs. If the energy delivered by the micropulse is adequate, RPE cells are selectively damaged by micro-vaporization that occurs around intracellular melanosomes of the RPE cells. Then, SRT stimulates the migration and proliferation of adjacent RPE cells into irradiated areas and improves metabolism at the SRT-treated area by restoring the RPE monolayer [21,22,23]. Since no scotoma was observed by microperimetry at the SRT-treated area in DME patients in previous studies, the photoreceptor-sparing SRT could be appropriate for treating DME while avoiding the complications of CLP [15, 24].
However, to titrate the appropriate pulse energy of SRT and 2RT, it is typically mandatory to perform test spots outside the macular area prior to the treatment spot at the macula. Although each new laser has different thresholds for damaging RPE cells due to different laser settings and parameters, microperimetry and optical coherence tomography (OCT) findings from clinical studies of DME have demonstrated that the new lasers preserve photoreceptors [14,15,16,17]. Several clinical studies have reported that 810 nm SMPL showed an improvement in best corrected visual acuity (BCVA) and a reduction in the macular thickness in patients with DME [12, 16, 17, 25, 26]. Recently, 2 clinical studies of the use of 810 nm or 577 nm SMPL have reported that the initial central foveal thickness (CFT) could influence the clinical outcomes. A good response to SMPL laser was achieved in cases with mild DME with a thinner pretreatment CFT [27, 28].
Considering that the effect of SRT is associated with RPE-derived factors similar to other reduced-power laser systems, we suspected that retinal thickness may also affect the efficacy of SRT, as it does in SMPL treatment. Thus, the aim of this study was to evaluate the efficacy of SRT in patients with DME, based on their baseline CFT.
Methods
We retrospectively reviewed the medical charts of 85 patients who had undergone SRT for DME from October 2014 to February 2016. This study adhered to the principles of the Declaration of Helsinki, and data collection was approved by the Institutional Review Board of Yeouido St. Mary’s Hospital of the Catholic University of Korea. All patients received an explanation of the potential risks and benefits of SRT, intravitreal anti-VEGF, and the local steroid. Written informed consent was obtained in all patients who chose SRT.
Patients with type 2 diabetes and clinically significant DME, according to ETDRS criteria, were included in the study [5]. An additional inclusion criterion was the availability of medical records for 6 months or more after SRT. Patients with a history of subtenon or intravitreal steroid treatment within 6 months prior to SRT laser, intravitreal anti-VEGF injection within 3 months prior to SRT, focal/grid or panretinal CLP within 1 year prior to SRT, or any intraocular surgery were excluded. Other exclusion criteria were a diagnosis of ocular disease affecting visual acuities, such as age-related macular degeneration and macular edema due to other retinal vascular diseases.
All patients were treated by a single surgeon (Y.J.R) and were followed up for 6 months or more after SRT. All SRT was performed by using the Q-switched Nd:YLF laser system (with a wavelength of 527 nm, a spot size of 200 μm, single micropulse duration of 1.7 μs, a pulse repetition rate of 100 Hz, and 15 micropulses per burst) (Medical Laser Center, Lübeck, Germany). An Ocular Mainster contact lens with a magnification of 1.05 was used for irradiation. Ten to 16 preliminary test spots with increasing pulse energy were applied to the temporal arcade (60–200 μJ) to titrate the individual optimal pulse energy level. Color fundus photography (CFP) and FFA was performed 1 h after test spot irradiation to determine the adequate pulse energy for treatment spots (Fig. 1). The adequate pulse energy was determined based on two endpoints, including ophthalmoscopically invisible and FFA visible lesions at the test spots, as previously described [13, 15]. The minimum pulse energy that produced an FFA visible lesion on test spots was chosen as the adequate pulse energy. Once the appropriate pulse energy for treatment spots was determined, SRT spots were applied with a one-spot-space by 200 μm horizontally and vertically at the area where the focal or diffuse leakage was shown by FFA. SRT laser was applied to the whole leakage area of macular edema, as confirmed by FFA, and spectral domain optical coherence tomography (SD-OCT). Since SRT spots were invisible during irradiation, the surgeon made an effort to maintain one-spot-spaced density. Since FFA was necessary for the titration of pulse energy, laboratory exams including renal function test and HbA1c were performed in all patients at baseline.
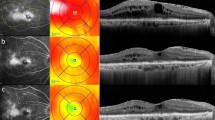
Serial changes in the selective retina therapy (SRT) spots on color fundus photography (CFP) and fundus autofluorescence (FAF). A 76-year-old woman with diabetic macular edema received panretinal conventional laser photocoagulation (CLP) in the right eye 5 years previously. No visible SRT spots were seen in the treated area (green circle) and tested area (yellow circle) on CFP at baseline: a 1 h, b 3 months, c 6 months, and d postirradiation. Retinal hard exudates at the SRT-treated region were markedly decreased by 6 months after SRT (d). No visible change was seen on FAF at baseline (e) and 1 h (f), 3 months (g), 6 months (h) postirradiation. Fundus fluorescein angiography (FFA) showed hyperfluorescence in old CLP spots at baseline (i). While 4 pairs of SRT test spots (100, 110, 120, 130 μJ) (yellow arrowheads) were visible around the inferior arcade on FFA, 1 pair of SRT spots (90 μJ) (green arrowhead) were invisible 1 h after SRT irradiation (j). Magnified image of SRT test spots (k). Sixty-seven SRT spots of 100 μJ were applied to cover the area of macular edema.
All patients also underwent a complete ophthalmologic examination, including slit-lamp biomicroscopy, SD-OCT (Cirrus HD-OCT with software version 6.0; Carl Zeiss Meditec, Dublin, CA, USA), CFP (CF-60UVi; Canon Inc., Japan), and fundus autofluorescence (FAF) (Heidelberg Retina Angiograph 2 [HRA2]; Heidelberg Engineering, Heidelberg, Germany) at baseline and again at 3 and 6 months after SRT. SD-OCT was used to obtain the macular scan in an area of 6 × 6 mm2 using the 512 × 128 macular cube scan protocol after pupil dilation. The nine ETDRS subfields, including central subfield thickness, were automatically analyzed with Cirrus software (version 6.0). CFP, FAF, and FFA (HRA2) were performed at baseline and at 1 h after test spot irradiation. BCVA was measured with a standard Snellen chart and was converted to the logarithm of the minimum angle of resolution (logMAR) value for analysis.
A single-session retreatment was performed at 3 months after initial SRT if there was no reduction in CFT. Retreatment was performed to cover the whole edematous area according to the same laser parameters used for the first irradiation. Rescue treatment with 1.25 mg/0.05 mL intravitreal bevacizumab injections (Avastin; Roche, Switzerland) was performed if persistent DME or vision loss of 1 or more logMAR line were observed at 6 months after the initial SRT. All patients receiving rescue treatment were followed up at least 1 month after injection. Any adverse effects, such as retinal burns, retinal hemorrhage, and choroidal neovascularization, were recorded.
Patients were divided into two groups based on CFT at baseline. Group 1 was composed of eyes with a CFT < 400 μm, and group 2 was composed of eyes with a CFT ≥ 400 μm.
Statistical analysis
The change in CFT and BCVA from baseline to months 3 and 6 was assessed in each of the two groups by using paired t-tests. The Student’s t test was used to compare the baseline characteristics between the two groups in age, duration of diabetes, HbA1c, BCVA, and CFT. The chi-square test was used to compare the baseline characteristics in sex, type of macular edema, and the number of treatment naïve eyes. Statistical analyses were performed using SPSS version 18.0 (SPSS Inc., Chicago, IL, USA). A P value of < 0.05 was considered significant.
Results
Overall, we reviewed 98 eyes of 85 consecutive patients with clinically significant DME, who were treated with SRT laser. Of these, 72 eyes of 63 patients were finally included for the analysis according to inclusion and exclusion criteria. Thirty-five eyes of 31 patients were classified as group 1, and 37 eyes of 32 patients were classified as group 2. Overall, 68 eyes of 59 patients with a history of local steroid > 6 months (3 eyes), or intravitreal anti-VEGF injection > 3 months (38 eyes), or CLP > 1 year (31 eyes) prior to SRT were included based on inclusion/exclusion criteria; the number of treatment naïve eyes was 3 eyes (8.6%) in group 1 and 1 eye (2.7%) in group 2. There was no statistically significant difference in baseline characteristics between the two groups, except for mean BCVA and CFT (Table 1).
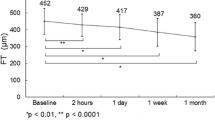
At 3 months after SRT, group 1 showed a mean reduction of 24.4 μm in CFT (from 368.8 ± 28.8 to 344.4 ± 32.5 μm; P < 0.001), whereas group 2 experienced no significant change in mean CFT (from 540.9 ± 87.6 to 533.4 ± 90.9 μm; P = 0.33). At 6 months after SRT, group 1 showed a mean reduction of 45.9 μm in CFT, to 322.9 ± 39.5 μm (P < 0.001), whereas group 2 again experienced no significant change in CFT, to 555.5 ± 93.4 μm (P = 0.10). At 6 months, CFT was reduced by > 5% in 65.7% of eyes in group 1, as compared to 8.1% of the eyes in group 2 (Fig. 2).
Group 1 experienced mean BCVA gains from 0.43 logMAR at baseline to 0.39 logMAR at month 3 (P = 0.06) and 0.3 logMAR at month 6 (P < 0.001), whereas group 2 showed no significant changes in mean BCVA at month 3 or 6 (P = 0.26, P = 0.91) (Table 2). At month 6, 74.3% of group 1 had gained ≥ 1 line logMAR visual acuity (VA) from baseline, and 2.9% of group 1 had lost ≥ 1 line logMAR VA; in contrast, 24.3% of group 2 had gained ≥ 1 line logMAR VA from baseline, and 40.5% of group 2 had lost ≥ 1 line logMAR VA (Fig. 3).
In group 1, additional SRT was performed in 6 of 35 eyes (17.1%) at 3 months, and rescue bevacizumab injection was performed in 1 eye (2.9%) at 6 months. In group 2, additional SRT was performed in 17 of 37 eyes (45.9%) at 3 months, and rescue bevacizumab injections were performed in 27 of 37 eyes (73%) at 6 months (Fig. 4). One month after rescue injections, the mean BCVA was improved from 0.54 logMAR (SD = 0.20) to 0.47 logMAR (SD = 0.20) (P < 0.001), and the mean CFT was reduced from 573.1 ± 91.4 μm to 484.71 ± 82.2 μm (P < 0.001).
In group 1, the mean pulse energy of first and additional SRT were 113.19 μJ (SD = 38.90) and 90.83 μJ (SD = 17.42), respectively, and the mean number of initial SRT spots and retreatment spots were 37.36 (SD = 25.56) and 29.33 (SD = 10.81), respectively. In group 2, the mean pulse energy of the first and additional SRT were 116.89 μJ (SD = 25.51) and 112.0 μJ (SD = 26.62 respectively, and the mean numbers of initial and retreatment spots were 58.79 (SD = 36.37), and 68.65(SD =26.62) respectively.
No adverse effects from SRT laser were observed in either group. All SRT spots were invisible during irradiation, and no evidence of photoreceptor damage was detected by CFP or OCT findings during the 6-month follow-up period. Additionally, no change in the SRT-treated region was observed on FAF during the follow-up period (Fig. 1).
Discussion
Although SRT monotherapy has demonstrated promising results for DME, previous studies did not investigate the effect of the initial CFT. CFT may influence patient outcomes, as it does in SMPL. Thus, we evaluated the efficacy of SRT for DME according to pretreatment CFT. We found that SRT had a limited effect on severe DME but was effective for improving BCVA and reducing CFT in mild DME cases during a 6-month follow-up period.
RPE-targeting lasers, such as SMPL and 2RT, have shown favorable results in clinical studies of DME [12, 14, 16, 17, 25, 26, 29, 30]. Interestingly, the patients evaluated in those clinical studies had DME with mean CFT values in the range of 275–379 μm. Since the mean baseline CFT prior to SMPL treatment was < 400 μm in these studies, the efficacy of SMPL for thicker CFT (≥ 400 μm) was not investigated. Recently, Mansouri et al. reported that 810-nm SMPL was effective in DME patients with pretreatment CFT < 400 μm, as compared to CFT ≥ 400 μm. Although all patients with ≥ 400 μm CFT received a rescue bevacizumab injection, no patients with CFT < 400 μm required rescue treatment from 6 to 12 months during follow-up [27]. In addition, Citirik M reported that 577 nm SMPL yielded a significant improvement in BCVA and a reduction in CFT in DME patients with < 300 μm pretreatment CFT; however, patients with CFT ≥ 300 μm experienced no significant change in CFT [28]. Although the measurements of central retinal thickness vary, depending on the different OCT devices or protocol implemented [31], many clinical studies showed that RPE-targeting lasers, such as SMPL and 2RT, tended to be more effective for mild DME patients with thinner initial CFT.
Unlike SMPL, the effect of SRT originates from retinal rejuvenation, similar to 2RT, in terms of RPE restoration after RPE damage. Although the mechanism of SRT remains unclear, the beneficial effect is known to be associated with the restoration of the outer blood-retinal barrier and the expression of MMPs, a particularly active form of MMP-2 and other cell mediators during the RPE healing process [21,22,23]. The role of active MMP-2 was known to improve hydraulic conductivity and transport capabilities through Bruch’s membrane [19, 21]. After SRT irradiation on an ex vivo model of RPE, a decrease of VEGF, a major angiogenic stimulator, and an increase of the pigment epithelium-derived factor, a potent angiogenic inhibitor, were shown 3 days after treatment. Additionally, the active form of MMP-2 was upregulated in the SRT lesion after treatment [21,22,23]. Theses cell mediators seem to be associated with the therapeutic effect of SRT for DME.
Roider et al. reported that SRT induced functional and anatomical improvement or stabilization in 84% of DME eyes with a mean baseline CFT of 276 μm at 6 months after SRT [13]. Considering that the efficacy of new RPE-targeting lasers tends to be greater in DME patients with overall baseline CFT < 400 μm, we divided patients in this study into 2 groups using a baseline CFT cut-off of 400 μm.
In this study, a mean CFT reduction of 45.9 μm and mean improvement of 0.13 logMAR VA was shown at 6 months in group 1; however, there was no significant improvement in the mean CFT and BCVA of group 2 at 6 months after SRT. While our previous pilot study showed that BCVA was maintained or improved in 88.2% of SRT-treated eyes with DME at 6 months, there was no significant improvement in reducing CFT. Although the SRT-treated eyes in the prior study had a mean baseline CFT of 351.1 μm (SD=110.2) [15], similar to the mean baseline CFT of 368.8 μm (SD=28.8) in group 1 in the current study, the baseline characteristics of patients were different because the range of CFT (221–585 μm) in the previous study was wider than that (283–398 μm) in group 1 of the present study.
In the current study, single-session retreatment was performed at 3 months because additional SRT was shown to influence the reduction of macular thickness positively in a previous study [15]. In group 1, six eyes (17.1%) received retreatment at 3 months and showed a reduction in CFT at 6 months (Fig. 5). Only one eye in group 1 received rescue anti-VEGF injections because of persistent DME at 6 months. Although 17 eyes (45.9%) in group 2 underwent retreatment at 3 months, all retreated eyes were given rescue anti-VEGF injections at 6 months after SRT. While the mean number of 9.9 additional retreatment spots were applied to cover the enlarged macular edema in group 2, retreatment was not effective for treating DME, unlike in group 1. Since 88.43 μm reduction in mean CFT and 0.07 logMAR improvement in BCVA were measured 1 month after rescue treatment, anti-VEGF injection was effective for non-responder to SRT in this study.
A 58-year-old man who presented with clinically significant diabetic macular edema (DME) in the right eye. a DME was observed on optical coherence tomography (OCT) at initial presentation. b At 3 months after selective retina therapy (SRT), retreatment was performed because of the persistent DME after the first SRT. c DME was markedly resolved 6 months after SRT.
Although the mean pulse energy was similar between the two groups, an average number of 21.4 spots more were needed in group 2 than in group 1 to cover the larger macular edema, according to the one-spot-spaced irradiation protocol. In our previous study, we demonstrated that increased autofluorescence in SRT spots noted 1 week after SRT had disappeared gradually by the 3-month follow-up [15]. Similar to the previous study, no change in FAF was observed at the 3- and 6-month follow-ups. No SRT-related adverse effects, such as retinal burns, retinal hemorrhages, and choroidal neovascularization, were noted in this study.
Even though the cause of a poor response to SRT in patients with thicker DME is not clear, several possible causes may be postulated. First, the beneficial effect of SRT is associated with the release of various cell mediators during RPE regeneration after SRT [21, 22]. Thicker DME might dilute the concentration of the cell mediators, as deduced from studies of SMPL [27, 28]. Second, since the minimum pulse energy of SRT is chosen according to the ophthalmoscopic and angiographic findings for test spots, any discrepancy between the healthy tested area and the edematous area could induce undertreatment. In the previous study, this discrepancy between the healthy test area and the thicker macula with sub-retinal fluid resulted in occasional undertreatment in patients with central serous chorioretinopathy (CSC). When adequate pulse energy was measured in CSC patients with large sub-retinal fluid by real-time dosimetry, an increase of pulse energy was necessary for proper treatment [32]. We speculate that medium pulse energy rather than minimum pulse energy might be more effective for thicker DME. Whereas inappropriately increased pulse energy for thicker DME can produce photoreceptor damage, an optimized dosimetry system might facilitate in fine-tuning the adequate pulse energy by monitoring the instant response of RPE to SRT in real-time [13, 15, 32, 33]. Under- or over-treatment might be avoided by using real-time dosimetry with complete optimization of dosimetry in the near future. Third, although adequate pulse energy was determined from angiographic features of the test spots, intra-individual differences in the distribution of pigment might potentially affect the appropriate treatment.
Our study had several limitations, including its retrospective study design, short-term postoperative follow-up period, the relatively small sample size, and the small number of treatment-naïve patients. In addition, although patients’ HbA1c levels were documented at baseline, glycemic control markers could not be assessed during the follow-up period.
In conclusion, the smaller CFT prior to treatment shows a higher effectiveness of SRT for DME in improving BCVA and reducing CFT, as well as decreasing the number of retreatments and rescue treatments in this study. Therefore, SRT could be a therapeutic option for mild DME, without the need for frequent anti-VEGF injections. To our knowledge, this is the first study to report the efficacy of SRT in accordance with baseline anatomical severity of diabetic macular edema. However, further prospective randomized clinical trials with larger sample sizes are necessary to evaluate the efficacy of SRT based on the anatomical severity of DME.
References
Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, Lin JK, Farzadfar F, Khang Y-H, Stevens GA, Rao M, Ali MK, Riley LM, Robinson CA, Ezzati M (2011) National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2·7 million participants. Lancet 378:31–40. https://doi.org/10.1016/s0140-6736(11)60679-x
Cheung N, Mitchell P, Wong TY (2010) Diabetic retinopathy. Lancet 376:124–136. https://doi.org/10.1016/s0140-6736(09)62124-3
Antcliff RJ, Marshall J (2009) The pathogenesis of edema in diabetic maculopathy. Semin Ophthalmol 14:223–232. https://doi.org/10.3109/08820539909069541
Tso MO (1982) Pathology of cystoid macular edema. Ophthalmology 89:902–915. https://doi.org/10.1016/s0161-6420(82)34698-9
group ETDRSr (1985) Photocoagulation for diabetic macular edema. Early treatment diabetic retinopathy study report number 1. Arch Ophthalmol 103:1796–1806
Lee CM, Olk RJ (1991) Modified grid laser photocoagulation for diffuse diabetic macular edema. Long-term visual results. Ophthalmology 98:1594–1602. https://doi.org/10.1016/s0161-6420(91)32082-7
Schatz H, Madeira D, McDonald HR, Johnson RN (1991) Progressive enlargement of laser scars following grid laser photocoagulation for diffuse diabetic macular edema. Arch Ophthalmol 109:1549–1551. https://doi.org/10.1001/archopht.1991.01080110085041
Lewis H, Schachat AP, Haimann MH, Haller JA, Quinlan P, von Fricken MA, Fine SL, Murphy RP (1990) Choroidal neovascularization after laser photocoagulation for diabetic macular edema. Ophthalmology 97:503–510; discussion 510-501. https://doi.org/10.1016/s0161-6420(90)32574-5
Pankratov MM (1990) Pulsed delivery of laser energy in experimental thermal retinal photocoagulation. Proc. SPIE 1202, laser-tissue interaction,
Chhablani J, Roh YJ, Jobling AI, Fletcher EL, Lek JJ, Bansal P, Guymer R, Luttrull JK (2018) Restorative retinal laser therapy: present state and future directions. Surv Ophthalmol 63:307–328. https://doi.org/10.1016/j.survophthal.2017.09.008
Sivaprasad S, Dorin G (2012) Subthreshold diode laser micropulse photocoagulation for the treatment of diabetic macular edema. Expert Rev Med Devices 9:189–197. https://doi.org/10.1586/erd.12.1
Laursen ML, Moeller F, Sander B, Sjoelie AK (2004) Subthreshold micropulse diode laser treatment in diabetic macular oedema. Br J Ophthalmol 88:1173–1179. https://doi.org/10.1136/bjo.2003.040949
Roider J, Liew SH, Klatt C, Elsner H, Poerksen E, Hillenkamp J, Brinkmann R, Birngruber R (2010) Selective retina therapy (SRT) for clinically significant diabetic macular edema. Graefes Arch Clin Exp Ophthalmol 248:1263–1272. https://doi.org/10.1007/s00417-010-1356-3
Pelosini L, Hamilton R, Mohamed M, Hamilton AM, Marshall J (2013) Retina rejuvenation therapy for diabetic macular edema: a pilot study. Retina 33:548–558. https://doi.org/10.1097/IAE.0b013e3182670fea
Park YG, Kim JR, Kang S, Seifert E, Theisen-Kunde D, Brinkmann R, Roh YJ (2016) Safety and efficacy of selective retina therapy (SRT) for the treatment of diabetic macular edema in Korean patients. Graefes Arch Clin Exp Ophthalmol 254:1703–1713. https://doi.org/10.1007/s00417-015-3262-1
Lavinsky D, Cardillo JA, Melo LA Jr, Dare A, Farah ME, Belfort R Jr (2011) Randomized clinical trial evaluating mETDRS versus normal or high-density micropulse photocoagulation for diabetic macular edema. Invest Ophthalmol Vis Sci 52:4314–4323. https://doi.org/10.1167/iovs.10-6828
Vujosevic S, Bottega E, Casciano M, Pilotto E, Convento E, Midena E (2010) Microperimetry and fundus autofluorescence in diabetic macular edema: subthreshold micropulse diode laser versus modified early treatment diabetic retinopathy study laser photocoagulation. Retina 30:908–916. https://doi.org/10.1097/IAE.0b013e3181c96986
Novack RL, Stefansson E, Hatchell DL (1990) The effect of photocoagulation on the oxygenation and ultrastructure of avascular retina. Exp Eye Res 50:289–296. https://doi.org/10.1016/0014-4835(90)90213-e
Ahir A, Guo L, Hussain AA, Marshall J (2002) Expression of metalloproteinases from human retinal pigment epithelial cells and their effects on the hydraulic conductivity of Bruch's membrane. Invest Ophthalmol Vis Sci 43:458–465
Sramek C, Mackanos M, Spitler R, Leung LS, Nomoto H, Contag CH, Palanker D (2011) Non-damaging retinal phototherapy: dynamic range of heat shock protein expression. Invest Ophthalmol Vis Sci 52:1780–1787. https://doi.org/10.1167/iovs.10-5917
Treumer F, Klettner A, Baltz J, Hussain AA, Miura Y, Brinkmann R, Roider J, Hillenkamp J (2012) Vectorial release of matrix metalloproteinases (MMPs) from porcine RPE-choroid explants following selective retina therapy (SRT): towards slowing the macular ageing process. Exp Eye Res 97:63–72. https://doi.org/10.1016/j.exer.2012.02.011
Richert E, Koinzer S, Tode J, Schlott K, Brinkmann R, Hillenkamp J, Klettner A, Roider J (2018) Release of different cell mediators during retinal pigment epithelium regeneration following selective retina therapy. Invest Ophthalmol Vis Sci 59:1323–1331. https://doi.org/10.1167/iovs.17-23163
Brinkmann R, Roider J, Birngruber R (2006) Selective retina therapy (SRT): a review on methods, techniques, preclinical and first clinical results. Bull Soc Belge Ophtalmol 302:51–69
Roider J, Brinkmann R, Wirbelauer C, Laqua H, Birngruber R (1999) Retinal sparing by selective retinal pigment epithelial photocoagulation. Arch Ophthalmol 117:1028–1034. https://doi.org/10.1001/archopht.117.8.1028
Luttrull JK, Musch DC, Mainster MA (2005) Subthreshold diode micropulse photocoagulation for the treatment of clinically significant diabetic macular oedema. Br J Ophthalmol 89:74–80. https://doi.org/10.1136/bjo.2004.051540
Luttrull JK, Sinclair SH (2014) Safety of transfoveal subthreshold diode micropulse laser for fovea-involving diabetic macular edema in eyes with good visual acuity. Retina 34:2010–2020. https://doi.org/10.1097/iae.0000000000000177
Mansouri A, Sampat KM, Malik KJ, Steiner JN, Glaser BM, Medscape (2014) Efficacy of subthreshold micropulse laser in the treatment of diabetic macular edema is influenced by pre-treatment central foveal thickness. Eye (Lond) 28:1418–1424. https://doi.org/10.1038/eye.2014.264
Citirik M (2018) The impact of central foveal thickness on the efficacy of subthreshold micropulse yellow laser photocoagulation in diabetic macular edema. Lasers Med Sci 34:907–912. https://doi.org/10.1007/s10103-018-2672-9
Casson RJ, Raymond G, Newland HS, Gilhotra JS, Gray TL (2012) Pilot randomized trial of a nanopulse retinal laser versus conventional photocoagulation for the treatment of diabetic macular oedema. Clin Exp Ophthalmol 40:604–610. https://doi.org/10.1111/j.1442-9071.2012.02756.x
Vujosevic S, Martini F, Longhin E, Convento E, Cavarzeran F, Midena E (2015) Subthreshold micropulse yellow laser versus subthreshold micropulse infrared laser in center-involving diabetic macular edema: morphologic and functional safety. Retina 35:1594–1603. https://doi.org/10.1097/iae.0000000000000521
Heussen FM, Ouyang Y, McDonnell EC, Narala R, Ruiz-Garcia H, Walsh AC, Sadda SR (2012) Comparison of manually corrected retinal thickness measurements from multiple spectral-domain optical coherence tomography instruments. Br J Ophthalmol 96:380–385. https://doi.org/10.1136/bjo.2010.201111
Park YG, Kang S, Kim M, Yoo N, Roh YJ (2017) Selective retina therapy with automatic real-time feedback-controlled dosimetry for chronic central serous chorioretinopathy in Korean patients. Graefes Arch Clin Exp Ophthalmol 255:1375–1383. https://doi.org/10.1007/s00417-017-3672-3
Minhee K, Park YG, Kang S, Roh YJ (2018) Comparison of the tissue response of selective retina therapy with or without real-time feedback-controlled dosimetry. Graefes Arch Clin Exp Ophthalmol 256:1639–1651. https://doi.org/10.1007/s00417-018-4067-9
Acknowledgments
Medical laser center Lübeck (MLL) provided SRT laser system.
Funding
This study was supported in part by the grant from South Korean Government’s Ministry of Trade, Industry and Energy (M000004912-00192937) and was supported in part by grant of the Institute of Clinical Medicine Research in the Yeouido St. Mary’s hospital, Catholic University of Korea.
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: Minhee Kim, Young-Jung Roh; Performed the treatment: Young-Jung Roh; Analyzed the data: Minhee Kim, Young-Jung Roh; Contributed reagents/materials/analysis tools: Minhee Kim, Young Gun Park, Seung Hee Jeon, Seung Yong Choi, Young-Jung Roh; Wrote the paper: Minhee Kim, Young Gun Park, Young-Jung Roh; Approved final version of the manuscript: Young-Jung Roh, Minhee Kim, Young Gun Park, Seung Hee Jeon, Seung Yong Choi.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study adhered to the principles of the Declaration of Helsinki and data collection was compliant with and approved by the Institutional Review Board of Yeouido St. Mary’s Hospital of the Catholic University of Korea.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Disclaimer
Medical laser center Lübeck (MLL) provided SRT laser system through international R&D project from South Korean Government’s Ministry of Trade, Industry and Energy (M000004912-00192937). MLL had no role in the design or conduct of this study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kim, M., Park, Y.G., Jeon, S.H. et al. The efficacy of selective retina therapy for diabetic macular edema based on pretreatment central foveal thickness. Lasers Med Sci 35, 1781–1790 (2020). https://doi.org/10.1007/s10103-020-02984-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-020-02984-6