Abstract
In this work, we present the efficacy of photodynamic therapy against yeast cells in an animal model. We tested two photosensitizers, methylene blue and protoporphyrin IX. Thirty-seven female BALB-c mice with a body mass of 20–25 g were used. To achieve persistent vaginitis, estrus was induced by subcutaneous injection of 0.1 mg/mL estradiol valerate applied weekly. Three days after pseudo-estrus, intravaginal inoculation with Candida albicans was performed. Mice were anesthetized with ketamine (80 mg/kg) and xylazine (10 mg/kg) by intraperitoneal injection before inoculation, and antimicrobial photodynamic therapy (aPDT) was performed 5 days after fungal inoculation. Two photosensitizers were tested, methylene blue (MB; 100 μM) and protoporphyrin IX (PpNetNI; 10 μM). Two custom-made LEDs emitting light at 660 and 630 nm at approximately 800 mW each were used for irradiation. The aPDT treatment reduced the fungal colony-forming units (CFUs) by one order of magnitude for the MB (p = 0.020) and PpNetNI (p = 0.018) photosensitizers. Seven days after the treatment, there were significantly fewer CFUs compared to the control group (p = 0.041 and p = 0.035 for MB and PpNetNI, respectively), but this was not increased compared to the initial number immediately after aPDT. Using aPDT as a therapeutic option to decrease fungal infection in a vaginal candidiasis model resulted in a significant reduction in the C. albicans population. Both photosensitizers were effective for preventing reinfection within 7 days. The aPDT also had no effect on the vaginal mucosa at the ultrastructural level. In addition to the fungicide effect, we observed reduced swelling and lack of the formation of abscesses, microabscesses coating the cornified epithelial layer, and the accumulation of neutrophils in the submucosa.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vulvovaginal candidiasis (VVC) is a superficial infection caused by species of Candida. It affects millions of women every year. Although Candida albicans is the main cause of VVC (85–90%), the identification of non-Candida albicans Candida (NCAC) species, such as C. glabrata, C. krusei, C. tropicalis, and C. parapsilosis, is increasing [1].
Vulvovaginal candidiasis affects around 75% of women of childbearing age at least once in their lifetime, and of these women, half will experience a second episode [2]. Patients usually report soreness, vaginal redness, dysuria, dyspareunia, and burning, often accompanied by vaginal discharge [3, 4]. VVC is considered one of the most neglected diseases in scientific research, as well as in medical history. Problems associated with antifungal effectiveness, drug safety, and microbial resistance limit the treatment of this fungal infection. The azole family has been used for the treatment of candidemia and localized Candida infection. Fluconazole acts on the molecular target 14-alpha-demethylase, a P450 enzyme, which is responsible for cholesterol production in mammalian liver cells. The adverse effects of azoles are related to blocking the activity of these enzymes, and consequently, high liver toxicity [5].
The current limitations of drugs for the treatment of vaginal candidiasis, combined with the prevalence of the infection, reinforce the need for different treatment approaches for symptomatic patients. Antimicrobial photodynamic therapy (aPDT) represents a potential new therapeutic adjuvant option that works by inactivating Candida cells using light of a certain wavelength and nontoxic photosensitizers (PS).
Antimicrobial photodynamic therapy is based on the interaction of harmless low-intensity visible light with a nontoxic dye (e.g., methylene blue) in the presence of oxygen. Lasers (most common light source) and light-emitting diode (LED) can present emissions in the range from 580 to 680 nm.
The effectiveness of aPDT depends on the right combination of PS and irradiation parameters [6]. At least four parameters must be considered in this interaction: low or no toxicity of the PS, efficiency bringing singlet oxygen, cell membrane permeability, and fast elimination of the PS after treatment.
Photodynamic therapies recently received FDA approval for ophthalmologic (treatment for age-related macular degeneration) [7].
In this work, we present an in vivo model to determine the effect of certain irradiation parameters on fungal yeast cells. Two different PS were used, PpNetNI (a cationic derivate of protoporphyrin IX) and methylene blue (MB). Microbiological analyses were performed to compare the inactivation of fungal cells on the day of treatment and 7 days after. Vaginal samples were collected to compare the histological and inflammatory effects at these two timepoints.
Materials and methods
Candida albicans (ATCC 90028) were grown on Saboraud dextrose agar (Acumedia® Lot 105961B expiry May 2017) under aerobic conditions for 24 h before the experiment. Cells were suspended in phosphate-buffered saline (PBS) and then inoculated intravaginally.
Animal model
The vulvovaginal candidiasis murine experimental model adopted in this study was previously described by Fidel in 2008 [9]. The Ethics Committee of Universidade Nove de Julho (protocol number 00025/2013) approved this work.
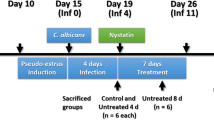
Thirty-seven female BALB-c mice with a body mass of 20–25 g were used in this study. All animals were housed under a 12-h light/dark cycle and had ad libitum access to food and water. To achieve persistent vaginitis, we induced estrus by the subcutaneous injection of 0.1 mg/mL estradiol valerate. Vaginal inoculation of C. albicans was performed 3 days after pseudo-estrus with approximately 1 × 106 yeast cells in 50 mL of PBS. Mice were anesthetized with ketamine (80 mg/kg) and xylazine (10 mg/kg) by intraperitoneal injection before inoculation (Fig. 2) and treatment [10]. Treatment with aPDT was performed 5 days after fungal inoculation.
Animals were divided into three groups. One group was treated with MB, one with PpNetNI, and a control group. Animals in the control group were inoculated with 50 μL of PBS inside the vagina (Fig. 1b) and left untreated. After the Candida infection, we performed a vaginal wash for microbiological analysis on day 0 (n = 8) and on day 7 (n = 7). The vaginal canals of control mice that were euthanized on day 0 were removed for analysis.
Animals in the MB group were inoculated with 50 μL of 100 μM MB solution inside the vagina (Fig. 1b), then irradiated with a laser at 660 nm. The laser head was directed at the entrance of the vaginal canal. On day 0, immediately after treatment, a vaginal wash was performed for microbiological analysis and the vaginal canal was removed for histological analyses (n = 6). Five other animals were kept alive until day 7, when a vaginal wash was performed for microbiological analysis.
Mice in the PpNetNI group were inoculated with 50 μL of 10 μM PpNetNI solution inside the vagina (Fig. 1b) and irradiated with a LED at 630 nm. Due to the size of the LED head, the irradiation for this group was transabdominal. After the treatment on day 0, we performed a vaginal wash for microbiological analysis and removed the vaginal canal for histological analyses (n = 6). Five other animals were kept alive until day 7, when a vaginal wash was performed for microbiological analysis.
Irradiation parameters
Due to the different absorption spectra of the PS (Fig. 1), different irradiation sources were used. A laser emitting at 660 nm was used for the MB group and, due to the lack of a commercial laser, a custom LED emitting at 630 nm was used for the PpNetNI group. The effect of aPDT is reliant on exposure time, and a longer irradiation time is more likely to inactivate the microorganism. The treatment of C. albicans vaginitis by aPDT in an animal model has previously been reported with 360 s of irradiation. In an attempt to increase the photodynamic effect, the irradiation time was doubled in comparison to these previous studies [11, 12]. The irradiation parameters are listed in Table 1.
Figure 2 shows the process of inoculation with C. albicans and the PS. We inserted the laser head into the vaginal canal to deliver the light; however, as the LED head was too large, LED irradiation was performed transabdominally, as shown in Fig. 1.
The longer optical path of the LED photons is partially compensated by its higher power.
Microbiological analysis
A vaginal wash with 50 μL PBS was performed to recover the yeast. The sample was serially diluted in PBS to achieve 10−1 to 10−3 of the original concentration; then, 10 μL of each group was streaked onto Sabouraud chloramphenicol agar plates (Acumedia®) in triplicate and incubated at 37 °C for 24 h.
Histomorphometrical analysis
The vaginal canal was removed for analysis. Longitudinal sections were cut, fixed, and then stained with hematoxylin and eosin (H&E). Additional histological sections were stained with periodic acid Schiff (PAS) in order to observe C. albicans perfusion in the epithelial layers.
A 2-μm longitudinal section of the vagina (from the vulva to the cervix) was analyzed to identify any microstructural changes. The neutrophil influx scores are presented in Table 2.
Statistical analysis
The colony-forming units (CFU) presented a log-normal distribution as analyzed by the Shapiro-Wilk test (p > 0.05). A one-tailed t test was used for analysis, and a difference was considered if the number of CFU determined for the groups that received aPDT was less than the control group. To maintain the global significance level at α = 0.05, all p values were corrected by the Hyan-Holm stepdown Bonferroni procedure. The histological scores were statistically analyzed using the Mann-Whitney test.
Results
Figure 3 shows that the model of C. albicans infection was successful, with 5.17 ± 0.63 log10 (CFU/mL) yeast recovered from vaginal wash of mice in the control group. The aPDT treatments achieved a reduction in fungal amount for both MB (p = 0.020) and PpNetNI (p = 0.018) groups. The results obtained 7 days after treatment showed 4.19 ± 0.21 log10 (CFU/mL) yeast recovered for MB (p = 0.041) and 4.37 ± 0.48 log10 (CFU/mL) for PpNetNI (p = 0.035), both of which were significantly different from the control group.
The histological analysis showed an intense inflammatory response in the proximal area of the vagina, mostly classified as score 3 according to the definition provided in Table 2, and the epithelium of the cervix womb showed neutrophil infiltration for all groups (control, MB, and PpNetNI). On the other hand, the groups that received aPDT showed only discrete neutrophil infiltrate in the entrance and medial area of the vagina (Fig. 4). No alteration in fibroblast organization nor necrosis was observed following aPDT.
Inflammatory response of the samples. The lowest score (0) represents a lack of neutrophils in the sample; in the score (1), it is possible to find mild: (1 to 10) neutrophils in the lumen and 1 to 5 microabscesses present in the epithelial layer; in the score (2), there are numerous neutrophils in the lumen and in the submucosa, along with numerous microabscesses; the highest score (3) was characterized by large abscess formation, microabscesses coating the cornified epithelial layer and numerous neutrophils in the submucosa, as defined in Table 2. The control group presented significant differences to the methylene blue (p = 0.041) and the PpNetNI (p = 0.027) groups
Figure 5 shows representative histological sections of mice in each group.
a Photomicrograph of the vaginal canal of mice in the control group showing numerous neutrophils in the upper epithelial layers (score 3), lumen and connective tissue (stained with hematoxylin and eosin [H&E], original magnification 160×). b Vaginal canal of mice in the control group showing Candida sp. hyphae in the superficial epithelial layer and in the lumen (stained with periodic acid Schiff [PAS], original magnification 160×). c Vaginal canal of mice in the methylene blue (MB) group with few neutrophils in the epithelia and lumen (score 1) (stained with H&E, original magnification 160×). d Vaginal canal of mice in the MB group showing Candida sp. hyphae in the superficial epithelial layer and in the lumen, but no neutrophils (score 0) (stained with PAS, original magnification 160×). e Vaginal canal of mice in the PpNetNI group with scant neutrophils in the upper epithelial layers and lumen (stained with H&E, original magnification 160×). f Vaginal canal of mice in the PpNetNI group with Candida sp. hyphae in the superficial epithelial layer and in the lumen (stained with PAS, original magnification 160×)
Discussion
Many authors have described the antifungal effects of aPDT [8, 13,14,15,16]. In this study, we applied a different approach to evaluate the interaction among the microorganisms and the inflammatory response and defense in the vaginal environment of the host 7 days after treatment. Some authors [17, 18] reported a 3 log fungal reduction in in vitro models. However, the goal of these studies was usually to achieve a complete fungicidal effect, whereas the current study aimed to reduce the fungal infection.
Antimicrobial photodynamic therapy is only effective in the presence of light. Furthermore, as opposed to a systemic drug-based antifungal treatment, aPDT acts locally at the site of PS application and irradiation. This is an advantage in the case of localized infections, facilitating treatment of the infected site without compromising the microbiota of the host.
Antimicrobial photodynamic therapy (with the PS used for this work) is not specific when it comes to the species of Candida or even the microorganisms that colonize the vagina. Therefore, it is likely that aPDT altered the overall microbiota of the vaginal canal of mice in the current study. Nevertheless, similar effects are expected when an azole-based treatment is administered [19], but with the added side effect of liver toxicity. In clinical practice, changes in the vaginal microbiota after antibiotic treatment show a trend toward recolonization of the vagina by commensal species leading to re-establishment of vaginal homeostasis [19].
The PS uptake may also influence the selectivity of the treatment. Following a certain period of contact in the dark, the microorganism can begin to clear PS molecules from the cytoplasm. Phenotiazinium dyes, for instance, are substrates for the ABC pump of Candida and can affect the killing rate for this yeast [18, 20, 21].
As the vaginal environment is normally colonized by C. albicans, a complete fungicidal effect may be deleterious to the microbiota that regularly colonize the vagina; thus, the 1 log reduction in this model is more suitable than complete sterilization of the vaginal canal. Nevertheless, more studies are needed to investigate to behaver of vaginal microbiota after PDT and how it impacts the ecology and bacterial recolonization.
Studies in the literature using similar PS and radiation parameters in vitro are in good agreement with the findings of this work [17]. The effect of efflux systems on aPDT suggests that multidrug resistance (MDR) inhibitors could be used with phenothiazinium salts to improve the efficacy of aPDT. Moreover, blocking multidrug efflux pumps with specific inhibitors has been shown to increase antimicrobial photoinactivation, and C. albicans may be affected by efflux pump blockers [18, 21]. Aside from aPDT, C. albicans may also be affected by efflux pump blockers [22]. However, the literature lacks data on the effect of efflux pumps and MDR inhibitors on cationic PS in animal models. The current study shows that it is possible to achieve about 1 log reduction in C. albicans using aPDT with MB or PpNetNI.
Although variations in the radiation parameters and PS may influence the outcome of photodynamic therapy, specific characteristics of the in vitro infection, such as the organization of the C. albicans in biofilms, plays an important role and may decrease the effectiveness of the aPDT in comparison with in vitro studies [17, 23].
Methylene blue is an inexpensive but efficient photosensitizer with low toxicity in humans and animals. In this murine model, we obtained a significant reduction in yeast immediately after MB application (p = 0.020). Seven days after the procedure, the population of C. albicans had still not increased at the infection site (p = 0.041), suggesting that the aPDT was also effective in the longer term.
The photosensitizer PpNetNI was recently synthesized, and the current study presents the first results for its application in an in vivo model. Despite the little information available for PpNetNI, porphyries usually present a highly efficient photodynamic effect [23].
Using PpNetNI and a 630-nm high-power LED, we obtained a significant reduction in the number of fungal colonies immediately after the application (p = 0.018). In addition, the number of colonies were still diminished 7 days after the procedure in comparison with the control group (p = 0.035). Although performing aPDT on day 0 did not eradicate the yeast, it was unable to recolonize the microenvironment within 7 days.
Histological analysis of samples collected immediately after the procedure showed a score of zero at every site, indicating that there were no deleterious effects in the tissue architecture at the microstructure level due to the aPDT (Fig. 4).
Overgrowth of Candida triggers an inflammatory response in the vaginal wall, which cause the symptoms of the disease. The ability of Candida to grow in a biofilm is related to its pathogenesis and virulence [10, 24]. In our study, we were unable to visualize or analyze if there was a biofilm formed at the evaluated site. However, aPDT can disrupt the biofilm structure in vitro.
Aside from the fungicidal effect of the aPDT, the residual effect of the photobiomodulation (i.e., some photons were not absorbed by the PS and its energy was transferred to the host, producing a photobiomodulation effect) also modulated the neutrophilic inflammatory infiltrate. Figure 4 shows large abscess formation, microabscesses coating the cornified epithelial layer, and numerous neutrophils in the submucosa at 45% of the sites in control animals, while only 26 and 30% were observed in the aPDT groups treated with MB and PpNetNI, respectively. Meanwhile, about 70% of the aPDT samples were free from neutrophils and presented no ultrastructural changes, while only 52% of the control group presented the same conditions. These differences were statistically significant with p = 0.041 and p = 0.027 for aPDT with MB and PpNetNI, respectively. In addition, the yeast that survived aPDT was expected to recolonize the host soon after treatment; however, the yeast population remained unaltered 7 days after the procedure. Further studies are needed to elucidate the mechanisms underlying the prolonged response of the host to maintain the low yeast population.
Conclusions
In conclusion, the use of aPDT as a therapeutic option to decrease the fungal infection in a vaginal candidiasis model resulted in a significant reduction in the amount of C. albicans. The tested photosensitizers, methylene blue and PpNetNI, were also effective in preventing the infection from returning within 7 days. There were no changes in the vaginal mucosa at the ultrastructural level due to aPDT.
Aside from the fungicidal effect, we observed significantly reduced swelling and prevented the formation of abscesses, microabscesses coating the cornified epithelial layer, and the infiltration of neutrophils in the submucosa, likely due to the photobiomodulation (PMB) from the residual photons.
By extrapolating these findings to clinical practice, photodynamic therapy may represent an alternative treatment (or combined with fluconazole) to treat vaginal candidiasis. It could, in theory, decrease the infection in the acute phase, minimizing the symptoms and allowing enough time for the patient’s immune response system to eradicate the fungi without the need for drugs.
References
Gonçalves B, Ferreira C, Alves CT, Henriques M, Azeredo J, Silva S (2015) vulvovaginal candidiasis: epidemiology, microbiology and risk factors. Crit Rev Microbiol 21:1–23
Chew SY, Than LT (2016) Vulvovaginal candidosis: contemporary challenges and the future of prophylactic and therapeutic approaches. Mycoses 59(5):262–273
Mendling W, Brasch J, Cornely OA, Effendy I, Friese K, Ginter-Hanselmayer G, Hof H, Mayser P, Mylonas I, Ruhnke M, Schaller M, Weissenbacher ER (2015) Vulvovaginal candidosis (AWMF 015/072), S2k (excluding chronic mucocutaneous candidosis). Mycoses 58:1–15
Peters BM, Yano J, Noverr MC, Fidel PL Jr (2014) Candida vaginitis: when opportunism knocks, the host responds. PLoS Pathog 10:e1003965
Carrillo-Muñoz AJ, Giusiano G, Ezkurra PA, Quindós G (2006) Antifungal agents: mode of action in yeast cells. Rev Esp Quimioter 19:130–139
Eckert LO (1998) Vulvovaginal candidiasis: clinical manifestations, risk factors, management algorithm. Obstet Gynecol 92(5):757–765
Gallemore RP, Boyer DS (2006) PDT or anti-VEGF for AMD treatment. Rev of Ophthalmology
Machado-de-Sena RM, Corrêa L, Kato IT, Prates RA, Senna AM, Santos CC, Picanço DA, Ribeiro MS (2014) Photodynamic therapy has antifungal effect and reduces inflammatory signals in Candida albicans-induced murine vaginitis. Photodiagn Photodyn Ther 11:275–282
Naglik JR, Fidel PL Jr, Odds FC (2008) Animal models of mucosal Candida infection. FEMS Microbiol Lett:129–139
Black CA, Eyers FM, Russell A, Dunkley ML, Clancy RL, Beagley KW (1999) Increased severity of Candida vaginitis in BALB/c nu/nu mice versus the parent strain is not abrogated by adoptive transfer of T cell enriched lymphocytes. J Reprod Immunol 45:1–18
Sousa AS, Prates RA, de Santi ME, Lopes RG, Bussadori SK, Ferreira LR, Deana AM (2016) Photodynamic inactivation of Candida albicans biofilm: influence of the radiant energy and photosensitizer charge. Photodiagn Photodyn Ther 14:111–114
Alvarenga LH, Prates RA, Yoshimura TM, Kato IT, Suzuki LC, Ribeiro MS, Ferreira LR, Pereira SA, Martinez EF, Saba-Chujfi E (2015) Aggregatibacter actinomycetemcomitans biofilm can be inactivated by methylene blue-mediated photodynamic therapy. Photodiagn Photodyn Ther 12:131–135
Kato IT, Prates RA, Sabino CP, Fuchs BB, Tegos GP, Mylonakis E, Hamblin MR, Ribeiro MS (2013) Antimicrobial photodynamic inactivation inhibits Candida albicans virulence factors and reduces in vivo pathogenicity. Antimicrob Agents Chemother 57:445–451
Calzavara-pinton P, Rossi MT, Sala R, Venturini M (2012) Photodynamic antifungal chemotherapy. Photochem Photobiol 88:512–522
Mima EG, Pavarina AC, Dovigo LN, Vergani CE, Costa CA, Kurachi C, Bagnato VS (2010) Susceptibility of Candida albicans to photodynamic therapy in a murine model of oral candidosis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 109:392–401
Hamblin MR (2004) Photodynamic therapy: a new antimicrobial approach to infectious disease? Photochem Photobiol Sci 3(5):436–450
Ferreira LR, Sousa AS, Alvarenga LH, Deana AM, de Santi MEOS, Kato IT, Leal CRL, Ribeiro MS, Prates RA (2016) Antimicrobial photodynamic therapy on Candida albicans pre-treated by fluconazole delayed yeast inactivation. Photodiagn Photodyn Ther 7:15–25
Prates RA, Kato IT, Ribeiro MS, Tegos GP, Hamblin MR (2011) Influence of multidrug efflux systems on methylene blue-mediated photodynamic inactivation of Candida albicans. J Antimicrob Chemother 66(7):1525–1532
Ling Z, Liu X, Chen W, Luo Y, Yuan L, Xia Y, Nelson KE, Huang S, Zhang S, Wang Y, Yuan J, Li L, Xiang C (2013) The restoration of the vaginal microbiota after treatment for bacterial vaginosis with metronidazole or probiotics. Microb Ecol 65:773–780
Rineh A, Dolla NK, Ball AR, Magana M, Bremner JB, Hamblin MR, Tegos GP, Kelso MJ (2017) Attaching the NorA efflux pump inhibitor INF55 to methylene blue enhances antimicrobial photodynamic inactivation of methicillin-resistant Staphylococcus aureus in vitro and in vivo. ACS Infect Dis 3:756–766
Tegos GP, Hamblin MR (2006) Phenothiazinium antimicrobial photosensitizers are substrates of bacterial multidrug resistance pumps. Antimicrob Agents Chemother 50:196–203
G.P. Tegos; K. Masago, F. Aziz, A. Higginbotham, F.R. Stermitz, M.R Hamblin (2008) Antimicrob Agents Chemother. 52(9):3202–9
Silveira LB, Prates RA, Novelli MD, Marigo HA, Garrocho AA, Amorim JC, Sousa GR, Pinotti M, Ribeiro MS (2008) Investigation of mast cells in human gingiva following low-intensity laser irradiation. Photomed Laser Surg 26(4):315–321
Bozó A, Domán M, Majoros L, Kardos G, Varga I, Kovács R (2016) The in vitro and in vivo efficacy of fluconazole in combination with farnesol against Candida albicans isolates using a murine vulvovaginitis model. J Microbiol 54:753–760
Funding
The work was partially funded by the São Paulo Research Foundation (FAPESP) grant 08/57721-3.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The Ethics Committee of Universidade Nove de Julho (protocol number 00025/2013) approved this work.
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
The Ethics Committee of Universidade Nove de Julho (protocol number 00025/2013) approved this study.
Informed consent
This work involved an animal study; therefore, obtaining informed consent was not necessary.
Rights and permissions
About this article
Cite this article
de Santi, M.E.S.O., Prates, R.A., França, C.M. et al. Antimicrobial photodynamic therapy as a new approach for the treatment of vulvovaginal candidiasis: preliminary results. Lasers Med Sci 33, 1925–1931 (2018). https://doi.org/10.1007/s10103-018-2557-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-018-2557-y