Abstract
The aim of this study was to assess the effects of repeated applications of antimicrobial photodynamic therapy (aPDT) on the non-surgical periodontal treatment of residual pockets. This work was performed and reported according to the Cochrane and PRISMA recommendations, respectively, and registered at the PROSPERO registry (number CRD42017058403). An extensive search of the biomedical literature was conducted on four databases from January 1960 to August 2018, followed by hand searching. Analysis of the quality of the selected studies was based on the risk of bias. Only two randomised controlled clinical trials (RCTs) met the inclusion criteria although they had unclear risk of bias. One study showed that repeated applications of aPDT in association with conventional non-surgical treatment during periodontal maintenance improved all clinical outcomes after 6 months. The other study, which assessed the effects of repeated applications of aPDT in association with ultrasound debridement on periodontal pathogens, showed no significant reduction of the main pathogens after 3–6 months but reported reductions of probing pocket depth and C-reactive protein after 3 and 6 months, respectively, compared to mechanical therapy alone. Concluding, it was not possible to state that repeated applications of aPDT, in association with non-surgical treatment of residual pockets, have effective clinical effects in the periodontal maintenance therapy. Although one can consider that aPDT is a promising adjuvant therapy, it is still necessary to carry out more RCTs with low risk of bias in order to confirm or refute the benefits of multiple applications for residual periodontal pockets.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Periodontal diseases are highly prevalent in adults, with recent studies performed in Latin America reporting a high prevalence, reaching more than 90% of the population [1]. Periodontitis is one of the forms of periodontal diseases and has a high prevalence in the United States, affecting almost half of the population (i.e. 45.9%) older than 30 years old [2]. This form results from an inflammation of the supporting dental structures in response to chronic infections caused by various periodontal pathogens [3].
In fact, bacterial biofilm plays a key role in the aetiology of periodontitis. Specifically, these microorganisms and their virulence factors can induce the release of pro-inflammatory cytokines, provoking an inflammatory response and generating alveolar bone loss [4]. In a more advanced stage, periodontitis leads to loss of the tooth, thus reducing the quality of life of the patients and affecting their general health [5]. In general, biofilm is beneficial to the host by providing colonisation resistance against exogenous pathogens and by interacting with the immune system at a level compatible with health [3]. However, the colonisation of subgingival area results in shifts in the bacterial composition of the biofilm, introducing or enhancing the level of periodontophatic bacteria that may initiate disease [3, 4]. Subgingival microbiota is dominated by different kinds of gram-negative rods such as Prevotella species, Porphyromonas gingivalis, and Fusobacterium nucleatum and also including motile bacteria and spirochetes located at the external portion of the biofilm in direct relation to the pocket tissue [3, 4]. Furthermore, the majority of the bacteria are anaerobic and have a proteolytic metabolism. These are favoured by the local anaerobic conditions in the periodontal pocket rich in gingival crevicular fluid, which is a tissue exudate that contains some proteins and blood products [3]. The biofilm and the ongoing inflammation will gradually result in deepening of the periodontal pockets, degradation of the bone, and ultimately loss of teeth [4].
The main objective of the periodontal therapy is to eliminate bacterial deposits on the root surfaces of the teeth by means of mechanical treatment, that is, scaling and root planing (SRP) [6]. However, it is difficult to eliminate periodontal pathogens from the deepest areas of the periodontal pockets, or into the adjacent soft tissue, root cement, and dentinal tubules [7]. Periodontal pathogens, such as P. gingivalis, Tannerella forsythia and Treponema denticola, have the ability to invade gingival epithelial cells which may enable them to cause inflammation within the tissue and also protect these pathogens from mechanical removal [8]. Systemic antibiotics in conjunction with scaling and root planing (SRP) can offer an additional benefit over SRP in such situation [6]. Unfortunately, the regular use of antibiotics in periodontal treatment is not advisable [9] because they could lead to the development of antimicrobial resistance or may promote the overgrowth of new pathogens [10]. Therefore, the limitations of conventional treatment open space for new treatment approaches.
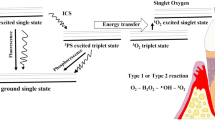
Antimicrobial photodynamic therapy (aPDT) is a proposal for treatment of periodontitis, which involves the use of low-power laser or LED in association with a non-toxic photo-sensibiliser (FS) to reduce the amount of periodontal pathogens. aPDT uses a laser light with an appropriate wavelength, in the presence of oxygen, to activate the photosensitizer (PS) [7]. Free radicals of singlet oxygen are formed by changing the energy status of PS molecules, which destroy the membrane, the mitochondria or the nuclei of cells [11]. In addition to bactericidal effects of aPDT, the diode laser application added benefits to periodontal healing because of its biostimulative effects [7]. The PS excited triplet can undergo two types of reactions: it can react directly with a substrate, like the cell membrane or a molecule, and transfer a proton or an electron to form a radical anion or radical cation, respectively [12]. These radicals may further react with oxygen to produce reactive oxygen species (type 1 reaction). Alternatively, in a type 2 reaction, the triplet PS can transfer its energy directly to molecular oxygen to form excited-state singlet oxygen. Both reactions can occur in the same time, and the success of these processes depends on the type of PS used, the concentrations of substrate and oxygen [12].
Some studies investigated the effect of a single application of aPDT in the treatment of periodontitis, showing conflicting results [13,14,15]. Compared to SRP alone, the association of a single application of aPDT resulted in greater reductions of bleeding on probing (BoP) rates [13]. On the other hand, some studies found no additional improvement in the reduction of clinical parameters [14, 15].
More recently, patients with periodontitis undergoing support periodontal therapy have been treated with non-surgical subgingival debridement in association with 2, 3 or 5 applications of aPDT [7, 9, 11]. However, up to now, no systematic reviews demonstrating whether repeated applications of aPDT provide additional benefits to the non-surgical periodontal treatment of residual pockets have been published.
The aim of this study was to assess the effects of repeated applications of aPDT on the non-surgical periodontal treatment of residual pockets.
Material and methods
This systematic review was conducted according to the criteria established by Cochrane [16] and reported following the PRISMA guidelines [17]. It was registered at the PROSPERO registry under the number CRD42017058403. The following question was developed with PICO format [18]: “Do repeated applications of aPDT in association with non-surgical periodontal treatment have superior effects on residual pockets compared to non-surgical treatment of CP alone?”
Search strategy
An extensive search of the biomedical literature was performed on the databases MEDLINE (PubMed), LILACS, Cochrane Central Register of Controlled Trials (CENTRAL) and Elsevier (Science Direct) by using appropriate keywords and titles related to photodynamic therapy and non-surgical periodontal therapy. Combinations of Boolean operators “OR” and “AND” were used for searching the following terms: Photodynamic therapy; Antimicrobial Photodynamic therapy; Photo-chemotherapy; Periodontal Disease; Periodontitis; Chronic Periodontitis; Residual Pockets; Root Planing; Scaling and Root Planing; Non-surgical periodontal therapy and supportive periodontal therapy, from January 1960 to August 2018.
Other related studies were identified by hand searching reference lists of studies. This strategy was shown to be effective for identification of clinical studies which sometimes are not found on electronic databases [19]. Moreover, each theme was manually sought from the major journals of periodontology and laser therapy published in the last 15 years, namely: “Journal of Clinical Periodontology”, “Journal of Periodontology”, “Journal of Periodontal Research”, “Clinical Oral Investigations”, “Journal of Dental Research, Laser Medical Science”, “Journal of Dental Lasers”, “International Journal of Laser Dentistry”, “Journal of Laser Dentistry”, “The Journal of Oral Laser Applications, Laser in Dentistry and Lasers in Dental Science”.
Study design
This systematic review included only original randomised controlled clinical trials with at least a 6-month follow-up. Articles were excluded according to the following criteria: duplicate study, no control group or no publication in journals. The reasons for rejecting the study during selection were recorded.
Participants
Patients diagnosed with Periodontitis according to a classification system [20] and without age restriction were included. Exclusion criteria were: diabetes or other systemic diseases and no presence of residual pockets.
Intervention
Repeated applications of aPDT (2 or more) in association with non-surgical periodontal treatment of residual pockets.
Comparison/control
Non-surgical periodontal treatment (scaling and root planing performed manually or ultrasonic debridement).
Outcomes
The primary outcome of interest was the change in the clinical attachment level (CAL), whereas reductions of probing pocket depth (PPD), BoP, levels of periodontal pathogens and biological markers were secondary outcomes.
Data selection and extraction
During the selection process, two independent reviewers obtained data on population, interventions, outcomes and follow-up periods of the studies. Attempts to get in contact with the authors were made in order to verify still-open questions.
Of the chosen articles, the complete ones were analysed before consensual decision-making on their inclusion or exclusion.
Assessment of the risk of bias
Assessment of the risk of bias in the included studies was based on the Cochrane criteria [16] as follows: (1) random sequence generation; (2) allocation concealment, (3) blinding of participants and personnel, (4) blinding of outcome assessment, (5) incomplete outcome data, (6) selective reporting and (7) other bias.
After collecting these items, the studies were classified as of “low risk” (i.e. low risk of bias for all major points), “high risk” (i.e. high risk of bias for one or more points) and “unclear risk” (i.e. unclear risk of bias for one or more major points).
Data were summarised in a flowchart and tables in order to facilitate the description of the analyses performed.
Results
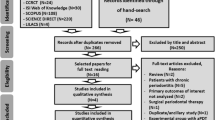
Electronic search resulted in the identification of a total of 632 titles and abstracts. Only one article was found after hand searching. A total of 250 studies remained after exclusion of duplicates. By using titles and abstracts, 60 studies were selected after applying the inclusion criteria, and of these, 17 were complete studies which were selected for eligibility. Fifteen studies were excluded after being fully read, and the reasons for exclusion are listed in Table 1. Therefore, only two studies were selected for qualitative analysis. Figure 1 describes the flowchart of the studies identified, selected and evaluated for review according to the eligibility criteria.
Flowchart of the process of identification and selection of the studies (PRISMA) [17]
A general view of the articles excluded and the reasons for their rejection are listed in Table 1.
Two studies were included in the qualitative analysis [9, 11]. Due to a reduced number of studies selected, it was not possible to perform a meta-analysis.
The study by Muller Campanile et al. [9] was classified as of “unclear risk of bias” because the blinding of the patients was not clear. However, the method used for generating and concealing the assignment sequence was cited and the examiner and practitioners were blinded to the results and to the treatments provided, respectively. There were no missing outcome data. All patients but one completed the study (Fig. 2).
Risk of bias in the studies selected (Cochrane) [16]
The study by Lulic et al. [11] was also classified as of “unclear risk of bias” because the blinding of outcome assessment was not mentioned. On the other hand, randomisation and assignment of patients were cited and the description of these procedures was included. Patients, researchers and oral hygienist were all blinded to the energy configuration and activation or not of the laser point used in the aPDT after intense training. There were no missing outcome data. All patients completed the study (Fig. 2).
Finally, none of the included studies were registered in ClinicalTrials.gov which hampered the evaluation of Selective Reporting criteria.
The risk of bias of the included studies is listed in Fig. 2
The characteristics and results of the selected studies are listed in Tables 2 and 3.
Discussion
The objective of this study was to describe the additional effects of repeated applications of aPDT in association with non-surgical periodontal treatment of residual pockets.
In the past decade, several clinical studies assessed the effects of aPDT in the periodontal therapy [7, 9, 11, 13,14,15, 21,22,23,24,25,26,27,28,29,30,31,32,33,34]. The fact is that the reestablishment of a subgingival environment compatible to periodontal health is essential for preventing both recolonisation by putative periodontal pathogens and recurrence of the disease [35].
Nevertheless, the anatomical difficulties found during SRP suggest the need to use other therapeutic modalities aiming at microbial control. As aPDT has been associated with diode laser for photo-biomodulation of tissues, this approach might further benefit patients with systemic impairment [24]. Moreover, anti-inflammatory and bio-modulating properties of low-power laser can facilitate both the process of proliferation and the healing of inflamed periodontal tissues [7], thus contributing to the treatment of residual pockets.
Some authors showed statistically significant results for aPDT in association with SRP, which increased CAL and reduced BoP after 3 months of treatment [36, 37]. However, Balata et al. [38] reported no additional clinical benefits when a single application of aPDT in the initial periodontal therapy was tested in comparison to ultrasound. In the treatment of residual pockets, the improvement seems to only occur for decreasing BoP in already-treated sites in maintenance patients [7, 33, 39,40,41].
In addition to clinical studies, some systematic reviews and meta-analyses assessed the effect of a single application of aPDT alone or in conjunction with treatment of chronic periodontitis, showing inconclusive controversial results regarding the clinical advantages of their use [42,43,44]. A recent systematic review concluded that the use of a single application of aPDT in association with SRP improves clinical parameters in the maintenance of residual pockets [42] but there is no evidence yet supporting its effectiveness in the medium and long terms [43]. Another review concluded that a single application of aPDT is not superior to SRP alone as an alternative therapy [44].
These controversial results seem to suggest that a single application of aPDT may not be enough to promote an additional benefit to SRP. Some authors have suggested that repeated applications of aPDT, associated with non-surgical periodontal treatment, can be a more advantageous approach in the treatment of residual pockets [9, 11]. The present study was carried out to assess whether this hypothesis can be confirmed.
Initially, up to now, there is no systematic review describing the effects of multiple applications of aPDT associated with non-surgical periodontal treatment of residual pockets. Literature search was conducted after defining the objective of the study, followed by selection of articles, acquisition of data, evaluation of methodology quality and synthesis of data. After this process, two articles were included for discussion.
The study conducted by Lulic et al. [11] demonstrated that SRP promoted a great reduction in PPD, significant increase in CAL and decrease in BoP after five applications of aPDT associated to SRP for a period of 2 weeks (days 0, 1, 2, 7, 14) compared to SRP alone. The results in the experimental groups were statistically significant after 6 months, improving the clinical outcomes for residual pockets (≥ 5 mm) during periodontal maintenance. After 3 months, only percentages of BoP decreased. Clinical advantages were also observed as all the parameters improved after 12 months of follow-up, suggesting that the use of multiple applications of aPDT in association with SRP should be recommended for treatment of residual pockets.
In the study by Muller Campanile et al. [9], three therapeutic modalities were compared between groups A, B and C. In the groups receiving two (A) and one (B) application of aPDT during 1-week period, statistically significant differences were reported in the reduction of PPD after 3 months compared to the non-irradiated group (C). After 6 months, however, the differences were non-significant regarding all the clinical outcomes, differently from the results found in the study by Lulic et al. [11]. As for the microbiological effects, there was no reduction of six periodontal pathogenic microorganisms (P. gingivalis, Aggregatibacter actinomycetemcomitans, T. forsythia, T. denticola, Prevotella intermedia, Parvimonas micra), which were evaluated by means of DNA probe and PCR-based assays in three groups analysed after 3 and 6 months.
The risk of bias assessed in the studies by Muller Campanile et al. [9] and Lulic et al. [11] were classified according to the Cochrane criteria [16]. After assessment of all points regarding the quality of the methodology applied in each of the studies, both were considered as of unclear risk of bias. Lulic et al. [11] showed clearly that researchers, patients and oral hygienist were blinded to the use of laser. However, such a finding was contrary to Muller Campanile et al. [9], who reported only the blinding of researchers and examiner of the results. However, the blinding of outcome assessment was not found in Lulic et al. [11]. Furthermore, none of the studies were registered in ClinicalTrials.gov . So, it was not possible to identify if there was selective report or not.
In the two studies included in the present systematic review, both smokers and non-smokers participated in the experimental and control groups. Lulic et al. [11] found greater clinical advantages in their sample of ten patients, of whom eight were non-smokers. On the other hand, Muller Campanile et al. [9] used a much larger sample consisting of 27 patients, of whom one was ex-smoker and 14 were non-smokers. The fact that the study by Lulic et al. [11] used a sample, although small, with a majority of non-smokers suggests that the improvement in all clinical parameters after 6 months may also be related to the non-smoking habit.
Muller Campanile et al. [9] assessed the effect of one and two applications of aPDT plus US on the reduction of pathogenic microbiota, reporting no positive results compared to US alone. This also suggests that the presence of smoker patients can contribute to the maintenance of residual pockets, resulting in non-effective outcomes. Other clinical studies have shown a great clinical improvement of the patients, with aPDT plus SRP reducing periodontal pathogens in non-smoker patients compared to the use of SRP alone [7]. This finding was reiterated by a long-term study conducted by Matuliene et al. [44], who concluded that residual pockets were related to the smoking habit. Similarly, Rieder et al. [45] confirmed the dose-dependent relationship between smoking habit and presence of residual pockets in periodontal patients undergoing support therapy.
The biological effects were also assessed, but only by Muller Campanile et al. [9], who showed very significant results in the reduction of important biological biomarkers. A significant decrease of C-reactive protein (CRP), amyloid A, fibrinogen, procalcitonin and alpha-2 macroglobulin was demonstrated in this study after 6 months of treatment. When the authors assessed the groups separately, PCR was lower only in the group with two applications of aPDT (A). The effects of aPDT on inflammatory mediators involved in the pathogenesis of periodontal disease are not yet well understood and there are currently a few studies assessing these aspects [46]. Significant reductions in the levels of inflammatory cytokines in the gingival sulcus fluid following non-surgical periodontal treatment with aPDT were demonstrated elsewhere, thus indicating a significant clinical improvement of the periodontal tissues [30, 46]. In contrast, Pourabas et al. [15] assessed the effects of a single application of aPDT associated with SRP and concluded that there were no additional benefits in the clinical parameters or inflammatory markers TNF-α, Interleucina1-β and metalloprotein matrix after 3 months of treatment.
With regard to the action of the photosensibiliser (PS), the phenothiazine dye (i.e. methylene blue) was used at a concentration of 10 mg/ml in the two studies analysed [9, 11] regarding the periodontal pockets. In addition to being the most used currently, this approach has been shown by other clinical studies to have better bactericidal and bacteriostatic effects. Both methylene blue (MB) and toluidine blue (TB) are phenothiazine compounds available at concentrations of 10 mg/ml or 100 mg/ml, which are effective for inactivating periodontal pathogenic Gram-positive and Gram-negative bacteria, thus making PS the treatment of choice for periodontitis [47]. Phenothiazine dyes are naturally cationic and have been widely used in aPDT for inactivating a great variety of Gram-positive microorganisms, Gram-negative bacteria and also fungi cells [48]. The pre-irradiation time, after application of the dye to periodontal pockets, was different in the two studies as Lulic et al. [11] used a pre-irradiation time of 3 min, whereas Muller Campanile et al. [9] used 1 min only. Although the microbiological effects have not been assessed in the study by Lulic et al. [11], the longer pre-irradiation time may explain the better clinical results. On the other hand, the study by Muller Campanile et al. [9] showed no significant microbiological results and used a shorter time of pre-irradiation (i.e. 1 min), differently from a study assessing the microbiological effects of aPDT plus SRP by using a pre-irradiation time of 3 min, reporting positive results [7]. The longer time of pre-irradiation time seems to represent more positive clinical outcomes compared to a shorter one [25, 28]. After the pre-irradiation time, optical fibres were adapted to the laser points and then inserted into the residual pockets. Lasers were activated and the pockets irradiated. In the two studies, low-power diode lasers were used in the red light spectrum with wavelength of 670 nm and irradiation time of 60 s. It should be also emphasised that laser potencies (75 mW and 280 mW) and energy densities were different in both studies, perhaps explaining the differences in the results reported [9, 11]. The possible correlation between laser energy density and reduction of periodontal pathogens by using aPDT needs to be better determined.
In conclusion, the two studies included in this review were found to be very heterogeneous due to their clear differences related to the eligibility criteria, outcomes assessed and evaluation of the results reported. Thus, it is suggested that similar clinical protocols should be tested in a larger number of patients by means of randomised clinical trials with better methodological quality in order to confirm the clinical, microbiological and anti-inflammatory benefits of the multiple applications of aPDT associated with non-surgical mechanical treatment of residual periodontal pockets.
Conclusion
From the studies included in the present systematic review, it was not possible to state that repeated applications of aPDT, in association with non-surgical treatment of residual pockets, have effective clinical effects in the periodontal maintenance therapy. Although one can consider that aPDT is a promising adjuvant therapy, it is still necessary to carry out more RCTs with low risk of bias in order to confirm or refute the benefits of multiple applications for residual periodontal pockets.
References
Oppermann RV, Haas AN, Rösing CK, Susin C (2015) Epidemiology of periodontal diseases in adults from Latin America. Periodontol 67:13–33
Eke PI, Dye BA, Wei L, Slade GD, Thornton-Evans GO, Borgnakke WS, Taylor GW, Page RC, Beck JD, Genco RJ (2015) Update on prevalence of periodontitis in adults in the United States: NHANES 2009 to 2012. J Periodontol 86:611–622
Larsen T, Fiehn NE (2017) Dental biofilm infections - an update. APMIS 125:376–384
Huang N, Gibson FC 3rd (2014) Immuno-pathogenesis of periodontal disease: current and emerging paradigms. Curr Oral Health Rep 1:124–132
Wright CD, McNeil DW, Edwards CB, Crout RJ, Neiswanger K, Shaffer JR, Marazita ML (2017) Periodontal status and quality of life: impact of fear of pain and dental fear. Pain Res Manag 2017(5491923):9
Zandbergen D, Slot DE, Niederman R, Van der Weijden FA (2016) The concomitant administration of systemic amoxicillin and metronidazole compared to scaling and root planing alone in treating periodontitis: a systematic review. BMC Oral Health 16:27
Petelin M, Perkič K, Seme K, Gašpirc B (2015) Effect of repeated adjunctive antimicrobial photodynamic therapy on subgingival periodontal pathogens in the treatment of chronic periodontitis. Lasers Med Sci 30:1647–1656
Ji S, Choi YS, Choi Y (2015) Bacterial invasion and persistence: critical events in the pathogenesis of periodontitis? J Periodontal Res 50:570–585
Muller Campanile VS, Giannopoulou C, Campanile G, Cancela JA, Mombelli A (2015) Single or repeated antimicrobial photodynamic therapy as adjunct to ultrasonic debridement in residual periodontal pockets: clinical, microbiological, and local biological effects. Lasers Med Sci 30:27–34
Rams TE, Degener JE, Van Winkelhoff AJ (2014) Antibiotic resistance in human chronic periodontitis microbiota. J Periodontol 85:160–169
Lulic M, Leiggener Görög I, Salvi GE, Ramseier CA, Mattheos N, Lang NP (2009) One-year outcomes of repeated adjunctive photodynamic therapy during periodontal maintenance: a proof-of-principle randomized-controlled clinical trial. J Clin Periodontol 36:661–666
Castano AP, Demidova TN, Hamblin MR (2004) Mechanisms in photodynamic therapy: part one-photosensitizers, photochemistry and cellular localization. Photodiagn Photodyn Ther 1:279–293
Betsy J, Prasanth CS, Baiju KV, Prasanthila J, Subhash N (2014) Efficacy of antimicrobial photodynamic therapy in management of chronic periodontitis: a randomized controlled clinical trial. J Clin Periodontol 41:573–571
Theodoro LH, Silva SP, Pires JR, Soares GH, Pontes AE, Zuza EP, Spolidório DM, de Toledo BE, Garcia VG (2012) Clinical and microbiological effects of photodynamic therapy associated with nonsurgical periodontal treatment. A 6-month follow-up. Lasers Med Sci 27:687–693
Pourabas R, Kashefimehr A, Rahmanpour N, Babaloo Z, Kishen A, Tenenbaum HC, Azarpazhooh A (2014) Effects of photodynamic therapy on clinical and gingival crevicular fluid inflammatory biomarkers in chronic periodontitis: a Split-mouth randomized clinical trial. J Periodontol 85:1222–1229
Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA (2011) Cochrane Bias methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343:d5928
Moher D, Liberati A, Tetzlaff J, Altman DG, Prisma Group (2010) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 8:336–341
Schardt C, Adams MB, Owens T, Keitz S, Fontelo P (2007) Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med Inform Decis Mak 7:16
Bickley SR, Harrison JE (2003) How to find the evidence. J Orthod 30:72–78
G Caton J, Armitage G, Berglundh T, Chapple ILC, Jepsen S, S Kornman K, L Mealey B, Papapanou PN, Sanz M, S Tonetti M (2018) A new classification scheme for periodontal and peri-implant diseases and conditions - introduction and key changes from the 1999 classification. J Periodontol 89(Suppl 1):S1–S8
Carvalho VF, Andrade PVC, Rodrigues MF, Hirata MH, Hirata RDC, Pannuti CM, De Micheli G, Conde MC (2015) Antimicrobial photodynamic effect to treat residual pockets in periodontal patients: a randomized controlled clinical trial. J Clin Periodontol 42:440–447
Andrade PV, Euzebio Alves VT, de Carvalho VF, De Franco Rodrigues M, Pannuti CM, Holzhausen M, De Micheli G, Conde MC (2017) Photodynamic therapy decrease immune-inflammatory mediators level s during periodontal maintenance. Lasers Med Sci 32:9–17
Bassir SH, Moslemi N, Jamali R, Mashmouly S, Fekrazad R, Chiniforush N, Shamshiri AR, Nowzari H (2013) Photoactivated disinfection using light-emitting diode as an adjunct in the management of chronic periodontitis: a pilot double-blind split-mouth randomized clinical trial. J Clin Periodontol 40:65–72
Sreedhar A, Sarkar I, Rajan P, Pai J, Malagi S, Kamath V, Barmappa R (2015) Comparative evaluation of the efficacy of curcumin gel with and without photoactivation as an adjunct to scaling and root planing in the treatment of chronic periodontitis: a split mouth clinical and microbiological study. J Nat Sci Biol Med 6:102–109
Franco EJ, Pogue RE, Sakamoto LH, Cavalcante LL, Carvalho DR, de Andrade RV (2014) Increased expression of genes after periodontal treatment with photodynamic therapy. Photodiagn Photodyn Ther 11:41–47
Ge L, Shu R, Li Y, Li C, Luo L, Song Z, Xie Y, Liu D (2011) Adjunctive effect of photodynamic therapy to scaling and root planing in the treatment of chronic periodontitis. Photomed Laser Surg 29:33–37
Ge LH, Shu R, Shen MH (2008) Effect of photodynamic therapy on IL-1beta and MMP-8 in gingival crevicular fluid of chronic periodontitis. Shanghai Kou Qiang Yi Xue 17:10–14
Giannelli M, Formigli L, Lorenzini L, Bani D (2012) Combined photoablative and photodynamic diode laser therapy as an adjunct to non-surgical periodontal treatment: a randomized split-mouth clinical trial. J Clin Periodontol 39:962–970
Corrêa MG, Oliveira DH, Saraceni CH, Ribeiro FV, Pimentel SP, Cirano FR, Casarin RC (2016) Short term microbiological effects of photodynamic therapy in nonsurgical periodontal treatment of residual pockets: a splitmouth RCT. Lasers Surg Med 48:944–950
Kolbe MF, Ribeiro FV, Luchesi VH, Casarin RC, Sallum EA, Nociti FH Jr, Ambrosano GM, Cirano FR, Pimentel SP, Casati MZ (2014) Photodynamic therapy during supportive periodontal care: clinical, microbiologic, immunoinflammatory, and patient centered performance in a split-mouth randomized clinical trial. J Periodontol 85:277–286
Campos GN, Pimentel SP, Ribeiro FV, Casarin RC, Cirano FR, Saraceni CH, Casati MZ (2013) The adjunctive effect of photodynamic therapy for residual pockets in single-rooted teeth: a randomized controlled clinical trial. Lasers Med Sci 28:317–324
Cappuyns I, Cionca N, Wick P, Giannopoulou C, Mombelli A (2012) Treatment of residual pockets with photodynamic therapy, diode laser, or deep scaling. A randomized, split-mouth controlled clinical trial. Lasers Med Sci 27:979–986
Chondros P, Nikolidakis D, Christodoulides N, Rössler R, Gutknecht N, Sculean A (2009) Photodynamic therapy as adjunct to non-surgical periodontal treatment in patients on periodontal maintenance: a randomized controlled clinical trial. Lasers Med Sci 24:681–688
Goh EX, Tan KS, Chan YH, Lim LP (2017) Effects of root debridement and adjunctive photodynamic therapy in residual pockets of patients on supportive periodontal therapy: a randomized split-mouth trial. Photodiagn Photodyn Ther 18:342–348
Zijnge V, Meijer HF, Lie MA, Tromp JA, Degener JE, Harmsen HJ, Abbas F (2010) The recolonization hypothesis in a full-mouth or multiple-session treatment protocol: a blinded randomized clinical trial. J Clin Periodontol 37:518–525
Andersen R, Loebel N, Hammond D, Wilson M (2007) Treatment of periodontal disease by photodisinfection compared to scaling and root planing. J Clin Dent 18:34–38
Braun A, Dehn C, Krause F, Jepsen S (2008) Short-term clinical effects of adjunctive antimicrobial photodynamic therapy in periodontal treatment: a randomized clinical trial. J Clin Periodontol 35:877–884
Balata ML, de Andrade LP, Santos DB, Cavalcanti AN, Tunes Uda R, Ribeiro Édel P, Bittencourt S (2013) Photodynamic therapy associated with full-mouth ultrasonic debridement in the treatment of severe chronic periodontitis: a randomized controlled clinical trial. J Appl Oral Sci 21:208–214
Christodoulides N, Nikolidakis D, Chondros P, Becker J, Schwarz F, Rossler R, Sculean A (2008) Photodynamic therapy as an adjunct to non-surgical periodontal treatment: a randomized controlled clinical trial. J Periodontol 79:1638–1644
Dzink JL, Socransky SS, Haffajee AD (1998) The predominant cultivable microbiota of active and inactive lesions of destructive periodontal diseases. J Clin Periodontol 15:316–323
Xue D, Zhao Y (2017) Clinical effectiveness of adjunctive antimicrobial photodynamic therapy for residual pockets during supportive periodontal therapy: A systematic review and meta-analysis. Photodiagnosis Photodyn Ther 17:127–133
Sgolastra FF, Petrucci A, Severino M, Graziani F, Gatto R, Monaco A (2013) Photodynamic therapy in the treatment of chronic periodontitis: a systematic review and meta-analysis. Lasers Med Sci 28:669–682
Azarpazhooh A, Shah OS, Tenenbaum HC, Goldberg MB (2010) The effect of photodynamic therapy for periodontitis: a systematic review and meta-analysis. J Periodontol 81:4–14
Matuliene G, Pjetursson BE, Salvi GE, Schmidlin K, Brägger U, Zwahlen M, Lang NP (2008) Influence of residual pockets on progression of periodontitis and tooth loss: results after 11 years of maintenance. J Clin Periodontol 35:685–695
Rieder C, Joss A, Lang NP (2004) Influence of compliance and smoking habits on the outcomes of supportive periodontal therapy (SPT) in a private practice. Oral Health Prev Dent 2:89–94
Giannopoulou C, Cappuyns I, Cancela J, Cionca N, Mombelli A (2012) Effect of photodynamic therapy, diode laser and deep scaling on cytokine and acute-phase protein levels in gingival crevicular fluid of residual periodontal pockets. J Periodontol 83:1018–1027
Qin YL, Luan XL, Sheng YQ, Zhou CN, Zang ZG (2008) Comparison of toluidine blue-mediated photodynamic therapy and conventional scaling treatment for periodontitis in rats. J Periodontal Res 43:162–167
Dai T, Huang YY, Hamblin MR (2009) Photodynamic therapy for localized infections--state of the art. Photodiagn Photodyn Ther 6:170–188
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest statement
The authors declare that they have no conflict of interest.
Ethical approval
Not applicable (systematic review).
Rights and permissions
About this article
Cite this article
Franco, T.P.M., Dos Santos, A.P.P. & Canabarro, A. The effects of repeated applications of antimicrobial photodynamic therapy in the treatment of residual periodontal pockets: a systematic review. Lasers Med Sci 34, 855–863 (2019). https://doi.org/10.1007/s10103-018-02703-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-018-02703-2