Abstract
Although magnesium and magnesium alloys are considered biocompatible and biodegradable, they suffer from poor corrosion performance in the human body environment. In light of this, surface modification via rapid surface melting of AZ31B Mg alloy using a continuous-wave Nd:YAG laser was conducted. Laser processing was performed with laser energy ranging from 1.06 to 3.18 J/mm2. The corrosion behavior in simulated body fluid of laser surface-treated and untreated AZ31B Mg alloy samples was evaluated using electrochemical technique. The effect of laser surface treatment on phase and microstructure evolution was evaluated using X-ray diffraction and scanning electron microscopy. Microstructure examination revealed grain refinement as well as formation and uniform distribution of Mg17Al12 phase along the grain boundary for laser surface-treated samples. Evolution of such unique microstructure during laser surface treatment indicated enhancement in the corrosion resistance of laser surface-treated samples compared to untreated alloy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bio-implant materials are commonly metallic materials, such as stainless steel and cobalt chromium-, and titanium-based alloys, on account of their optimum properties (strength, corrosion resistance, and biocompatibility) compared to ceramic and polymeric materials [1]. However, the release of toxic ions when in use for a long term as a result of reaction with body fluids and mechanical incompatibility in the form of mismatch between elastic moduli of the bone and these alloys has been reported to cause failure in tissue development and bone loss during the process of bone regeneration [2–4]. Furthermore, in some select bioapplications, the non-degradability of the above-mentioned alloys leads to the requirement of successive surgery to remove the implant after tissue recovery, resulting in extra cost, time, and inconvenience [5, 6]. In order to address these issues, magnesium (Mg) and its alloys are considered as potential biomaterials due to their desirable properties. These alloys possess good biocompatibility and biodegradable characteristics by exhibiting similar elastic modulus, yield strength, and density to that of the human bone [7–9].
However, Mg and its alloys suffer from poor corrosion resistance. They are particularly susceptible to micro-galvanic corrosion and pitting corrosion [10–12]. These attacks are severe in Cl− ion-containing environments such as body fluid [13]. The surface oxide film of Mg(OH)2 reacts with Cl− ions in body fluid to form a soluble MgCl2 compound, thus further aggravating the pitting process. In view of this, Mg is commonly alloyed with suitable alloying elements in order to enhance corrosion resistance. Among the Mg alloys, AZ series alloys are potential implant materials due to their better mechanical and corrosion properties compared to pure Mg [14]. Additionally, it has been reported that Al content has to be kept optimum in order to maintain strength and corrosion resistance without hampering the biological performance of the alloy [15–17]. Excess amounts of Al have been reported to react with phosphate within the body, which might cause dementia, pointing towards careful control of the composition [18]. AZ31 alloy, which is one of the commercially popular Mg alloys, is a promising candidate for implant application on account of the reduced Al content and at the same time possessing good strength and corrosion resistance [17]. In recent years, increasing research is being focused on investigating the bioactivity and bio-corrosion behavior of AZ31 in simulated body fluid (SBF). Song et al. pointed out the growth of a hydroxyapatite layer on AZ31 surface after immersion in SBF, indicating the biocompatible nature of AZ31 [19]. In another study, Lopez et al. showed that grain refinement of AZ31 resulted in better corrosion resistance in SBF compared to a coarse-grained material [20]. Li et al. focused on micro-arc oxidation coatings on AZ31 and reported an increase in the corrosion resistance of the coated samples in SBF [21].
Besides alloying, surface modifications can also improve the corrosion resistance of Mg alloys. In view of this, several techniques have been explored, such as, but not limited to, laser surface melting (LSM) [22, 23], electroplating [24], and anodizing [25]. Among these techniques, LSM, and laser processing in general, provides some unique advantages, such as the highly controllable nature of processing, rapid processing, and excellent repeatability [26]. In addition, the rapid melting and cooling rates inherently associated with LSM help in achieving refined microstructures. As a result, a good biofunctional surface can be produced without disturbing the bulk properties [27–30]. A previous report by the present research group has pointed towards the excellent bio-wettability of laser surface-treated AZ31 with refined grain structure within the surface, further bolstering AZ31 as a suitable bio-implant material. In order to develop deeper understanding, the current work as a succession to the previous study [31] investigated the microstructure evolution and in vitro corrosion behavior in SBF solution of laser surface-melted AZ31B alloy. Various laser processing conditions were explored. The present study further strengthened laser processing as one of the feasible methods to improve the performance of Mg-based bio-implant materials.
Materials and methods
AZ31B alloy was procured in an as-cast condition with nominal composition (in weight percent) as follows: 2.89 Al, 0.92 Zn, 0.05 Mn, 0.01 Si, 0.002 Cu, 0.001 Ni, 0.004 Fe, and balance Mg. Prior to laser surface modification, the AZ31B coupons were cut into rectangular blocks of dimension 50 × 50 × 7 mm. These blocks were ground using 400 SiC grit paper followed by rinsing with methanol to obtain a clean surface. LSM on polished coupons was carried out with the various processing parameters listed in Table 1. A continuous-wave 3-kW Nd:YAG laser (IPG Photonics, model no. YLS-3000) was employed for this purpose. The characteristic laser processing parameters were as follows: beam diameter, 0.6 mm; wavelength, 1064 nm; and power distribution mode, Gaussian, TEM00. A laser scanning speed of 500 mm/s and a laser track overlap of 0.15 mm were used in all the processing conditions. Laser track overlap was necessary to obtain a full coverage of the surface. The laser input power was incremented from 250 to 700 W to obtain various laser energy densities on the sample surface (Table 1). Ar was used as the shielding gas during the entire laser processing experiments to avoid surface oxidation during processing.
Phases in the as-cast and LSM samples were identified using X-ray diffraction (XRD) technique. A Rigaku Ultima diffractometer with Cu Kα radiation (wavelength = 1.5 Å), operating at 40 kV and 40 mA, was employed to carry out XRD analysis. Samples were scanned in the 2θ range of 20–70° using a step size of 0.025 and a scan rate of 1.5°/mm. Phase identification was carried out with the help of JADE software incorporating standard powder diffraction files.
Microstructure examination was conducted in the cross-section perpendicular to the laser track. The cross-sections of samples were cut into smaller rectangular blocks (5 × 3 × 7 mm). A slow-speed diamond-laced wafer cutting machine operated at 300 rpm and 100 g of normal load was used to achieve stress-free cuts. Furthermore, an oil-based cutting fluid was employed to avoid heat generation during cutting. This arrangement was adopted to avoid any microstructural changes in the material during the cutting process. The cut samples were mounted in epoxy molds. These mounted samples were ground using a series of (120–1200 grit) SiC abrasive papers. A smooth mirror finish on the sample cross-section was obtained by employing 1–0.03 μm Al2O3 water-based abrasive suspension. The prepared samples were then cleaned by ultrasonic cleaning setup in succession with deionized water and methanol as the media. Prior to the microstructural investigation, the prepared samples were etched by acetic picric solution (5 mL acetic acid + 6 g picric acid + 100 mL ethyl alcohol + 10 mL H2O) to delineate the microstructural features [32]. Microstructures were investigated using FEI Quanta 200 environmental scanning electron microscope (SEM) equipped with energy-dispersive spectroscopy (EDS).
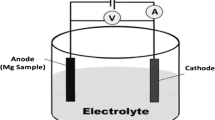
The corrosion performance of as-cast and all LSM samples in SBF solution was evaluated in a standard 250-mL three-electrode electrochemical cell. The working electrode, saturated calomel electrode (SCE), was used as a reference electrode, and platinum wire served as a counter electrode. SBF was employed as the electrolyte. The method of preparation of SBF has been described elsewhere [33]. All the samples were polished to a roughness of ∼4 μm prior to the corrosion testing. An area of 0.25 cm2 was exposed to the SBF solution. Prior to the corrosion tests, the surface potential of the working electrode was stabilized for a period of 1 h. Potentiodynamic polarization scans were recorded on all the samples using a BioLogic® SP-300 potentiostat/galvanostat. These scans were carried out within the voltage scanning range from −200 to +800 mV with respect to the corrosion potential in the anodic direction. A voltage scan rate of 1.0 mV/s was employed. Values of corrosion current (i corr), corrosion potential (E corr), and pitting potential (E pitt) were extracted from potentiodynamic polarization plots. All the electrochemical tests were performed at room temperature (25 °C). The experiments were repeated at least three times to examine the reproducibility of the results. After the corrosion tests, corroded samples were ultrasonically cleaned with methanol for 5 min, and then microstructure of the corroded surface was observed using SEM.
Results and discussion
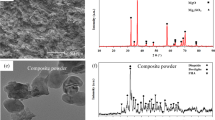
XRD analysis revealed the presence of α-Mg for the as-cast sample and α-Mg and β (Mg17Al12) phases for all LSM samples (Fig. 1). With an increase in the laser processing energy density, the intensity of β-phase peaks increased significantly (encircled regions in Fig. 1). Given that all the experimental conditions during XRD experiments were kept the same, the increase in intensity is likely an outcome of the increased volume fraction of β-phase. This increase in the volume fraction of β-phase was estimated according to Eq. 1 [34] and listed in Table 2. Equation 1 is based on the integrated peak area, which can be measured by taking the maximum peak intensity and the peak width at half maximum height. The amount of β-phase marginally increased for the LSM samples with an increase in the laser energy density.
Microstructure analyses of the as-cast sample revealed the presence of equiaxed α grains with an average grain size of 10 ± 1.2 μm (Fig. 2a). No second phase was observed in the SEM analysis for the as-cast sample, supporting the XRD observations (Fig. 1). The average depth of the melted region for the LSM samples increased from 125 to 199 μm with increase in the laser energy density from 1.06 to 3.18 J/mm2, respectively (Fig. 2b–d). In the case of LSM samples, equiaxed grains (dark phase) were revealed with a secondary gray phase distributed along the grain boundary (insets of Fig. 2b–d) in the back-scattered electron (BSE) images. This pointed towards the presence of higher atomic number elements (majorly Al in the present case) at the grain boundary region. The LSM samples clearly underwent grain refinement (average grain size in the range of 1.08–1.19 μm; Fig. 2b–d) compared to the as-cast sample. The refinement could be attributed to rapid melting followed by quenching during laser material interaction for a short amount of time (of the order of milliseconds), as observed in the literature [31].
Elemental analysis using EDS revealed Al enrichment at the grain boundary (Fig. 3), as observed in the BSE images (Fig. 2b–d). For the 3.18-J/mm2 condition sample, the average Al content in grains (1.66 wt%) was much lower than that in the grain boundaries (3.07 wt%). According to the results obtained in the XRD analysis (Fig. 1 and Table 2), it can be reasonably assumed that the Al-rich grain boundary regions are likely to be a β-phase. Development of surface microstructures can be attributed to kinetics of the laser processing. As soon as the surface reached the melting temperature, Al and Mg were completely miscible in each other [31]. This was followed by self-rapid quenching inherently associated with the laser [35] processing. As a result, Al was retained in the solid solution. As the beam moved away from the concerned location, reheating of the laser-melted region took place during the subsequent number of passes. This could have resulted in the precipitation and redistribution of the β-phase at the grain boundary. Development of such a host of microstructures had a clear influence on the corrosion behavior of LSM samples in the SBF medium.
Effect of LSM on corrosion performance
The influence of microstructure development during LSM on corrosion behavior is depicted in Tafel plots obtained during potentiodynamic polarization tests in SBF (Fig. 4). The primary observation suggested a shift in E corr value towards the positive side for LSM samples (Table 3). The value of i corr extracted from these Tafel plots clearly indicated a reduction in corrosion current with increase in laser energy density for the LSM samples (Table 3). These observations preliminarily indicated improvement in corrosion resistance for the LSM samples compared to the as-cast sample in SBF.
Further investigation of the Tafel plots (Fig. 4) revealed that the cathodic slope (β c) for all the samples remained within a narrow range (92–111 mV), indicating a similar rate of cathodic reaction for all the samples. On the contrary, the anodic slope (β a) was distinctly higher for the LSM samples (Table 3), indicating anodic dissolution to occur at slower rates for the LSM samples. The anodic part also possessed a passivation region. The passivation range defined by the difference between E pit (the point at which passive film starts to break) and E corr increased with laser energy density for the LSM samples (Table 3). The passivation behavior is likely due to the formation of a thin Mg(OH)2 surface layer during the corrosion process. This further confirmed the increased corrosion resistance of the LSM samples.
Such a behavior can be correlated to the microstructure development during surface melting by laser. Song et al. [10] reported that the corrosion potential of the β-phase is higher than that of the α-phase. It was also shown that the β-phase precipitated at the grain boundary posed a barrier to corrosion in NaCl solution in the case of AZ91. For the same alloy, on the other hand, the β-phase precipitated within the grain region worsened the corrosion resistance [10, 36, 37]. From a microstructure point of view, the process of corrosion occurs preferentially in the grain boundary due to the high surface energy possessed by these regions, making them anodic with respect to the grain interior. However, in the present case, the microstructure consisted of a β-phase around refined equiaxed grains of α. In addition, the volume fraction of the β-phase, which was calculated from the XRD spectra (Table 2), and the thickness of the melting zone (Fig. 2b–d) were the highest for the 3.18-J/mm2 sample. Considering the nobler corrosion potential of the β-phase than the α-phase [18], the β-phase can abate anodic effects of the grain boundaries and work as a barrier to restrain the process of corrosion, pitting in particular [10, 38].
The role of Al content was further pointed out by Coy et al., who demonstrated that the LSM-treated AZ91D Mg alloy generated a modified surface layer with ∼10 wt% content of aluminum with remarkably enhanced corrosion resistance in 3.5 wt% NaCl solution [22]. In contrast, Banerjee et al. showed that the LSM-treated ZE41 Mg alloy, which has no Al content, experienced severely poor corrosion performance compared to the untreated ZE41 Mg [39], indicating the importance of Al content. Hence, the improvement of the corrosion resistance due to the LSM process in the present case on AZ31B Mg alloy is likely to be correlated to the refined equiaxed α grains with a homogeneous and continuous β-phase network along the grain boundaries. These combined effects led to the delay in pitting process and allowed more time to form a passive layer on the sample surface.
The corroded surface of the as-cast sample indicated extensive pitting and network cracks which covered the whole surface, pointing towards aggressive corrosion attack (Fig. 5a). For the sample corresponding to a laser energy density of 1.06 J/mm2, the corroded surface had pits, crack network, and a small amount of smooth surface area representing the passive region. With further increase in laser energy density, the fraction of the smooth area increased and the crack network reduced (Fig. 5c). It indicates that the passive layer became more stable in the SBF solution. The sample treated with an energy density of 3.18 J/mm2 possessed the majority of the smooth regions on the corroded surface (Fig. 5d). Furthermore, there was a substantial reduction in the number of pits compared to all other samples (Fig. 5). In addition, the pits appeared much shallower in nature for this processing condition. These observations, along with the results of the potentiodynamic polarization measurements, indicated the highest corrosion resistance for the sample laser treated with an energy density of 3.18 J/mm2.
Conclusions
The present study on the effect of LSM on the corrosion behavior of AZ31B Mg alloy indicated that the corrosion resistance of AZ31B in SBF can be improved by the LSM process. The results of XRD, SEM, and EDS revealed equiaxed refined grains of α-Mg surrounded by a continuous network of β-phase (Mg17Al12) precipitated along the grain boundaries within the laser-melted region. With increasing laser energy density from 1.06 to 3.18 J/mm2, the depth of the melted region increased from 125 to 198 μm. The grain sizes within the laser-melted region were much finer (1.19, 1.09, and 1.08 μm for laser energy densities of 1.06, 2.12, and 3.18 J/mm2, respectively) compared to 10 μm for the as-cast sample. Development of such a microstructure resulted in an increase in corrosion resistance, as indicated by the potentiodynamic polarization measurements. The corrosion potential was higher (within the range from −1.45 to −1.48 V) for all the LSM samples than for the as-cast sample (−1.57 V). The corrosion current density reduced with an increase in laser energy density, from 12.14 μA/cm2 for the as-cast sample to 5.82 μA/cm2 for the sample laser surface-melted with an energy density of 3.18 J/mm2. Further, the passivation range increased with an increase in laser energy density from 0.14 V for the as-cast sample to 0.23 V for the sample laser surface-melted with 3.18 J/mm2.
References
Saris NL, Mervaala E, Karppanen H et al (2000) Magnesium: an update on physiological, clinical and analytical aspects. Clin Chim Acta 294(1):1–26
Zeng R, Dietzel W, Witte F et al (2008) Progress and challenge for magnesium alloys as biomaterials. Adv Eng Mater 10(8):B3–B14
Cohen J (1998) Current concepts review. Corrosion of metal orthopaedic implants. J Bone Joint Surg Am 80(10):1554
Puleo DA, Huh WW (1995) Acute toxicity of metal ions in cultures of osteogenic cells derived from bone marrow stromal cells. J Appl Biomater 6(2):109–116
Witte F, Kaese V, Haferkamp H et al (2005) In vivo corrosion of four magnesium alloys and the associated bone response. Biomaterials 26(17):3557–3563
Zhou W, Shen T, Aung NN (2010) Effect of heat treatment on corrosion behaviour of magnesium alloy AZ91D in simulated body fluid. Corros Sci 52(3):1035–1041
Li L, Gao J, Wang Y (2004) Evaluation of cyto-toxicity and corrosion behavior of alkali-heat-treated magnesium in simulated body fluid. Surf Coat Technol 185(1):92–98
Han G, Lee J, Kim Y et al (2012) Preferred crystallographic pitting corrosion of pure magnesium in Hanks’ solution. Corros Sci 63:316–322
Dahotre NB, Joshi SS (2016) Machining of bone and hard tissues. Springer International, Switzerland
Song GL, Atrens A (1999) Corrosion mechanisms of magnesium alloys. Adv Eng Mater 1(1):11–33
Shrikant JS (2014) Development of cast magnesium alloys with improved strength. Master’s thesis, Indian Institute of Science, Bangalore
Joshi S, Mohan M, Seshan S et al (2013) Effect of addition of Al & Ca and heat treatment on the cast Mg-6Zn alloy. Mater Sci Forum 765:33–37
Zhang X, Zhang K, Deng X et al (2012) Corrosion behavior of Mg–Y alloy in NaCl aqueous solution. Prog Nat Sci: Mater Int 22(2):169–174
Nakama D, Katoh K, Tokisue H (2008) Some characteristics of AZ31/AZ91 dissimilar magnesium alloy deposit by friction surfacing. Mater Trans 49(5):1137–41
Walton JR (2011) Bioavailable aluminum: its effects on human health. In: Nriagu JO (ed) Encyclopedia of environmental health. Elsevier, Burlington, pp 331–342
Homayun B, Afshar A (2014) Microstructure, mechanical properties, corrosion behavior and cytotoxicity of Mg–Zn–Al–Ca alloys as biodegradable materials. J Alloys Compounds 607:1–10
Zhang L, Zhang J, Chen C et al (2015) Advances in microarc oxidation coated AZ31 Mg alloys for biomedical applications. Corros Sci 91:7–28
Song G (2007) Control of biodegradation of biocompatible magnesium alloys. Corros Sci 49(4):1696–701
Song Y, Han E, Shan D et al (2012) The role of second phases in the corrosion behavior of Mg–5Zn alloy. Corros Sci 60:238–245
Alvarez-Lopez M, Pereda MD, del Valle JA et al (2010) Corrosion behaviour of AZ31 magnesium alloy with different grain sizes in simulated biological fluids. Acta Biomater 6(5):1763–1771
Sreekanth D, Rameshbabu N (2012) Development and characterization of MgO/hydroxyapatite composite coating on AZ31 magnesium alloy by plasma electrolytic oxidation coupled with electrophoretic deposition. Mater Lett 68:439–442
Coy AE, Viejo F, Garcia-Garcia FJ et al (2010) Effect of excimer laser surface melting on the microstructure and corrosion performance of the die cast AZ91D magnesium alloy. Corros Sci 52(2):387–397
Taltavull C, Torres B, Lopez AJ et al (2014) Corrosion behaviour of laser surface melted magnesium alloy AZ91D. Mater Des 57:40–50
Li W, Zhu L, Shan D (2006) Effects of low temperature thermal treatment on zinc and/or tin plated coatings of AZ91D magnesium alloy. Surf Coat Technol 201(6):2768–75
Cao F, Cao J, Zhang Z et al (2007) Plasma electrolytic oxidation of AZ91D magnesium alloy with different additives and its corrosion behavior. Mater Corros 58(9):696–703
Joshi SS, Katakam S, Singh Arora H et al (2016) Amorphous coatings and surfaces on structural materials. Crit Rev Solid State Mater Sci 41(1):1–46
Abbas G, Liu Z, Skeldon P (2005) Corrosion behaviour of laser-melted magnesium alloys. Appl Surf Sci 247(1–4):347–353
Trdan U, Grum J (2012) Evaluation of corrosion resistance of AA6082-T651 aluminium alloy after laser shock peening by means of cyclic polarisation and ElS methods. Corros Sci 59:324–333
Mondal AK, Kumar S, Blawert C et al (2008) Effect of laser surface treatment on corrosion and wear resistance of ACM720 Mg alloy. Surf Coat Technol 202(14):3187–98
Gao Y, Wang C, Yao M et al (2007) Corrosion behavior of laser melted AZ91HP magnesium alloy. Mater Corros 58(6):463–466
Ho YH, Vora HD, Dahotre NB (2015) Laser surface modification of AZ31B Mg alloy for bio-wettability. J Biomater Appl 29(7):915–928
Berglund I (2010) Design and evaluation of a biodegradable magnesium alloy for use as an implant material. Master’s thesis, Karolinska Institute, Stockholm
Dahotre NB, Paital SR, Samant AN et al (2010) Wetting behaviour of laser synthetic surface microtextures on Ti–6Al–4V for bioapplication. Phil Trans R Soc A 368(1917):1863–1889
Norrish K, Taylor R (1962) Quantitative analysis by X-ray diffraction. Clay Miner Bull 5(28):98–109
Joshi SS, Gkriniari AV, Katakam S et al (2015) Dynamic crystallization during non-isothermal laser treatment of Fe–Si–B metallic glass. J Phys D 48(49):495501
Song G, Atrens A, Wu X et al (1998) Corrosion behaviour of AZ21, AZ501 and AZ91 in sodium chloride. Corros Sci 40(10):1769–1791
Zhao M, Liu M, Song G et al (2008) Influence of the β-phase morphology on the corrosion of the Mg alloy AZ91. Corros Sci 50(7):1939–1953
Salman S, Ichino R, Okido M (2010) A comparative electrochemical study of AZ31 and AZ91 magnesium alloy. Int J Corros 2010:1–7
Banerjee PC, Raman RKS, Durandet Y et al (2011) Electrochemical investigation of the influence of laser surface melting on the microstructure and corrosion behaviour of ZE41 magnesium alloy—an EIS based study. Corros Sci 53(4):1505–1514
Acknowledgements
The authors wish to acknowledge the Center for Advanced Research and Technology (CART) at the University of North Texas for providing access to the microstructure characterization facilities. The authors also thank Dr. H.D. Vora for helping in laser processing of the samples.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Role of funding source
There was no funding provided from the external sources as well as internal sources towards the research work presented in the manuscript.
Research involving human participants and/or animals
Neither animals nor human subjects were involved in the research work presented in the manuscript.
Informed consent
No human subjects were involved in the research work presented in the manuscript. Therefore, informed consent is not applicable.
Rights and permissions
About this article
Cite this article
Wu, TC., Ho, YH., Joshi, S.S. et al. Microstructure and corrosion behavior of laser surface-treated AZ31B Mg bio-implant material. Lasers Med Sci 32, 797–803 (2017). https://doi.org/10.1007/s10103-017-2174-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-017-2174-1