Abstract
The aim of this study was to evaluate the preventive and/or reparative effects of low-level laser therapy (LLLT) on orthodontically induced inflammatory root resorption (OIIRR) in rats. Thirty rats were divided into four groups (short-term control (SC), short-term laser (SL), long-term control (LC), long-term laser (LL)). In all groups, the left first molar was moved mesially for 11 days. At the end of this period, the rats in groups SC and SL were killed in order to observe the resorption lacunas and to evaluate whether LLLT had any positive effect on root resorption. The groups LC and LL were remained for a healing period of 14 days in order to observe spontaneous repair of the resorption areas and investigate whether LLLT had reparative effects on root resorption. A Ga-Al-As diode laser (Doris, CTL-1106MX, Warsaw, Poland) with a wavelength of 820 nm was used. In SL group, the first molars were irradiated with the dose of 4.8 J/cm2 (50 mW, 12 s, 0.6 J) on every other day during force application. In LL group, the irradiation period was started on the day of appliance removal and the first molars were irradiated with the dose of 4.8 J/cm2 on every other day for the next 14 days. LLLT significantly increased the number of osteoblasts and fibroblasts, and inflammatory response in SL group in comparison with SC group (P = .001). The amount of resorption did not represent any difference between the two groups (P = .16). In LL group, LLLT significantly increased the number of fibroblasts and decreased the amount of resorption in comparison with LC group (P = .001; P = .02). Both parameters indicating the reparative and the resorptive processes were found to be increased by LLLT applied during orthodontic force load. LLLT applied after termination of the orthodontic force significantly alleyed resorption and enhanced/accelerated the healing of OIIRR. LLLT has significant reparative effects on OIIRR while it is not possible to say that it definitely has a preventive effect.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Root resorption, an undesirable complication of orthodontic treatment, is considered as a side effect of the cellular activity associated with the removal of necrotic tissue in an over-compressed periodontal ligament [1]. Among treated adolescents, some form of orthodontically induced inflammatory root resorption (OIIRR) incidence is reported as 93 % while 10–20 % of the cases had severe resorption of >3 mm [2, 3]. Several chemicals/pharmacological agents (PGE2, calcium gluconate, doxycycline, L-thyroxin, bisphosphonates, tetracycline, fluoride) have been tested to prevent or to repair this pathological condition [4–7]. Although most of them were found effective, they did not get into clinical use due to their possible systemic or adverse effects on orthodontic tooth movement.
The biostimulatory effects of low-level laser therapy (LLLT) on several processes have been investigated since 1971, and it has been found very beneficial in treatment of inflammatory problems [8]. With the molecular absorption of laser light, different cellular organelles are affected and several cellular responses are triggered creating anti-inflammatory or regenerative changes [9, 10]. There are several studies in literature that reported the positive outcomes of 800–830-nm LLLT applied in various fields [11]. Osteoblast and osteoclast differentiation, the expression of receptor activator of nuclear factor kappa ligand (RANKL)- and RANK-positive cells, and bone remodeling [12] were increased during orthodontic tooth movement, and collagen synthesis was stimulated [12, 13] via the therapy. Furthermore, Bicakci et al. [10] and Eslamian et al. [14] found that LLLT was efficient in reducing orthodontic post-adjustment pain.
As is well known in orthodontics, the immediate response of the periodontal ligament to mechanical force is inflammatory causing not only orthodontic tooth movement but also some amount of root resorption. This resorption starts as small lacunae, deepens gradually, and ends up with flattening of root apex or shortening of root length. Depending on the reparative effects of LLLT, it was thought that this therapy could be useful for preventing or repairing root resorption.
In this regard, the aim of this study was to evaluate the preventive and/or reparative effects of LLLT on orthodontically induced inflammatory root resorption (OIIRR) in rats.
Material and methods
Study sample
Twelve-week-old, 30 male Wistar albino rats (weighing 185 ± 10 g) were obtained from the animal laboratory of Cumhuriyet University Faculty of Medicine. The experimental protocol was approved by the Animal Research Ethics Committee of Cumhuriyet University. Guidelines for using laboratory animals were strictly followed throughout the study. All animals were housed in a 12-h light/dark environment at the constant temperature of 23 °C and fed a standard pellet diet with tap water ad libitum. The rats were monitored during the experiment, and all animals were weighed on every day of experiment. The animals were randomly divided into four groups.
Short-term control group (group SC)
The maxillary left first molars of seven animals were moved mesially for 11 days. All animals in this group were killed just after the mesialization period in order to observe the resorption lacunas induced by mechanical force.
Long-term control group (group LC)
In this group, the same mesialization procedure was performed for 11 days. Afterward, the appliances were removed and the first molars were stabilized for 14 days of healing period. At the end of this period, the animals were killed and histological investigations were performed in order to observe spontaneous repair of the resorption lacunas.
Short-term laser group (group SL)
The mesialization of maxillary left first molars of seven animals was provided, and the first molars were irradiated on every other day with the dose of 4.8 J/cm2 starting from the first day. The animals were killed after 11 days. The aim of this group was to evaluate whether LLLT had any preventive effect on root resorption or not.
Long-term laser group (group LL)
In this group, maxillary left first molars were mesialized as it was in other groups for 11 days. On the day of appliance removal and stabilization, the irradiation period was started. For the next 14 days, the first molars were irradiated on every other day with the dose of 4.8 J/cm2. The aim of this group was to investigate whether LLLT had reparative effects on root resorption or not.
Control group (C)
The contralateral maxillary right first molars of seven rats which were not moved served as the controls to represent physiological situation at the related tissues.
Appliance design
Appliances were attached to maxillary incisors and upper left first molars under anesthesia with xylasine (Rompun-Bayer, Lever-Kusen, Germany, 3 mg/kg) and ketamine (Ketalar-Pfizer, USA, 90 mg/kg) combination. The appliance comprised a Ni-Ti closed coil spring (Rocky Mountain Orthodontics, Denver, CO, USA) and ligature wires. The coil springs were cut into 5-mm pieces and fixed with 0.015-in ligature wires (Dentaurum, Ispringen, Germany) distally to the maxillary first molars and anteriorly to the incisors (Fig. 1). A groove at the level of the gingival papilla was prepared on the distal sides of the incisor teeth using a stainless steel disc for retention of the ligature wires. The ligature wires around the teeth were sealed with composite resins to increase the stability of the appliance. The force obtained from the coil spring was calibrated with a gauge to 50 g and was not reactivated until the end of the experimental period.
In the long-term groups (LC and LL), the appliance was removed, and composite resin (Transbond XT, 3 M/Unitek, Monrovia, CA, USA) was placed in the interdental space between the left first and second molars to provide stability.
Laser irradiation
A Ga-Al-As diode laser (Doris, CTL-1106MX, Warsaw, Poland) with a wavelength of 820 nm was used. The irradiation was performed with continuous waves by a fiber applicator 2 mm in diameter (Doris, CTL-2241) on every other day during the irradiation period with the parameters represented in Table 1. The animals were anesthetized for each laser exposure session. The mucosa covering the root of the tooth was irradiated from four points (two points at buccal, two at the palatinal side).
Histological preparation
After the animals were killed with an overdose of anesthetic (200 mg/kg sodium pentobarbital; Petothal, Abbot, ABD), the appliance and tooth-bearing segments of the maxilla were immediately dissected and kept in 10 % formalin solution for 48 h. The specimens were decalcified in 10 % disodium ethylenediaminetetraacetic acid (pH 7.4) solution for 6 weeks, and then a routine paraffin procedure was performed. Briefly, tissue samples were dehydrated through graded alcohol series and cleared in xylene and embedded in paraffin. Five-micrometer para-sagittal sections were cut and prepared for both histochemical and indirect immunohistochemical stainings. The slide showing the greatest length of the distopalatal root and four adjacent slides were alternatively stained with H&E, anti-RANKL, and anti-osteoprotegerin (OPG) immunostaining system.
Histochemical evaluation
The area of investigation was the mesial aspect of the distopalatal root and adjacent structures, including the inter-radicular alveolar bone septum. Hematoxylin–eosin (H&E) stain was applied for histochemical evaluation of number of osteoblasts and fibroblasts, osteoclasts, capillaries, and resorption areas. The deckle-edged cells with an eosinophilic cytoplasm, placed in Howship’s lacunae that had been resorbing bony tissue, were considered as osteoclasts. The oval-shaped active cells with a dark basophilic cytoplasm were considered as osteoblasts. The scores of osteoblasts were determined as follows: 1 to 10 cells, mild (+); 10 to 20 cells, moderate (++); 20 to 30 cells, strong (+++); and more than 30 cells, very strong (++++). The areas lined with endothelium that contained blood cells were considered as capillaries.
Immunohistochemical evaluation
In this study, immunolocalization of RANKL and OPG was examined by indirect immunohistochemistry. For this purpose, the avidin–biotin peroxidase system was used. Anti-receptor activator of nuclear factor kappa ligand (anti-RANKL) (1:100 dilution, Santa Cruz, sc-7628, Ca, USA), anti-osteoprotegerin (anti-OPG) (1:100 dilution, Santa Cruz, sc-8468, Ca/USA), and, as a secondary antibody, ABC staining system (Santa Cruz, sc-2023, Ca, USA) were used. The presence of a red-brown precipitate indicated positive findings for the primary antibodies. The observer (SI), blinded to clinical information, evaluated the staining scores, and the mean values of the staining intensities were graded semi-quantitatively as mild (+), moderate (++), strong (+++), or very strong (++++).
Statistical analysis
Data analysis was performed by SPSS for Windows, version 16 (SPSS Inc, Chicago, IL). Means and standard deviations for number of osteoclasts were calculated and compared with Kruskal-Wallis test. Pairwise comparisons were made by Mann-Whitney U test. The parameters representing number of osteoblasts and fibroblasts, number and width of capillaries, inflammatory cell infiltration, resorption, RANKL, and OPG immunoreactivities were defined as the scores indicating intensities. The scores of the groups were compared with Fisher’s exact test. A value of P < .05 was considered statistically significant.
Results
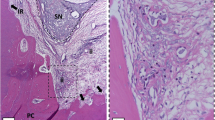
The animals did not show obvious signs of systemic illness throughout the study period. Deep mucosal infection, dehiscence, or other adverse effects were not encountered in any of the animals in our study. Weight loss did not increase to 10 % in all groups throughout the study. Descriptive statistics and statistical comparisons of groups for number of osteoclasts are represented in Table 2. The scores indicating the intensities and comparison of the scores are represented in Table 3 and Table 4. Photomicrographs stained with H&E and those immunostained with anti-RANKL and anti-OPG are represented in Fig. 2.
Photomicrographs stained with H&E and immunostained with anti-RANKL and anti-OPG primary antibody and scored according to the number of cells and capillaries, amount of resorption, and the staining intensity as mild (+), moderate (++), strong (+++), or very strong (++++); (×400 original magnification; fields, ×4 original magnification). Follow the integrity of cement layer; the groups SL and LC represented extensive resorption of the root surface, and the integrity of cementum was impaired. The integrity of cementum was ensured in group LL. Arrows, multinucleated clastic cells in resorption lacunae. C cementum, D dentine, Pl periodontal ligament, R resorptive areas
Histological findings
Control group
At the control side of the maxilla, the teeth and supporting tissues showed a normal histological appearance. Only a few small lacunae were observed, indicating the physiological distal drift of the teeth.
Short-term control group
The signs of typical inflammatory response to orthodontic force were observed, such as mild increase in number of capillaries providing inflammatory cell infiltration. Also, the numbers of osteoclasts, osteoblasts, and fibroblasts were increased, but yet milder than they were in the other groups. The intensity of resorption was moderate generally. When the findings of group LC were considered, it might be thought that the inflammatory response to orthodontic force had just started in this group and would probably increase in time.
Short-term laser group
The highest increases in number of osteoclasts and capillaries, and relatively intense inflammatory response were observed in this group. Hence, the intensity of resorption was found considerably increased. These findings indicated that LLLT intensified the response to orthodontic force. On the other hand, the intensity of resorption and the amount of increase in number of osteoclasts in this group and in LC group did not represent any difference (P > .05). This might be interpreted as LLLT brought forward or accelerated the response period rather than intensifying it. The numbers of osteoblasts and fibroblasts were significantly increased in comparison to group SC (P = .001). This increase was similar to that in LL group as well (P > .05). This means that reparative/formation process was scheduled to an earlier time in this group by LLLT.
Long-term control group
The number of osteoclasts was increased notably and the most intense resorption was observed in this group. Also, the number and width of capillaries was found to be increased.
The numbers of osteoblasts and fibroblasts were increased moderately in this group while the increase was lower than that in LL group. As the photomicrographs represented, cement layer was disintegrated and resorption process was dominant to reparative process in this group.
Long-term laser group
The number of osteoclasts and the intensity of resorption represented the least increase in this group compared to other groups. The numbers of osteoblasts and fibroblasts were found notably increased. The findings indicated that reparative process was dominant to resorption process. The provided integrity of the cement layer and scarcely any resorption cavities could be followed at photomicrographs already.
Immunohistochemical findings
RANKL immunoreactivity
RANKL-positive fibroblasts, osteoblasts, and osteoclasts in surrounding tissue were observed in all groups. LC group presented very strong RANKL immunoreactivity, while strong and moderate immunoreactivities were observed in SL and LL groups, respectively (Table 3).
OPG immunoreactivity
OPG immunoreactivity was observed in fibroblasts, osteoblasts, and osteoclasts and it was very strong in LL group. OPG immunoreactivity gradually increased in groups SC, LC, SL, and LL, respectively. The difference among the groups was found to be statistically significant (P < .05; Table 3).
RANKL/OPG ratio
The LL group showed the lowest value for this ratio indicating that the osteoclastic activity was the least in this group. The ratio was found to be increased more and more in groups SL, LC, and SC, respectively.
Discussion
As an adverse outcome of orthodontic treatment, root resorption has been reported in 93 % of the treated adolescents, nearly 15 % being moderate to severe while 10–20 % of the cases had severe resorption of >3 mm [2, 3, 15]. Although interrupting orthodontic force application may stop the further resorption, it would end the treatment without achieving the final goals. Furthermore, a significant reduction in root length may cause an unfavorable crown/root ratio of the affected teeth with increased mobility and periodontal destruction inducing vulnerability to occlusal forces [16]. Up to the present, several pharmacological agents have been tested to inhibit orthodontically induced root resorption (OIIRR) [4–7]. However, none of them get into clinical use due to their possible systemic effects or adverse effects on orthodontic tooth movement. On the other hand, low-level laser therapy (LLLT) is a considerably inexpensive and harmless method with its effects being limited at the target tissue. In the present study, the effects of LLLT on root resorption were investigated histologically and immunohistochemically.
Orthodontic force induces local tissue degradation followed by a process with inflammatory characteristics while causing/creating tooth movement [17]. OIIRR, a side effect of tooth movement, is a sterile inflammatory process involving various tissues, cells, and certain known biological messengers [18, 19].
Foo et al. reported that OIIRR was started by orthodontic tooth movement and that with a continuing force, cementum repair could not occur [20]. The appliance system used in this study produced a continuous force over the experimental period and moved the teeth orthodontically inducing root resorption. Hellsing and Hammarstrom found definitive resorption sites forming within 1 week of appliance placement [21]. Similarly, Jager et al. evaluated the repair of root resorption after 9 days of force application [22]. Hence, an experimental period of 11 days was thought to be sufficient to produce resorption lacunas for investigation.
The amount of force varies from 10 to 100 g among studies evaluating OIIRR in a rat model [22–24]. As mostly experimented, the coil springs of 50 g were used for the mesial movement of the molars in the present study. Actually, this was a considerably heavy force which coincides to 1000 g for a human molar [25]. However, we considered the relatively short experimental period and aimed to cause significant resorption craters rather than smooth concavities in order to represent the possible early and late differences between groups.
The anti-inflammatory and therapeutic (biostimulative) properties of LLLT have been very well known since 1971. LLLT is a stimulator of the ongoing biological process in tissue and has been found to be effective in modulating cell activity and production of endogenous molecules, which are also involved in orthodontic tooth movement [26, 27]. The therapy has been found to be capable of acceleration of macrophage migration and increasing their phagocytosis, increasing production of cytokines, prostaglandins, and TGF-β, activation of BMP, stimulation of bone tissue metabolism, and improving neo-angiogenesis and flow in microcirculation during bone healing [8, 28, 29]. OIIRR is a part of the elimination of hyaline zone, and this process continues until all hyaline tissue is removed [30]. The first cells to be involved in this necrotic tissue removal are macrophage-like cells and multinucleated giant cells which may be osteoclasts or odontoclasts [17, 30]. In this study, the groups SC and SL represented typical OIIRR sites in addition to increased number of osteoclasts at the end of the force-applied experimental period. Although the number of osteoclasts was more increased in SL group, the difference in amount of resorption was not significant statistically. According to Karu et al., the mitochondrial cytochromes absorb the photon energy leading to increase in ATP synthesis and improvement of the potential activity of the cells [9]. Because osteoclasts are multinuclear cells with high mitochondrial activity, they are affected from low-level laser irradiation readily. Furthermore, Hentunen et al. reported that the bone matrix liberates a light dose-dependent protein that stimulates osteoclast formation [31]. On the other hand, the number of osteoblasts and fibroblasts, and OPG immunoreactivity, which could be regarded as the elements of formation process, were found significantly higher in SL group. This might explain the indifference in amount of resorption despite the increased number of osteoclasts. As is well known, LLLT stimulates the ongoing biological process in tissue. While being under the orthodontic force, the therapy increased the response of the tissue, the inflammatory process, osteoclastic activity, and also the osteoblastic activity, like it enhances the orthodontic tooth movement observed previously [32]. Hereby, we observed such a significant difference between short-term groups. Moreover, the parameters both indicating the formation process and the resorptive process were found similar statistically in SL and LC groups (P > .05). This might be interpreted as LLLT brought forward or accelerated the whole response of the tissues rather than intensifying it.
It has been previously reported that cementum repair starts 12 days after force termination [22, 33, 34], and the repair process was found to be increased over the first 4 weeks of retention, slowed in the fifth and sixth weeks, and finally reaching a plateau at the end of 6 weeks [35, 36]. Although Rygh had stated that reparative process occurred when the orthodontic force stopped or decreased below a certain level [37], Brudvik et al. and Cheng et al. reported that root resorption continued in the hyalinised tissue area for another 4 weeks even after the orthodontic force had ceased [30, 38]. Today, it has been known that resorption and repair processes occurred simultaneously and resorption diminished the amount of repair all along the initial phases of the retention [38]. In accordance with this, the intensity of resorption in LC group was found to be unabated at the end of the retention period in the present study while it was found to be dramatically decreased in LL group which could be attributed to the positive effect of LLLT on repair process. As we have already known that osteoclastic activity continues until all necrotic tissue has been resorbed [30], the results of the present study could be interpreted as LLLT provided prompt removal of the necrotic tissue due to its anti-inflammatory effects. Also, repair process might be improved with the regenerative effects of the therapy.
Evaluation of the long-term groups showed that the number of osteoclasts was significantly decreased in LL group while it increased in LC group being in contrast to short-term results. When LC group was considered as a continuation of SC group, it would be observed that the number of osteoclasts had been continuing to rise although the orthodontic force had been stopped. This inference is in accordance with the findings of Brudvik and Rygh [30], which meant that the resorptive process had still been going on dominantly. Meanwhile, in LL group, the decreased number of osteoclasts and considerably increased number of osteoblasts and fibroblasts indicated that the predominant process had been reparative rather than destructive that was provided by LLLT.
As an alternative method to define osteoclastic activity, it is possible to evaluate receptor activator for nuclear factor κ B ligand (RANKL), an osteoclast differentiation factor, which is essential for the induction of osteoclastogenesis. In the study of Low et al., it was demonstrated that mRNA of RANK was detected in tissues involved in root resorption following application of orthodontic forces [39]. Furthermore, Yamaguchi et al. reported that compressed periodontal ligament (PDL) cells obtained from tissues with severe external apical root resorption produced a large amount of RANKL that upregulated osteoclastogenesis [40]. Recently, osteoprotegerin (OPG), a novel inhibitor of osteoclastogenesis, was identified that inhibits osteoclast terminal differentiation from their progenitors, as well as the function of mature osteoclasts [41]. Kanzaki et al. stated that OPG gene transfer to periodontal tissue inhibited RANKL-mediated osteoclastogenesis and inhibited experimental tooth movement [42]. Based on these, Nakano et al. claimed that osteoclast and odontoclast differentiation in root resorption areas is critically regulated by RANKL, which is produced by PDL cells in response to orthodontic force and concluded that the OPG/RANKL/RANK system may play an important role in OIIRR [43]. When the groups of the present study were compared, RANKL/OPG ratio was found to be lowest in LL group and highest in the SC group indicating the degree/intensity of osteoclastic activity. Although the number of osteoclasts was higher in the SL group, the RANKL/OPG ratio of this group was found to be lower than that of groups SC and LC. This might depend on the fact that LLLT provoked also the OPG syntheses which could inhibit the activity of mature osteoclasts leading to reduced amount of resorption. This could clarify the controversy that the intensity of resorption was higher in SC group while the difference for number of osteoclasts was not statistically significant between SC group and LL group. The same explanation is valid for the situation that the intensity of resorption was higher in LC group than the SL group while the number of osteoclasts was higher in the latter group.
It has been stated that early repair of the resorption lacunae starts with the invasion of fibroblast-like cells from the surrounding periodontal ligament [30, 36, 44]. After detachment of clastic cells, an initial uncalcified cementoid repair matrix is deposited by repair cells, fibroblast-like and cementoblastic cells, and followed by periodontal ligament re-attachment [30, 44]. Also, osteoblasts synthesize and deposit non-collagenous proteins in cementum [22]. In this study, the lased groups represented the most increase in the number of osteoblasts and fibroblasts while SC group represented the least increase. That is to say that at early times of root resorption, repair process started slowly under normal conditions as it was observed in SC group. On the other hand, this process was considerably active in SL group as much as it was in LL group. This means that LLLT stimulated proliferation of osteoblastic and fibroblastic cells and enhanced the reparative process not only in LL group but also in SL group. Zaidi et al. claimed that osteoblasts and osteoclasts have hormonal interaction [45]. It has been revealed that osteoclastic activity may influence posterior osteoblastic activity and vice versa [46]. Similarly, the osteoblastic activity might be increased simultaneously in our lased groups. This situation may be interpreted as a healing effect of LLLT on root resorption.
The resorption process initially takes place at the periphery of the hyaline zone, where blood supply to the periodontal ligament exists or even increased. For overcoming this inflammatory response, inflammatory cell infiltration is provided via an increase in number and width of capillaries. In the present study, the increase in new capillary formation and inflammatory cell infiltration was found to be most in SL group indicating that the biological response to orthodontic force was increased with LLLT.
Depending on the findings of the present study, it was revealed that 14 days of repair period was not enough for perceptible amount of repair of OIIRR in a rat study model. If the period would be longer, healing would extend, resorption lacunas would be more repaired, and the effects of the therapy would be more distinct.
Conclusions
Under the conditions of this experiment, the following conclusions were drawn.
-
1.
Application of 50 g of mesializing force during 11 days caused significant OIIRR of the maxillary first molars of Wistar rats.
-
2.
Both parameters indicating the reparative and the resorptive processes were found to be increased by LLLT applied during orthodontic force load.
-
3.
LLLT applied after termination of the orthodontic force significantly allayed resorption and enhanced/accelerated the healing of OIIRR.
-
4.
LLLT has significant reparative effects on OIIRR while it is not possible to say that it definitely has a preventive effect.
Further studies investigating long-term repair process are needed for a better understanding of the effects of LLLT on OIIRR.
References
Reitan K (1951) The initial tissue reaction incident to orthodontic tooth movement as related to the influence of function; an experimental histologic study on animal and human material. Acta Odontol Scand 6:1–240
Kurol J, Owman-Moll P, Lundgren D (1996) Time-related root resorption after application of a controlled continuous orthodontic force. Am J Orthod Dentofacial Orthop 110(3):303–310
Levander E, Malmgren O (1988) Evaluation of the risk of root resorption during orthodontic treatment: a study of upper incisors. Eur J Orthod 10(1):30–38
Golub LM, Mc Namara TF, D’Angelo G, Greenwald RA, Ramamrthy NS (1987) A non-bacterial chemically-modified tetracycline inhibits mammalian collagenase activity. J Dent Res 66:1310–1314
Ong CKL, Walsh LJ, Harbrow D, Taverne AR, Symons AL (2000) Orthodontic tooth movement in the prednisolone-treated rat. Angle Orthod 70:118–125
Seifi M, Eslami B, Saffar AS (2003) The effect of Prostaglandin E2 and calcium gluconate on orthodontic tooth movement and root resorption in rats. Eur J Orthod 225:199–204
Baysal A, Uysal T, Ozdamar S, Kurt B, Kurt G (2010) Comparisons of the effects of systemic administration of l-thyroxine and doxycycline on orthodontically induced root resorption in rats. Eur J Orthod 32(5):496–504
Glinkowski W, Pokora L (2001) Lasers in therapy. Laser instruments, Centrum Techniki Laserowej. Warsaw, Poland: Laser instruments, Centrum Techniki Laserowej
Karu T (1999) Primary and secondary mechanisms of action of visible to near-IR radiation on cells. J Photochem Photobiol 49:1–17
Bicakci AA, Kocoglu-Altan B, Toker H, Mutaf I, Sumer Z (2012) Efficiency of low-level laser therapy in reducing pain induced by orthodontic forces. Photomed Laser Surg 30(8):460–465
Altan AB, Bicakci AA, Avunduk MC, Esen H (2014) The effect of dosage on the efficiency of LLLT in new bone formation at the expanded suture in rats. Lasers Med Sci Sep 17. [Epub ahead of print]
Fujita S, Yamaguchi M, Utsunomiya T, Yamamoto H, Kasai K (2008) Low-energy laser stimulates tooth movement velocity via expression of RANK and RANKL. Orthod Craniofac Res 11:143–155
Saito S, Shimizu N (1997) Stimulatory effects of low-power laser irradiation on bone regeneration in midpalatal suture during expansion in the rat. Am J Orthod Dentofacial Orthop 111:525–532
Eslamian L, Borzabadi-Farahani A, Hassanzadeh-Azhiri A, Badiee MR, Fekrazad R (2014) The effect of 810-nm low-level laser therapy on pain caused by orthodontic elastomeric separators. Lasers Med Sci 29(2):559–564
Linge L, Linge BO (1991) Patient characteristics and treatment variables associated with apical root resorption during orthodontic treatment. Am J Orthod Dentofacial Orthop 99(1):35–43
Huang GJ, Richmond S, Vig KWL (2011) Evidence-based orthodontics. Blackwell Publishing, UK
Brezniak N, Wasserstein A (2002) Orthodontically induced inflammatory root resorption. Part I: The basic science aspects. Angle Orthod 72:175–179
Krishnan V, Davidovitch Z (2006) Cellular, molecular, and tissue-level reactions to orthodontic force. Am J Orthod Dentofacial Orthop 129(4):469, e1-32
Meikle MC (2006) The tissue, cellular, and molecular regulation of orthodontic tooth movement: 100 years after Carl Sandstedt. Eur J Orthod 28(3):221–240
Foo M, Jones A, Darendeliler MA (2007) Physical properties of root cementum: Part 9. Effect of systemic fluoride intake on root resorption in rats. Am J Orthod Dentofacial Orthop 131:34–43
Hellsing E, Hammarstrom L (1996) The hyaline zone and associated root surface changes in experimental orthodontics in rats: a light and scanning electron microscope study. Eur J Orthod 18:11–18
Jäger A, Kunert D, Friesen T, Zhang D, Lossdörfer S, Götz W (2008) Cellular and extracellular factors in early root resorption repair in the rat. Eur J Orthod 30(4):336–345
Gonzales C, Hotokezaka H, Yoshimatsu M, Yozgatian JH, Darendeliler MA, Yoshida N (2008) Force magnitude and duration effects on amount of tooth movement and root resorption in the rat molar. Angle Orthod 78(3):502–509
Gonzales C, Hotokezaka H, Darendeliler MA, Yoshida N (2010) Repair of root resorption 2 to 16 weeks after the application of continuous forces on maxillary first molars in rats: a 2- and 3-dimensional quantitative evaluation. Am J Orthod Dentofacial Orthop 137(4):477–485
Ren Y, Maltha JC, Kuijpers-Jagtman AM (2004) The rat as a model for orthodontic tooth movement—a critical review and a proposed solution. Eur J Orthod 25:483–490
Chen JW, Zhou YC (1989) Effect of low level carbon dioxide laser radiation on biochemical metabolism of rabbit mandibular bone callus. Laser Therapy 1:83–88
Zhang L, Xing D, Gao X, Wu S (2009) Low-power laser irradiation promotes cell proliferation by activating PI3K/Akt pathway. J Cell Phys 219(3):553–562
Nicola RA, Jorgetti V, Rigau J, Pacheco MT, dos Reis LM, Zângaro RA (2003) Effect of low-power GaAlAs laser (660 nm) on bone structure and cell activity: an experimental animal study. Lasers Med Sci 18(2):89–94
Hirata S, Kitamura C, Fukushima H, Nakamichi I, Abiko Y, Terashita M, Jimi E (2010) Low-level laser irradiation enhances BMP-induced osteoblast differentiation by stimulating the BMP/Smad signaling pathway. J Cell Biochem 111(6):1445–1452
Brudvik P, Rygh P (1995) Transition and determinants of orthodontic root resorption-repair sequence. Eur J Orthod 17:177–188
Hentunen TA, Cunningham NS, Vuolteenaho O, Reddi AH, Vaananen HK (1994) Osteoclast recruiting activity in bone matrix. Bone Miner 25:183–198
Genc G, Kocadereli I, Tasar F, Kilinc K, El S, Sarkarati B (2013) Effect of low-level laser therapy (LLLT) on orthodontic tooth movement. Lasers Med Sci 28(1):41–47
Mavragani M, Brudvik P, Selvig KA (2005) Orthodontically induced root and alveolar bone resorption: inhibitory effect of systemic doxycycline administration in rats. Eur J Orthod 27:215–225
Götz W, Kunert D, Zhang D, Kawarizadeh A, Lossdörfer S, Jager A (2006) Insulin-like growth factor system components in the periodontium during tooth root resorption and early repair processes in the rat. Eur J Oral Sci 114:318–327
Owman-Moll P, Kurol J, Lundgren D (1995) Repair of orthodontically induced root resorption in adolescents. Angle Orthod 65:403–408
Vardimon AD, Graber TM, Pitaru S (1993) Repair process of external root resorption subsequent to palatal expansion treatment. Am J Orthod Dentofacial Orthop 103:120–130
Rygh P (1977) Orthodontic root resorption studied by electron microscopy. Angle Orthod 47:1–16
Cheng LL, Türk T, Elekdağ-Türk S, Jones AS, Petocz P, Darendeliler MA (2009) Physical properties of root cementum: Part 13. Repair of root resorption 4 and 8 weeks after the application of continuous light and heavy forces for 4 weeks: a microcomputed-tomography study. Am J Orthod Dentofacial Orthop 136(3):320, e1-10
Low E, Zoellner H, Kharbanda OP, Darendeliler MA (2005) Expression of mRNA for osteoprotegerin and receptor activator of nuclear factor kappa ß ligand (RANKL) during root resorption induced by the application of heavy orthodontic forces on rat molars. Am J Orthod Dentofacial Orthop 128:497–503
Yamaguchi M, Aihara N, Kojima T, Kasai K (2006) RANKL increase in compressed periodontal ligament cells from root resorption. J Dent Res 85:751–756
Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS et al (1997) Osteoprotegerin—a novel secreted protein involved in the regulation of bone density. Cell 89:309–319
Kanzaki H, Chiba M, Takahashi I, Haruyama N, Nishimura M, Mitani H (2004) Local OPG gene transfer to periodontal tissue inhibits orthodontic tooth movement. J Dent Res 83:920–925
Nakano Y, Yamaguchi M, Fujita S, Asano M, Saito K, Kasai K (2011) Expressions of RANKL/RANK and M-CSF/c-fms in root resorption lacunae in rat molar by heavy orthodontic force. Eur J Orthod 33(4):335–343
Brudvik P, Rygh P (1995) The repair of orthodontic root resorption: an ultrastructural study. Eur J Orthod 17:189–198
Zaidi M, Pazianas M, Shankar VS, Bax BE, Bax CM, Bevis PJ, Stevens C, Huang CL, Blake DR, Moonga BS (1993) Osteoclast function and its control. Exp Physiol 78:721–739
McDonald BR, Gowen M (1993) The cell biology of bone. Baillières Clin Rheumatol 7:421–443
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Altan, A.B., Bicakci, A.A., Mutaf, H.I. et al. The effects of low-level laser therapy on orthodontically induced root resorption. Lasers Med Sci 30, 2067–2076 (2015). https://doi.org/10.1007/s10103-015-1717-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-015-1717-6